INTRODUCTION
The frequency and severity of invasive Haemophilus influenzae type b (Hib) infection in infants and children aged <5 years, together with the development of well tolerated conjugate Hib vaccines which are effective on infants from the first months of life [Reference Decker1, Reference Peltola, Kilpi and Anttila2], led to the introduction of Hib vaccination in the French routine infant immunization schedule in January 1993 [3]. Boucher et al. [Reference Boucher4] estimated that more than 1000 invasive Hib infections occurred in France in children in 1992, including more than 600 cases of meningitis, leading to 30 deaths and more than 100 permanent sequelae. Dabernat & Delmas [Reference Dabernat and Delmas5] reported that Hib was responsible for 96·8% of cases of Haemophilus influenzae (Hi) meningitis diagnosed in children aged 0–5 years during 1984–1992. France uses a polyoside vaccine composed of the Hib capsule polyribosyl ribitol phosphate (PRP) conjugated to a tetanus anatoxin (PRP-T). This conjugation induces a thymus-dependent response ensuring stronger immunogenicity from the first months of life, as well as an immune memory.
Since 1993, primary vaccination has been recommended in infants aged 2 months, with an initial catch-up vaccination up to age 5 years. The French infant immunization schedule recommends three injections for primary vaccination, each 1 month apart (at 2, 3 and 4 months), and a single booster dose when the child is aged between 16 and 18 months. The PRP-T vaccine provides very high protection once primary vaccination is completed [Reference Decker1]. Initially marketed as a monovalent vaccine, it was included in 1994 [Reference Roure and Begue6] in pentavalent then hexavalent vaccine combinations (together with diphtheria, tetanus, pertussis, poliomyelitis, and later hepatitis B).
Up to 2001, the combined vaccine contained the whole-cell pertussis component. During 2002–2005, both whole-cell and acellular pertussis-containing vaccines were used for infant primary vaccination, although most infant vaccinations were performed with the acellular pertussis-containing vaccines from 2003 onwards. In 2006, the marketing of the whole-cell pertussis-containing vaccine was discontinued.
In France (excluding overseas administrative regions), the surveillance of community-acquired invasive infections (meningitis and bacteraemia), including those due to Hi, has been conducted by the Epibac network and the national Haemophilus Reference Center (Centre national de reference des Haemophilus influenzae; CNRH) since 1987 [Reference Georges7]. Fifteen years after the introduction of routine Hib vaccination in France, we used data collected by the Epibac network since 1991 to study the impact of the vaccine on the incidence of invasive Hi disease. CNRH data on capsular types were used to assess the possible emergence of capsular types other than type b.
METHODS
Data collection
Since 1987, the Epibac network has monitored community-acquired invasive infections (meningitis and bacteraemia) due to Hi, through a voluntary surveillance system based on a network which includes most French hospital laboratories. This network covers invasive infections associated with six bacterial species including Hi. Surveillance entails sending several reminders, via email and telephone, throughout the year in order to ensure thoroughness of reporting and high quality of data. Invasive Hi disease is defined by isolating the bacterium from cerebrospinal fluid (CSF) and/or blood (meningitis and bacteraemia, respectively). Isolation and identification are performed using standard bacteriological methods in the network's participating laboratories. The following information is collected for each case: the site and date of sampling, the age and sex of the patient and the name and address of the laboratory. The present analysis is based on Epibac data collected since 1991, when the data collection procedure started to differentiate isolated bacteraemia from bacteraemia associated with Hi isolation in CSF, thus allowing the total number of invasive infections to be calculated. At the same time, the quality of demographic data collection for cases improved and more reliable data about infant age (in months) became available. As vaccination began in January 1993, the years 1991 and 1992 were used as the pre-vaccination reference period. However, data on meningitis are also shown in the figures from 1987 as they were not affected by the 1990 change in the recording and reporting of bacteraemia. Laboratories belonging to the Epibac network have been requested to send invasive Hi isolates to the CNRH for serotyping and antibiotic susceptibility testing since 1987. However, the proportion of isolates sent by Epibac laboratories to the CNRH has not been monitored before 2000 and some isolates received by CNRH have been provided by non-Epibac participating laboratories, making the CNRH data not fully superimposable with the Epibac data. The two-source capture–recapture method we applied to Epibac and CNRH data in 2005 and 2006, showed that the average proportion for all ages of Epibac strains received by CNRH was 21% for all invasive isolates and 58% for CSF. Higher average proportions were found for children aged <15 years (37% and 75%, respectively).
The serotype was determined by agglutination with polyvalent and monovalent sera from BD Difco (France) and, starting in 2000, was confirmed by polymerase chain reaction (PCR), as described by Falla et al. [Reference Falla8]. In the present analysis, we analysed data on isolates from CSF and/or blood collected by the CNRH from 1991 to 2007 as the mandate of the original CNRH terminated in 2007, and the newly designated CNRH took several months to become operational [Reference Dabernat9–Reference Dabernat12].
The CNRH collected the vaccination status from clinicians for children aged <15 years when Hi was isolated from CSF or blood. Since 1999, this information has been available for most cases.
Vaccine coverage
In France, infant vaccination status is assessed through health certificates which are completed at age 24 months for all children, by their GP or paediatrician, and sent to their local health department. No data are available for conjugated monovalent Hib vaccine initial catch-up coverage in children up to age 5 years. The standard health certificate was revised in 1995, allowing three doses + booster Hib vaccination coverage to be monitored from 1998 onwards. Vaccination coverage for the three-dose primary vaccination has only been monitored since 2004 [Reference Fonteneau, Guthmann and Levy-Bruhl13].
Statistical methods
The annual incidence of Hi bacteraemia and meningitis is calculated using the number of cases reported to the Epibac network as the numerator and the French population covered by the network's participating hospitals as the denominator. The latter is estimated from the proportion of national public and private acute-care hospital admissions covered by participating laboratories. This proportion is computed using the National Hospital Annual Activities Database which is an exhaustive source of information regarding inpatient hospital stays, managed by the Directorate for Research, Studies, Evaluation and Statistics (DREES) at the Ministry of Health. Population data is issued each year by the National Institute for Statistics and Economic Studies (INSEE). Only hospitals which send data for the full 12-month period are considered as participating laboratories. This method allows adjustment of incidence estimates for the Epibac network coverage (Supplementary Fig. S1).
Stata v. 9.2 software (StataCorp, USA) was used for all analyses. Trends were identified using Poisson regression or negative binomial regression analysis when the distribution of data was overdispersed. Distributions were compared using the χ 2 test, and 95% confidence intervals (CIs) of annual or multiannual incidence rates were calculated using Poisson's law.
RESULTS
Laboratory participation in surveillance activity
The number of laboratories participating in the Epibac network increased gradually during the study period, from 129 in 1991 to 294 in 2008, corresponding to an increase in national Epibac coverage from 30% to 78% (Supplementary Fig. S1). The present analysis is based on an estimated national total of 9821 Hi cases after annual adjustment for participation, based on the 5755 cases of invasive Hi disease reported to Epibac during this period.
Vaccine coverage since 1998
Vaccination coverage at age 2 years (first four doses, including the first booster) was 79% when first estimated in 1998. It rose to 85% in 1999 and then remained stable between 86% and 87% up to 2004. Between 2004 and 2008, estimated vaccine coverage remained stable at 97% for the three-dose primary vaccination and increased from 87% to 89% for three doses + booster vaccination (Fig. 1) [13, http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Haemophilus-influenzae-b].

Fig. 1 [colour online]. Evolution of Haemophilus invasive disease incidence and vaccination coverage.
Epidemiology in the pre-vaccine era
In 1991–1992, a yearly average of about 1000 cases of invasive Hi disease (meningitis and bacteraemia) occurred in France, corresponding to an incidence of 1·8 cases/100000 inhabitants (95% CI 1·7–2·0) (Supplementary Fig. S1). Most cases (65%) involved children aged <5 years, 4% occurred in those aged between 5 and 14 years, and 31% in persons aged >14 years.
Meningitis accounted for nearly half of all reported invasive Hi infections, with an estimated 490 cases/year, corresponding to an incidence of 0·86 cases/100 000 inhabitants (95% CI 0·76-0·97) (Supplementary Fig. S1). Hi meningitis mainly affected children aged <5 years (93% of cases, 12·1/100 000 children), while 3% of cases occurred in persons aged between 5 and 14 years, and 4% in those aged >14 years.
The incidence of Hi bacteraemia in 1991–1992 was 0·9/100 000 inhabitants (95% CI 0·8–1·1) (Supplementary Fig. S1); 41% of cases occurred in children aged <5 years (5·7/100 000), 36% in individuals aged between 5 and 64 years (0·43/100 000) and 23% in people aged >64 years (1·5/100 000).
Epidemiology in the vaccine era
The incidence of invasive Hi disease in 1993–1995 was half of that observed in the pre-vaccination reference period 1991–1992 (P < 0·001), stabilizing thereafter with an average of about 0·78/100 000 from 1996 to 2008 (P = 0·77) (Supplementary Fig. S1). This reduction was largely due to the decrease in the incidence of meningitis, with a reduction of 90% during 1996–2008, compared to pre-vaccination period values. The incidence of bacteraemia decreased more moderately (25%) (P < 0·001) (Fig. 1). Overall, the age distribution of invasive Hi disease was reversed in relation to the pre-vaccination period: in 1996–2008, 12% of cases occurred in children aged <5 years, 3% in the 5–14 years age group, and 85% in those aged >15 years.
Meningitis, which represented 50% of invasive infections in the pre-vaccination period, stabilized at 12% during 1996–2008, with an average yearly incidence of 0·09/100 000 inhabitants (95% CI 0·079–0·094). Between 1991–92 and 1996–2008, a marked fall in the incidence of Hi meningitis (96%) in children aged <5 years led to the near-disappearance of this disease in young children. The incidence of Hi meningitis also decreased in children aged 5–14 years (P = 0·002), albeit less strongly (74%), while no significant change was noted in individuals aged >14 years (Fig. 2a).

Fig. 2. Incidence of Haemophilus influenzae invasive disease according to age and period, 1991–2008, Epibac Network, France. (a) Meningitis, (b) bacteraemia.
Between 1991–1992 and 1996–2008, the incidence of Hi bacteraemia decreased significantly in children aged <5 years (80%, P < 0·001), while it remained stable in individuals aged 5–64 years (P = 0·30) and increased in those aged ⩾65 years (P = 0·02). Trend tests, stratified for the ⩾65 years age group, confirmed this increase (Fig. 2b).
The possible indirect impact of vaccination was assessed by analysing changes in the incidence of invasive Hi disease in age groups not targeted by Hib vaccination. In children aged <3 months, the incidence of invasive Hi disease fell by 60% in 1996–2008 compared to the 1991–1992 pre-vaccination period (P < 0·001). This was the result of simultaneous reductions in the incidence of meningitis (from 8·5 to 1·1/100 000, P < 0·001) and of bacteraemia (from 22·6 to 11·4/100 000, P = 0·013) between the two periods. These decreases were not significant before 1996 (Figs 1 and 2).
Finally, in individuals aged >14 years, data showed a small although significant increase in incidence of invasive Hi disease between 1991–1992 and 1996–2008 (from 0·75 to 0·87/100 000, P = 0·043) but this increase was mainly observed in elderly people aged 75–89 years (from 1·8 to 2·8/100 000, P = 0·008).
CNRH analysis of Hi isolates from 1991 to 2007
During the whole 1991–2007 period, 1438 strains of Hi isolated from patients with invasive infection were received by the CNRH, including 322 during the 1991 and 1992 pre-vaccine period (Table 1).
Table 1. Serotype distribution of CNRH* isolates by age and site during the pre- and vaccine era

CNRH, National Reference Centre for Haemophilus; CSF, cerebrospinal fluid; NT, non-encapsulated isolates.
Data on age was available for all 1438 isolates: 80% and 39% were recovered from children aged <15 years in the pre-vaccine and vaccine eras, respectively. The isolates were recovered from CSF in 575 cases (40%), associated or not with positive blood culture, and by blood culture alone in 864 cases (60%).
The type was known for all the 1438 isolates, broken down as follows: 38% were type b, 7% were of another type [a (n = 3), d (n = 2), e (n = 26) or f (n = 65)], and 55% were non-encapsulated (35% of CSF isolates and 69% of blood isolates). In the non-type b encapsulated isolates, types e (27%) and f (67%) predominated, with no variation in the serotype distribution according to the sampling site (CSF vs. blood, P = 0·284). The proportion of type b isolates in isolates analysed by the CNRH decreased markedly between 1991–1992 and 1995–2007 [80% (257/322) and 16% (142/879), respectively, P < 0·001]. In the <15 years and ⩾16 years age groups these proportions decreased from 92% (237/259) to 35% (93/267) and from 32% (20/63) to 8% (49/612), respectively, in the same two periods.
During 1995–2007, encapsulated isolates were present equally in those aged >15 years (52%) and in those aged <15 years (48%), while most non-encapsulated isolates were recovered from patients aged >15 years (77% vs. 23%) (P < 0·001). Non-encapsulated isolates represented 82% (504/612) of strains isolated in the latter group.
Among non-type b isolates, the relative proportion of serotype f, other typable serotypes and non-encapsulated isolates did not vary between the pre-vaccination (9%, 0%, 91%) and late vaccination periods (1995–2007) (7%, 3%, 90%).
Vaccination status, collected since 1999, was available for 83% (132/160) of the children targeted by Hib vaccination, and for 91% (89/98) of those presenting with meningitis.
Among the 160 isolates associated with invasive infection recovered from children targeted by the Hib vaccination and sent to the CNRH between 1999 and 2007, 60 were type b. The immunization status was documented for 53 (88%) children, 30 of whom were partially vaccinated (n = 5) or unvaccinated (n = 25). Of these 30 children, 25 were aged ⩾5 months. The remaining 23 children were fully vaccinated for their age (38%). None of the children who had received three vaccine doses and a booster developed invasive Hib disease, but 21 children who had received the full three-dose primary series but had not reached the upper age for the recommended booster dose (18 months) developed the disease (Table 2). Two thirds of these 21 cases (n = 14) were in children between the ages of 12 and 18 months.
Table 2. Number of Hib isolates from children aged <15 years by vaccine status, origin of isolate and age (CNRH, 1999–2007)
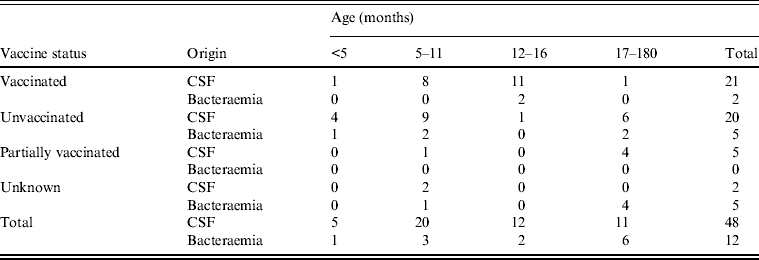
CNRH, National Reference Centre for Haemophilus.
In total, 34 (45%) of the 76 Hi isolates from children fully vaccinated according to age were encapsulated, 30% (n = 23) were type b, 15% had another capsular type (e in four cases and f in seven cases), and 55% (n = 42) were non-encapsulated.
DISCUSSION
Hib vaccination was introduced into the French immunization schedule in January 1993, mainly to protect children aged <5 years from the most severe Hib infections. Before this date, about half of the ∼1000 cases of invasive Hi disease diagnosed each year in France presented as meningitis, and most of the latter (93%) occurred in children aged <5 years. Using Epibac network data, we were able to assess the pre-vaccination situation and the impact of routine Hib infant immunization on the incidence of invasive Hi disease. Like many other countries where infant Hib vaccination is used [Reference Peltola, Kilpi and Anttila2, Reference Ribeiro14–Reference Ladhani19], the incidence of invasive Hi disease fell rapidly in France, decreasing by half within the first 2 years after the start of routine vaccination. Compared with the pre-vaccination reference period (1991–1992), the incidence of Hi meningitis fell markedly in children aged <5 years, by 95% in 1995, followed, slightly later, by a somewhat less marked fall (65% in 1996–2008) in children aged 5–14 years.
In addition to reducing the incidence of invasive Hib infection, Hib vaccination induces a reduction in pharyngeal carriage of the bacterium in vaccinated children, thereby reducing its circulation in the general population (herd effect). This fact might have protected the unvaccinated population [Reference Barbour and Mayon-White20, Reference McVernon21]. A 1999 study performed in three French districts which included 1683 children aged from 3 to 36 months (with 98·5% having received at least one dose of the Hib vaccine), showed that 41% of the children were colonized by non-encapsulated Hi strains, while none carried encapsulated Hib strains [Reference Dabernat22]. This underlines how rare Hib carriage is in the vaccine era in vaccinated children. Indeed, a delayed but significant reduction in the incidence of Hi meningitis and bacteraemia was observed in infants aged <3 months, who were too young to be vaccinated.
Between 1991 and 2008, the incidence of invasive diseases remained stable in the unvaccinated 15–64 years age groups, which favours the hypothesis that vaccination was responsible for the marked decrease in incidence in children.
In elderly subjects (>64 years), the incidence of bacteraemia slightly increased (4%). A similar increase in the incidence of invasive Hi disease in individuals aged ⩾65 years has been described in Europe [Reference Ladhani19] and the USA [Reference Dworkin, Park and Borchardt23] and, as was the case in France, was due to non-encapsulated strains. Such strains accounted for 82% of all strains isolated from invasive Hi cases in individuals aged >15 years, received by the CNRH during 1995–2007. No significant impact of vaccination was expected in this age range as Hib accounted for a limited proportion of Hi diseases in adults in the pre-vaccine era. However, the reason for the small increase remains unclear. It may reflect a switch, in children, from Hib carriage to carriage of other Hi strains able to induce invasive disease in the elderly. However, available evidence does not favour the hypothesis of a carriage replacement of Hib by other Hi serotypes in vaccinated children [Reference Barbour and Mayon-White20, Reference Takala24, Reference Ladhani25]. Population ageing could also contribute to increase the incidence of such infections in the most susceptible individuals. Finally, this increase could reflect an improved case ascertainment or case notification. We cannot rule out the fact that advances in technology and improved culture media performance may have contributed to the more frequent detection of Hi in blood culture after 1993. A change in the surveillance performance is very unlikely. First, our analysis already takes into account potential changes in the notification rate through yearly correction for thoroughness of reporting. Second, data provided by the participating laboratories are extracted from the same database they use for the recording of their routine diagnostic activity. It would be interesting to study Hi serotypes responsible for infections in elderly subjects and the severity of these infections.
Our analysis of CNRH data for 1999–2007, when the incidence of invasive Hi disease remained stable, confirmed that fully vaccinated children (three doses plus a booster) were highly protected, as no case of invasive Hib infection was identified in these children. More than half of the invasive Hib infections diagnosed in children up to age 15 years with documented vaccination status (30/53) occurred in children not appropriately vaccinated for their age. Of these, 25 cases occurred in children aged ⩾5 months and probably could have been prevented if those children had followed the recommended schedule. In addition, we identified 21 cases of invasive Hib infections which could be considered vaccination failures after primary vaccination, as they occurred in children aged 6–18 months who had received the three-dose primary vaccination series but had not yet reached the recommended upper age for the booster dose (18 months). Two thirds of the latter cases (n = 14) occurred in children aged between 12 and 18 months and could probably have been prevented by lowering the age of the first booster to the child's first birthday.
Following the initial decrease, we observed no upsurge in invasive Hi disease in the second year of life, contrary to the situation in the UK, at a time when children in this age group did not receive a booster dose [Reference McVernon, Ramsay and McLean26]. This supports the French choice of a vaccination strategy based on three doses in the first year of life, followed by a booster between 1 and 2 years [Reference Roure and Begue6].
The stable incidence rate in children observed from 1996 to 2008 confirms that Hib had not been replaced by another potentially invasive capsular type, a conclusion already drawn in 2000 in a study of bacterial meningitis in six French districts [Reference Dabernat9] and as previously reported by CNRH [Reference Dabernat and Delmas10, Reference Dabernat and Delmas11]. In particular, our analysis confirms a previous report concluding that the proportion of serotype f isolates among non-type b encapsulated Hi isolates received by the CNRH did not differ significantly between 1991–1992 and 1996–2007 (P = 0·99) [Reference Dabernat and Delmas5]. This conclusion differs from initial studies performed in Portugal and Brazil after the introduction of routine vaccination [Reference Bajanca and Caniça27, Reference Ribeiro28]. However, replacement of type b strains by type a in Brazil was shown to be a local and transient phenomenon which did not reach a level sufficiently large to call into question the Hib conjugate vaccination policy [Reference Ribeiro14]. Similarly, the increase in the number of non-encapsulated isolates in Portugal was also transient [Reference Ladhani25]. Finally, Ladhani et al. [Reference Ladhani25] analysed surveillance data from 1996 to 2006 from 14 EU countries and found no evidence of serotype replacement following Hib vaccination.
Our main conclusions, which go in the same direction, namely the major beneficial direct effect of Hib vaccination on the incidence of Hi invasive diseases in children, and the absence of replacement disease in all age groups should be be highlighted, especially at a time when certain sections of the public, and even some healthcare professionals, are questioning the benefits of vaccination. The quasi-disappearance, within just a few years, of a dreaded infantile disease is definitely a success of vaccination.
One of the strengths of our study is that it is based on a continuous surveillance network that has used the same methodology for more than 20 years and in which the exhaustiveness of reporting by participating laboratories, estimated on several occasions through a two- or three-source capture–recapture method, has always been high. It has always been >70% and even >80% since 1980, depending on the pathogen [Reference Hubert, Goulet and Rioux29–Reference Berger32]. It is important to point out that after correcting for the proportion of Hib diseases due to b serotypes in children aged <5 years (92·5%) (CNRH, data not shown), and for under-notification at that time (71%) [Reference Ribeiro28], the pre-vaccination total incidence of Hi disease in children aged <5 years estimated from data from the Epibac network (17·8/100 000), yields an estimate of 23 cases of Hib diseases/100 000, which fits well with the values found in various European countries [Reference Peltola33].
Our methodology for adjustment ensures that variations over time in the proportions of hospital laboratories actually participating in the surveillance are accounted for. It is reassuring that the comparison of the coverage of the Epibac network assessed through two independent methods (i.e. routine analysis and a specific analysis carried out within a three-source capture–recapture method) yielded very similar estimates for 2005 (79% and 81%, respectively) (Supplementary Fig. S1, [Reference Berger32]). Furthermore, unlike in other countries, the practice of lumbar puncture for suspected cases of meningitis has not been questioned in France in recent decades, something which could have induced a diagnostic bias, which in turn could affect the quality of surveillance. This is further confirmed by the stable incidence (or even slightly increasing trend) of meningococcal meningitis during the study period evidenced by the same Epibac data [Reference Georges7] and by the stable proportions of meningitis within all invasive meningococcal diseases notified through mandatory notification. This latter proportion varied between 72% and 85% during 1995–2007 with no downward trend (InVS, unpublished data).
The main limitation of our study is that about only 20% of Hi isolates recovered from cases with invasive infection and, more particularly, <50% from children up to age 15 years, were sent to CNRH for genotyping. However, the size of the Epibac dataset together with the large number of Hi isolates from children up to age 15 years that have been genotyped by CNRH since 1991 (about 1200 isolates) is sufficient to rule out any upsurge in Hib strains or indeed their replacement by other serotypes in children. Nevertheless, the continued existence of these risks, and the need for epidemiological surveillance of invasive Hi disease in adults, fully justifies the need for ongoing monitoring of these infections by the InVS, both through the Epibac network and through the ongoing efforts to increase the proportion of invasive strains sent to the CNRH.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813000083.
ACKNOWLEDGEMENTS
The authors thank all the biologists of the Epibac network for their helpful participation (list available at http://www.invs.sante.fr) as well as Dr Olivier Gaillot for his helpful comments on the manuscript. Our special thanks go to Edith Laurent for making the Epibac network function so effectively.
DECLARATION OF INTEREST
None.






