1. Introduction
During hospital admission, either in a psychiatric hospital, emergency department (ED) or at a general hospital ward, agitated behaviour (AB) is a challenging problem. Even more challenging is the management of AB in psychiatric outpatients as met by assertive outreach teams, community care or 24 u/7 psychiatric crisis services. In this paper, we understand agitation as a continuum ranging from severe excitement to agitation to aggression to violence, without clear demarcations between these states. At some point AB may become that unmanageable that the behaviour becomes risky or dangerous for the patient, others or staff members. When non-pharmacological interventions fail to resolve calmness, a psychiatrist or other doctor considers a pharmacological intervention often called rapid tranquillisation. Hereafter we use the term agitated behaviour (AB) for all behaviours necessitating an acute intervention psychopharmacological.
AB is associated with serious problems and challenges warranting rapid intervention. It represents a real danger for the individual involved. Indeed, AB can be a very stressful and may become life-threatening due to physical exhaustion. Next, AB may threaten the safety of other people involved whether it is family or medical staff. Finally, AB complicates the assessment and evaluation of the underlying somatic and psychiatric problems or disease.
The primary goal of any intervention towards AB is to ensure safety, facilitate assessment of underlying problems and prevent further escalation, through achieving calmness and collaboration [Reference Allen and Currier1, Reference Allen, Currier, Carpenter, Ross and Docherty2]. Both psychosocial and pharmacological interventions need to be considered [Reference Allison and Moncrieff3]. The aim of acute pharmacological interventions are to reach calmness and cooperativeness within a short timeframe of maximum 2 h [Reference Canas4, Reference Garriga, Pacchiarotti, Kasper, Zeller, Allen and Vazquez5].
Cochrane meta-analyses compared the effects of several drugs, each time comparing one drug with several other drugs [Reference Belgamwar and Fenton6–Reference Allen and Currier10]. The use of olanzapine or haloperidol plus promethazine is most favoured (see Table 1).
However, weaknesses remain in these meta-analyses that hamper clinical translations and guidance. First, in these meta-analyses one medication is compared with several other prescriptions and effect sizes are calculated. Although this is a statically sound method the clinical significance is only restricted to those medications compared with control medication. It has no meaning in towards the other medications that are not directly compared statistically. Next, the number of included studies (and the number of participants) is very small, questioning generalisability. Finally, in the real clinical world, differences remain between clinical centres, medical specialities (emergency physicians versus psychiatrists), regions and countries remains (11 De Fruyt, 2004 #182, 12).
The objective of the current paper is to provide an overview and meta-analysis on the use of pharmacological interventions in the management of AB. Primary outcome is change in AB at 120 min (2 h) and each drug is analyse separately. A systematic review and meta-analysis measuring the level of change on scales assessing AB is conducted. Second, a systematic review of the number and severity of adverse effects of the various medications to evaluate safety aspects of the medications used for rapid tranquilisation is conducted. Finally, recommendations for clinical use and future research projects are proposed.
2. Methods
2.1. Inclusion criteria and study evaluation
We identified randomised controlled trials with subjects randomised into intervention groups classified per medication to treat acute agitation.
2.1.1. The inclusion criteria were
1. Agitation
2. Psychiatric disorder or intoxication
3. Rapid tranquilisation or pharmacological intervention
4. Setting: ED in general hospital, ward in mental hospital or mixed context.
5. Randomised control trial, controlled clinical trial, clinical trial or Phase IV clinical trial with adequate control group.
6. Raw follow-up data of period of 2 h.
7. End date December 31st 2017
Patients with a delirium were excluded from the study, as these patients have a clear organic origin and good protocols exist. Children or adolescents under 18 years of age were searched separately with the same search string but age limit < 18 years. Data needed to be presented with raw outcome variables of the scale used per timeframe. Only studies including data within 2 h are included. When studies presented only effect sizes or p-values, authors were contacted to receive raw data.
2.1.2. Exclusion criteria were
Studies only presenting data of more than 2 h and, studies that only reporting effect sizes, only indicating statistical significant difference by mentioning p-values or effect sizes without raw data, were excluded.
2.2. Outcome scales
Primary outcome was change on well-accepted rating scales; PANNS-EC (Positive and Negative Symptom Scale – Excitement Components, also called the PEC) [Reference Kay, Fiszbein and Opler13], ACES (Agitation-Calmness Evaluation Scale; a scale developed by Eli-Lilly pharmaceuticals) and the OASS (Overt Agitation Severity Scale) [Reference Baldaca, Sanches, Cordeiro and Jackowski14], mean minutes of reaching calmness and repeated medication within two hours. The various RCT’s that study rapid tranquillisation used a variety of scales and outcome measures to assess the effect of the intervention.
PANSS-EC: A clinical scale assessing agitation level in patients. PANSS-EC is a subscore of 5 items derived from the PANSS [Reference Kay, Fiszbein and Opler13] that are associated with agitation: poor impulse control, tension, hostility, uncooperativeness and excitement. The PANSS-EC has become accepted as the scale for assessing agitation [Reference Leucht, Kane, Kissling, Hamann, Etschel and Engel15]. Validity and reliability have been demonstrated showing a strong correlation with the CGI and ACES in agitated patients [Reference Montoya, Valladares, Lizan, San, Escobar and Paz16]. The PANSS-EC and CGI are linearly correlated with average increase of 3.4 point (p < 0.001) and linearly inversely correlated with ACES of 5.5 points (P < 0.001). Crohnbach’s alpha was 0.86 [Reference Montoya, Valladares, Lizan, San, Escobar and Paz16].
ACES: The ACES consists of a single item rating overall agitation and sedation. It has a 9-point Likert scale: 1 – marked agitation, 2 – moderate agitation, 3 – mild agitation, 4 – normal behaviour, 5 – mild calmness, 6 – moderate calmness, 7 – marked calmness, 8 – deep sleep, 9 – unarousable. This scale has convergent validity and reliability compared with PANSS-EC [Reference Montoya, Valladares, Lizan, San, Escobar and Paz16–Reference Meehan, Wang, David, Nisivoccia, Jones and Beasley18]. Spearman correlation with PANNS-EC showed correlation coefficients of 0.73 – 0.8. The Crohnbach’ alpha varied from 0.86 (at admission) till 0.9 (at discharge) (16)
OASS: The OASS contains 47 observable characteristics of agitation, which are subcategorised into 12 behaviourally related units. Each subcategory is scored with likert-scale of 0 - no symptoms, 1- indicating mild symptoms to 4 - indicating very severe symptoms. The OASS exclusively rates observable manifestations of agitation. Interrater reliability is 0.97 (at 15 min) and 0.91 after 1 h, whereas validity 0.81 compared with PAS (Pittsburg Agiation Scale ([Reference Rosen, Burgio, Kollar, Cain, Allison and Fogleman19])) suggesting reasonable reliability and validity [Reference Yudofsky, Kopecky, Kunik, Silver and Endicott20].
2.3. Study quality assessment
Quality assessment was based upon the MOOSE checklist, which summarises recommendations of an expert panel for reporting meta-analyses and systematic reviews of observational studies [Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie21]. Methodological issues evaluated with the checklist were; the presence of a clearly focused study question, an appropriate study type, an adequate recruitment of patients and controls, an unbiased measurement of outcomes, the identification of statistical control of important confounding factors, the completeness of follow-up and the precision of estimates.
Table 1 Overview of data from Cochrane metanalysis on rapid traquillsiation.


All papers were reviewed by independent researchers (MB and EB), studying the papers closely on methodology and outcome measure based on the MOOSE checklist criteria. In case of doubt papers were discussed with IW and consensus reached. Additionally JdF checked the completeness of the search.
2.4. Data sources and search strategy
A systematic search was performed in Pubmed and Embase search libraries. The search terms in Pubmed were: ((((((((((("Psychomotor Agitation"[Mesh]) OR Psychomotor Agitation) OR Agitation) OR Acute agitation)) AND ((("Drug Therapy"[Mesh]) OR Drug Therapy) OR Pharmacological treatment)) AND (((("Mental Disorders"[Mesh]) OR Mental Disorders) OR psychiatric disorders) OR intoxication)) AND ((Therapy/Broad[filter]) AND (acute agitation AND mental disorder))) NOT (("Review"[Publication Type]) OR Review)) NOT (("Case Reports"[Publication Type]) OR Case Reports)) NOT (("Delirium"[Mesh]) OR Delirium)) NOT (("Pain"[Mesh]) OR Pain) Filters: Humans; Adult: 19+ years.
The search in EMBASE was: (((Acute agitation and lorazepam) or (Acute agitation and midazolam) or (Acute agitation and haloperidol) or (Acute agitation and olanzapine) or (Acute agitation and droperidol) or (Acute agitation and loxapine) or (Acute agitation and quetiapine) or (Acute agitation and aripiprazole) or (Acute agitation and ziprasidone) or (Acute agitation and lurasidone) or (Acute agitation and levopromazine) or (Acute agitation and risperidone)) not Review not Case reports not Delirium not Pain). Date of inclusion was till 01-01-2018.
First authors were contacted in case of missing or ambiguous information, or in case of only presenting p-values or only effect sizes. In case papers were not in the library of Maastricht University, first authors were also contacted for the requested article.
2.5. Data extraction
For each drug baseline data as number of patients, age (years), mean dose (in mg) and route of administration (oral, inhalation, intramuscular or intravenous administration) and diagnosis are noted in the data base. For each drug baseline and follow-up data of PANSS-EC, CGI and ACES are noted: at baseline, at 15–20 min, 30 min, 60 min, 90 min and 120 min of. For each drug the mean duration of becoming calm in minutes is noted per medication as well as the percentage of patients reaching calmness in 2 h and the percentage of patients that needed repeated medications within 2 h are noted. The reported percentage of adverse events is reported for each drug.
2.6. Outcomes
In the systematic review part of this manuscript the descriptive data per drug and publication are noted of the following variables: dosage number of patients, diagnosis, administration route, raw data of the psychometric scales (for the consecutive time intervals at follow-up), recall of a doctor within 2 h and the percentage of the adverse effects after 2 h are noted. The main outcome of the meta-analysis is the change on PANSS-EC, CGI and ACES at 2 h follow-up.
2.7. Statistical analyses
All analyses were performed using Stata [Reference Statacorp22]. To examine the outcomes per antipsychotic for each scale (PANSS-EC, ACES and CGI, all at 120 min), the Stata command metan [Reference Bradburn, Deeks, Altman and SJAC23] generated forest plots including pooled estimates (absolute changes) with their corresponding 95% confidence interval (95% CI).
Computation of summary effects was carried out under the random-effects model, in which Tau was estimated using the DerSimonian-Laird method. Heterogeneity analyses were carried out using the chi-square, I-square, and Tau-square statistics. Tau-square estimates the total amount of variability (heterogeneity) among the effect sizes, but does not differentiate between sources. Heterogeneity may be due to random or systematic differences between the estimated effect sizes. I-square estimates the proportion of the total variability in the effect size estimates that is due to heterogeneity among the true effects.
3. Results
The Pubmed search yielded 167 citations. The Embase search yielded 58 citations. Using backward citation tracking resulted in 15 extra studies. For further information the PRISMA flow diagram (Fig. 1). Full screening resulted in a rejection of 61 papers because these papers did not study rapid tranquillisation, presented only data only beyond the 2 h’ time period, appeared to be a review paper, a case report only, no data per medication but only medication groups, only report of effect size no raw data on PANNS-EC, ACES or CGI (see appendix: all excluded papers and reason of exclusion). Seven papers met all inclusion criteria and qualitative data, but presented no raw data and contacting authors did not result in retrieving these data. Studies reported effect sizes or gave p-values only. Ultimately 54 papers were used for data extraction (see Fig. 1).
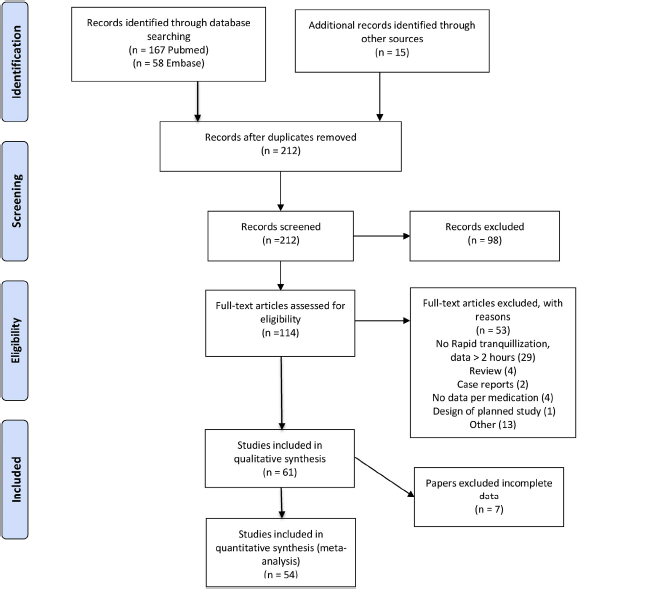
Fig. 1. PRISMA 2009 Flow Diagram: Rapid tranquilisation.
For more information, visit www.prisma---statement.org.
3.1. Drugs included
In total of seventeen drugs or combinations of drugs RCT were included. These RCT comprise 8829 subjects Table 2.
3.2. Reduction of agitated behaviour
Lorazepam has a reduction of 7 points on the PANNS-EC, for haloperidol the reduction is between 7 and 8 points, the reduction with haloperidol plus promethazine is assessed in only 1 study but shows a reduction of 15 point after 2 h. The combination of haloperidol plus lorazepam shows a reduction of 8–10 points after 2 h. The combination of haloperidol with midazolam results in a reduction of 15 points after 90 min (only 1 study). Levopromazine is used in two studies in a more elderly population, resulting in a decrease of 5–6 points. The reduction with aripiprazole is between 7 and 8 points with one exception where only 3 points reduction is reported [Reference De Filippis, Cuomo, Lionetto, Janiri, Simmaco and Caloro24]. Olanzapine shows a decrease around 7 and 10 points on the PANNS-EC. Risperidone shows a reduction of PANNS-EC in 2 h of 7–8 points in two papers [Reference Hatta, Kawabata, Yoshida, Hamakawa, Wakajima and Furuta25, Reference Lim, Kim, Pae, Lee, Lee and Paik26], and one study reports a reduction of 14 points after two hours [Reference Walther, Moggi, Horn, Moskvitin, Abderhalden and Maier27]. Addition of lorazepam of clonazepam to risperidone does not result in extra decrease on the PANSS-EC score. Ziprasidone shows a reduction of PANS-EC score of 3 – 15. Loxapine which is used through nasal inhalation results in 9–11 points reduction. Finally, placebo also shows some reduction after two hours on PANSS-EC of 2–6 points.
3.3. Primary outcome decreasing agitated behaviour, meta-analytic findings
Not all RCT’s, discussed in the systematic overview (see Table 3), could be used for meta-analysis. Only studies that provided PANSS-EC, ACES or CGI including standard deviations at baseline and at follow-up could be included (see Figs. 2–4, supplement tables S1 – S3 and supplement figures S2, S3 and S4). Twelve studies were eligible for the meta-analysis based on the PANNS-EC. The changes after 2 h’ follow-up are presented in Fig. 2. Risperidone shows the most robust change of >14 points on the PANSS-EC after two hours. Followed by olanzapine, aripiprazole and haloperidol plus lorazepam. All these drugs resulted in a decrease on the PANNS of > 8 points two hours after the drug intervention. Risperidone plus lorazepam, lorazepam, risperidone plus clonazepam and haloperidol resulted in a modest decrease of agitation of 6–8 point decrease on the PANSS-EC. Levopromazine, ziprasidone and placebo hardly showed any clinical relevant decrease of agitated behaviour.
Table 2 Overview of included drugs, number of studies and included subjects.

For more detailed information see table S2 (PANNS-EC meta-analyses data). Here the forest plots are presented per medication.
Seven drugs were eligible for the meta-analysis assessing ACES after 2 h. Haloperidol plus lorazepam showed the strongest change as measured with the ACES, followed by lorazepam, olanzapine, aripiprazole, haloperidol, levopromazine and placebo. Given that the strongest reduction of ACES les than 2.5 points on a scale of 0–5, this seems a rather moderate effect in reaching calmness.
In the meta-analysis using data of the CGI only 4 drugs are available for meta-analysis. The largest changes were related with olanzapine, followed by haloperidol, aripiprazole and ziprasidone. The level of change dos not differ strongly between olanzapine, haloperidol and aripiprazole.
The Figs. 3 and 4 show the results of changes at 2 h’ follow-up with ACES respectively CGI.).
For more detailed information see the forest plots (table S3 (ACES meta-analyses data) and table S4 (CGI meta-analyses data)).
3.4. Percentage of patients reaching calmness (see Table 3)
3.4.1. Benzodiazepines
With lorazepam 63–88% is calm at 120 min. About 78% reaches calmness within 15–20 minutes.
With midazolam only 1 study reports that 95% reaches calmness after 120 min [Reference Huf and oboTC33], whereas 55–89% reaches calmness in 15–20 min.
3.4.2. Antipsychotics (with additional medication)
Haloperidol shows that after 120 min 60–89% reaches calmness [Reference Andrezina, Marcus, Oren, Manos, Stock and Carson36, Reference Huf, Coutinho, Adams and Group43, Reference Wright, Birkett, David, Meehan, Ferchland and Alaka46]. In the short time (15–20 min) the percentage of patients reaching calmness between 55%–92% [Reference Huf, Coutinho, Adams and Group43].
The combination of haloperidol plus promethazine has a strong effect of 89–97% of the patients reaches calmness in 2 h. In the short term 67–91% reaches calmness within 15–20 min.
Studies with droperidol only report short term outcome data. About 53–92% reaches calmness with 15–20 minutes and one study report 96% of the patients has reached calmness after 60 min [Reference Richards, Derlet and Duncan31].
Only one study reports data on the combination of droperidol plus midazolam through IV administration, where 89% reaches calmness with 15–20 min and 98% after 60% minutes [Reference Taylor, Yap, Knott, Taylor, Phillips and Karro54].
Aripiprazole results in calmness in 60–84% of the patients after 120 min.
Olanzapine results in 73–91% of the patients in calmness after 2 h. One study reports that 66% of the patients reaches calmness after 15–20 min by IV administration.
Ziprasidone 29–90% of the patients reaches calmness after 2 h.
For loxapine reaching calmness varied from 66 to 74% within 2 h.
Placebo results in 28–44% of the patients in calmness after 2 h.
3.5. Mean duration reaching calmness
Some studies reported the mean time in minutes that patients reached calmness. The major administration route is IM. Only the study of Taylor [Reference Taylor, Yap, Knott, Taylor, Phillips and Karro54] used IV administration. Loxapin is inhaled. The only study assessing oral administration is with risperidone plus lorazepam [Reference Currier and Simpson49]. For lorazepam 1 study reported that calmness is reached after 48 min. Midazolam shows a mean time of 20–24 minutes. With haloperidol, the mean duration of reaching calmness is only given in 1 study and is 30 min [Reference Calver, Drinkwater and Isbister29]. The combination of haloperidol plus promethazine is results in calmness at 20–30 minutes. Adding lorazepam to haloperidol results in mean time of 44 min [Reference Currier and Simpson49]. The combination of haloperidol plus midazolam is quite fast and is reaches calmness in about 10 min [Reference Calver, Drinkwater and Isbister29]. The mean time with droperidol is about 8–25 min. Adding midazolam results in reaching calmness in 25 min, although one intravenous (IV) administration results in reaching calmness within 5 min [Reference Taylor, Yap, Knott, Taylor, Phillips and Karro54]. Olanzapine results in calmness with 11–30 min, be noted that the 11 min’ period is by IV administration. Risperidone plus lorazepam resulted in reaching calmness within 43 min. Loxapine intranasal administration results in reaching calmness in about 57–67 min. No data are available for aripiprazole, risperidone, levopromazine, ziprasidone or placebo.
3.6. Adverse effects
Description of the unwanted effects related to the medications varies quite strongly (see Table 3 for detailed information).
3.6.1. Oversedation
Some medication is related with oversedation although the numbers vary; lorazepam 10%, haloperidol 0–36%, haloperidol plus promethazine 3%, haloperidol plus lorazepam 13–70% haloperidol plus midazolam 40%, droperidol 1% of the cases, aripiprazole 4–9%, olanzapine 3–13%, risperidone 13%, risperidone plus lorazepam 13%, levopromazine 8%, ziprasidone 10%, loxapine 11–13% and placebo 2–10%.
3.6.2. Movement disorders
3.6.2.1. Benzodiazepines
The reported number of patients with movement disorders, more specific EPS, dystonia and akathisia, is absent with lorazepam, with only 1 study that report data on akathisia which is in 2% of the cases. For midazolam, no reports of movement disorders are given.
3.6.2.2. Antipsychotics
Haloperidol shows increased number of patients with movement disorders, EPS in 6–55% of the cases, reports of acute dystonia is between 0–17% and akathisia is reported in 8–46%. Haloperidol plus promethazine varies highly; percentages of EPS are between 0–74%, acute dystonia absent and akathisia is not reported.
Table 3 All drugs included: outcome and adverse effect overview.











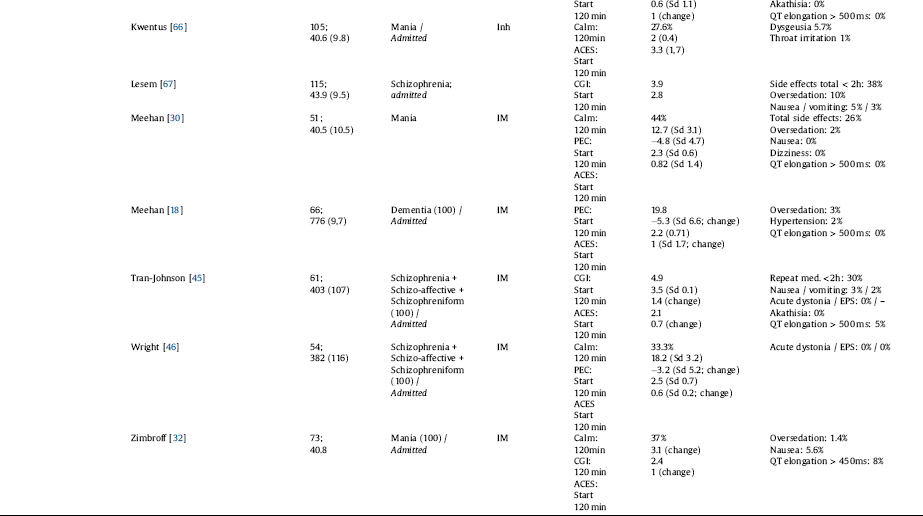
Haloperidol plus lorazepam shows some reports of EPS of 5%, acute dystonia of 3%.
Haloperidol plus midazolam has a percentage of acute dystonia 10% and EPS of 44%. Droperidol is mild in movement disorders with no reports of EPS, acute dystonia in 0–1% and no reports of akathisia. Adding midazolam to droperidol does not change these outcomes. For aripiprazole, there is one study that reports EPS (2%), acute dystonia is about 1–2% and akathisia is around 3%.
Olanzapine results in low rates of movement disorders; EPS in 0–5%, acute dystonia in 0–4% and akathisia in 0–2% of the cases.
Risperidone, the rates are modest EPS 6–8% and acute dystonia 2%. Adding lorazepam or clonazepam does not change the percentages of EPS or acute dystonia.
Levopromazine does not result in EPS or acute dystonia but akathisia is reported in 8% of the cases. Ziprasidone is does not result in acute dystonia and EPS, except for 1 study that reports EPS in 52% of the cases [Reference Mantovani, Labate, Sponholz, de Azevedo Marques and Guapo47]. For loxapine intranasal administration there are no reports of movement disorders. Finally, placebo results in some movement disorders EPS in 2–7%, but no reports of acute dystonia of akathisia.
3.6.3. Cardiovascular adverse effects
QT-elongation is QT-time > 500 ms, which increases the risk of arrhythmias.
3.6.3.1. Benzodiazepines
Absent in lorazepam except for 1 study that report that QT-elongation is present in 7% of the cases [Reference Zimbroff, Marcus, Manos, Stock, McQuade and Auby32]. Midazolam results in between 3–7% of the cases in QT-elongation.
3.6.3.2. Antipsychotics
Haloperidol shows QT-elongation in 0–6% of the cases. Droperidol in 1–6%. Studies addressing QT-time elongation in droperidol are presented in Table 4 (see Table 4). Adding midazolam to droperidol results in a percentage of 1–14%.
Aripiprazole results in 0–6% of the cases having QT-elongation. For olanzapine, the percentages vary between 0 and 3%. Placebo does not result in QT-elongation except in 2 studies with 5% and 8% of the cases showing QT-elongation [Reference Zimbroff, Marcus, Manos, Stock, McQuade and Auby32, Reference Tran-Johnson, Sack, Marcus, Auby, McQuade and Oren45].
3.6.4. Hypotension / hypertension
Hypertension is mentioned for some drugs (see Table 3).
Hypotension is more apparent with midazolam in 5% of the cases, haloperidol in 0–17%, haloperidol plus promethazine in 10%, haloperidol plus lorazepam one study reports hypotension in 3% of the cases. Haloperidol plus midazolam hypotension is reported in 10% of the patients. For droperidol, the percentage of hypotension is 0–4%. Adding midazolam to droperidol the percentage of cases with hypotension increases up to 41%, although another study reports only 2% of the cases develop hypotension. Olanzapine results in 0–4% of the cases in hypotension. Levopromazine resulted in hypertension in 3% and hypotension in 16% of the patients.
3.6.5. Hypoventilation
Midazolam increases the rate of saturation problems in those who intoxicated with alcohol. Between 1 and 30% of the cases that are reported that needed ventilation support.
3.6.6. Throat irritations
Loxapine shows some small increase in dysgeusia and throat irritation of respectively 4–17% and 1–7%.
4. Discussion
Pharmacological intervention in patients with agitated behaviour is a serious event, whether this is at an emergency department, in a ward of a psychiatric hospital or in a outpatient setting. The current study provides an overview and meta-analysis of several pharmacological interventions.
The outcomes in the current meta-analysis and systematic review suggests that haloperidol plus promethazine is strongest in decreasing the agitation measured with PANSS-EC and the percentage of patient that reached calmness in 2 h. Also olanzapine showed significant changes in reaching calmness measured with PANNS-EC, ACES and CGI. Both medications are relatively mild in the side-effects profile. These finding confirms results of the previous mentioned Cochrane reviews [Reference Huf, Alexander, Gandhi and Allen7, Reference Ostinelli, Brooke-Powney, Li and Adams9]. For the other medications the results are heterogeneous.
Midazolam and droperidol are both effective. These medications reach calmness very fast, even in minutes if administered IV or IM within. However, the sustainability of the effect is weak. IM administration of midazolam needs quite often repeated administration. The side effect profile shows the possibility of reaching oversedation and ventilation problems. Therefore, midazolam is more suitable for use at ED, where safety measures are available, but where fast interventions are needed as well. Droperidol administered intravenous results in calmness very fast, and remains reasonably fast via the intramuscular route. There are no results whether these effects last at 2 h. Droperidol has been abandoned for some years because of QT-time prolongation. However, recent studies have shown that the prevalence of exceeding unsafe QT-times is rare and not more than with other antipsychotics (see also table 3) (8). The problem of QT-elongation in droperidol appears a rather smaller problem and not more prevalent compared to other antipsychotics.
Risperidone, haloperidol plus lorazepam and aripiprazole show good effects at one or more of the scale in reaching calmness. Although, some either the number of studies or the number of patients is rather small, the side effects are more problematic or as in the case or aripiprazole is it only tested in patients in a manic episode.
According a recent expert consensus paper in dealing with aggression, the ideal medication should reach calmness without oversedate [Reference Garriga, Pacchiarotti, Kasper, Zeller, Allen and Vazquez5]. Considering this, the often- used haloperidol plus lorazepam or medications like midazolam are not first choice.
In discussion on rapid tranquillisation it is always about speed, effect and safety. The circumstances define whether speed in reaching calmness is more prevailing or whether collaboration in reaching calmness preferred. In cases of an ED the context asks sooner for IV intervention, as safety is more easily in jeopardy [Reference Jibson72]. Given the risk of respiratory adverse effects, IV administration is only safe to use for ED, as specific monitoring of physical parameters is required. Here midazolam or droperidol plus midazolam are good options as they act sedative within minutes. Generally, intravenous (IV) administration is much faster that intramuscular (IM) administration. Oral medication is slowest in reducing agitated behaviour, but preferred in situations where collaboration is important and safety has other intervention options [Reference Garriga, Pacchiarotti, Kasper, Zeller, Allen and Vazquez5]. Most studies chose the route of IM. RCT’s studying the effects of oral medication is studied in only 6 studies, all involving risperidone [Reference Hatta, Kawabata, Yoshida, Hamakawa, Wakajima and Furuta25, Reference Lim, Kim, Pae, Lee, Lee and Paik26, Reference Fang, Chen, Li, Wu, Li and Liu42, Reference Currier and Simpson49, Reference Currier, Chou, Feifel, Bossie, Turkoz and Mahmoud50, Reference Yildiz, Turgay, Alpay and Sachs52]. Several consensus papers advocate oral medication, or if not otherwise possible IM. However, most studies contradict this advice as they almost all are based on IM administration [Reference Garriga, Pacchiarotti, Kasper, Zeller, Allen and Vazquez5, Reference Rocca, Villari and Bogetto73–Reference NICE75]. In clinical practice taking oral medication is preferred as it adds to the patient’s feeling of control and autonomy and potentially allows for a better patient staff collaboration. So, these advantages need to be balanced with accepting the possible delay in reaching calmness using oral administration.
Of interest is loxapine, as the route of administration is inhalation. It is thought to have two benefits. First alike oral administration it restores or keeps collaboration intact. Second it serves as an alternative to those patient who are reluctant in taking IM injections [Reference Allen, Feifel, Lesem, Zimbroff, Ross and Munzar65] However, the results show that the effects rather weak in reaching calmness at 2 h.

Fig. 2. Weighted Mean Changes with PANNS-‐EC.
Per medication the weighted mean change of PANSS--‐EC score. Between brackets the number of RCT’s available and the number study subjects.
Guidelines advocate the use of second-generation antipsychotics [Reference NICE75], but despite these guidelines doctors preferably use the older antipsychotics or benzodiazepines [Reference Bervoets, Roelant, De Fruyt, Demunter, Dekeyser and Vandenbussche11, Reference Wilson, Minassian, Bahramzi, Campillo and Vilke76]. Most guidelines or reviews are only descriptive and offer an overview of the opportunities of pharmacological interventions [Reference Garriga, Pacchiarotti, Kasper, Zeller, Allen and Vazquez5, Reference Jibson72, Reference Rocca, Villari and Bogetto73, Reference Hockenhull, Whittington, Leitner, Barr, McGuire and Cherry77, Reference Pratt, Chandler-Oats, Nelstrop, Branford and Pereira78]. Apparently, clinicians rely heavily on clinical experience based evidence rather than thorough clinical studies. The level of evidence based on the Cochrane reviews is rather low.
Despite a specific search no studies have been found on rapid tranquillisation in children and adolescents that meet the inclusion criteria. Also the number of studies about the old age patients is limited to 4 studies [Reference Meehan, Zhang, David, Tohen, Janicak and Small30, Reference Suzuki, Gen and Takahashi44, Reference Rappaport, Marcus, Manos, McQuade and Oren57, Reference Suzuki and Gen61]. Agitated behaviour in the old ages is most likely a problem as well complicated by dosage issues and a greater liability of complicating adverse effects.
4.1. Limitations
Different limitations must be considered in interpreting our results. The number of studies and subjects is limited. This is complicated by the use of different psychometric assessment tools. Although the different scales are probably comparable, the results are not completely comparative. There is a clear need for a uniform set of outcome-variables, e.g. PANNS-EC reduction, number of patients that reaches calmness, mean score of reaching calmness at 2 h. In a separate paper we will come up with a proposal for a minimal set of data and data presentation.
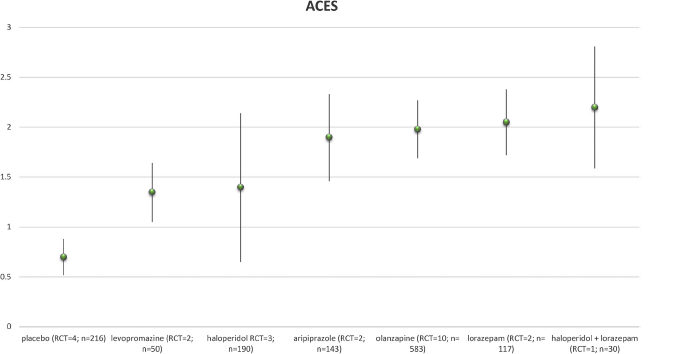
Fig. 3. Weighted Mean Changes with ACES.
Per medication the weighted mean change of ACES score. Between brackets the number of RCT’s available and the number study subjects.

Fig. 4. Weighted Mean Changes with CGI.
Per medication the weighted mean change of CGI score. Between brackets the number of RCT’s available and the number study subjects.
The meta-analytic approach needs data that are clear and up to a certain standard. A fair number of studies did not provide these complete data, i.e. raw data of changes including standard deviation. Contacting the authors did not result in new viable information.
The meta-analysis is only possible in those studies that presents baseline data and data after 2 h or data on the level of change assessed by the psychometric scales. This results in only a small proportion of drugs eligible for a meta-analytic approach. Finally, the review notes the percentages of adverse effects, so the safety of the specific drugs can be judged.
The studies also report primary outcome and adverse effects at different time points. Assessment of speed of onset is important. However, time points vary between studies. However the assessment of the various time are is not entirely uniform. So for meta-analysis the results to heterogeneous. Second, it is not the main outcome of this study.
The assessment and report of adverse effects varies from 2 h till the occurrence of adverse effects within 24 h.
Studies on midazolam and droperidol defined the end-point at 60 min. This hampers the comparability at 2 h. May be the medications show a less numbers of patients being calm at 2 h.
This systematic review and meta-analysis does not allow for direct comparison between the various drugs, as this is not formally tested.
There are no studies that test directly whether differences between administration route, IV versus IM of IM versus oral. Therefore in remains a point of discussion what route is to favour. However, the vast majority of the studies here are based on IM administration. Therefore a comparison between drugs is possible.
5. Conclusions and recommendations
Agitated or aggressive patients impedes the diagnostic and treatment process. A pharmacological intervention as rapid tranquillisation aims to reach calmness and restore contact within two hours. Haloperidol plus promethazine or olanzapine might be first choice drugs and are very well suited for use in hospital or outpatient interventions. This advice is in line with other guidelines [Reference NICE75]. At an ED the context asks for a more rapid onset of calmness and medical safety equipment is at hand allowing midazolam, droperidol or droperidol plus midazolam IV or IM to be used, medications that reaches calmness very fast but also need medical attention. In case of diagnostic insecurity or the probability of suspected contra-indications, lorazepam is a safe alternative. These recommendations are restricted to adult population only, as there are no studies on juveniles and adolescents or elderly people. Future research and publication wold benefit from a comprehensive and uniform assessment procedure and presentation of data.
Table 4 QT-time elongation in droperidol.

Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
Maarten Bak Declarations of interest: Janssen Pharmaceuticals the Netherlands sponsor of yearly conference
Irene Weltens Declarations of interest: none
Chris Bervoets Declarations of interest: none
Jurgen De Fruyt, Declarations of interest: has been a consultant for and conducted clinical research supported
by Janssen-Cilag.
Jerzy Samochowiec, Declarations of interest: none
Andrea Fiorillo,
Gaia Sampogna,
Przemyslaw Bienkowski,
Received speakers’ honoraria or travel support from:
Abbott, Adamed, Angelini, Apotex, Astellas, BGP Products, BMS, Bioton, Chiesi, Gedeon Richter, plus pharma, Ipsen, Janssen, Krka, Lilly, Lundbeck, Mylan, Pfizer, Polfa Tarchomin, Polpharma, Promed, Sandoz, Sanofi, Servier, Takeda, Teva, Valeant, Zentiva
W. Ulrich Preuss,
Blazej Misiak, Declarations of interest: none
Dorota Frydecka,
Agnieszka Samochowiec, Declarations of interest: none
Emma Bak Declarations of interest: none
Marjan Drukker Declarations of interest: none
Geert Dom Declarations of interest: none.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurpsy.2019.01.014.











Comments
No Comments have been published for this article.