Introduction
Antimicrobial resistance has emerged as a serious public health threat. Carbapenems serve as an important choice in treating bacterial infections caused by multidrug-resistant (MDR) bacteria because of their wide spectrum of activity against bacteria with extended-spectrum beta-lactamase (ESBL) and Amp-C enzymes. In the 2021 Global Antimicrobial Resistance and Use Surveillance System report, carbapenems were included in the ’watch list’ of antibiotics and were recommended to be protected as the last line of defense due to the risk of resistance development. 1
Treatment of infections caused by carbapenem-resistant pathogens is challenging due to the limited availability of effective antimicrobials, thus judicious carbapenem use is essential to prevent the emergence of carbapenem resistance. Several studies focusing on the use of carbapenems have often included audit and feedback within the context of the Antimicrobial Stewardship Programme. Reference Wong and Spellberg2–Reference Goodlet and Nailor7 To the best of our knowledge, no study has evaluated the effect of a hospital-specific guideline alone on the appropriate use of carbapenems and its impact on patient outcomes.
In our hospital, infection disease consultation and approval of certain broad-spectrum antibiotics are the standard. To ensure standardization among consultants, hospital-specific guidelines were developed in 2019. In this study, we compared the pre-guideline period (pre-GP) with the post-guideline period (post-GP) in terms of appropriateness of carbapenem prescribing practices and their effect on patient management.
Materials and methods
Study design
The study was designed as a quasi-experimental study. Hospital-specific treatment guidelines were introduced in April 2019. The guidelines can be accessed in English via the link ’https://enfeksiyon.hacettepe.edu.tr/en’. Updates were communicated both verbally and in writing. Using guidelines was made mandatory for all infectious disease (ID) physicians. Patients who used carbapenems for any infection in 2018 (pre-GP) were included as the control group, and those in 2020 (post-GP) were the intervention group. In addition to the guideline, a future study will evaluate the effect of the feedback audit after 2020.
Clinical setting
Hacettepe University Hospital has 1,040 ward beds, six intensive care units (ICUs) with a total of 143 beds.
An infection prevention and control plan for the management of patients colonized or infected with MDR organisms was already in practice at the time of the study. Throughout the study period, clinical specimens for microbiological evaluation were collected at the bedside. Blood samples were processed using the BACTEC 9240 blood culture system (Becton Dickinson, Cockeysville, MD, USA). Bacterial species isolated from all samples were identified using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry and conventional tests. Antibiotic susceptibility profiles of the isolates were determined by BD Phoenix™ automated susceptibility testing system (Becton Dickinson and Company BD, USA), Kirby-Bauer disk diffusion susceptibility test, or antimicrobial gradient test. Antibiotic susceptibility results were interpreted according to European Committee on Antimicrobial Susceptibility Testing recommendations. 8 Fecal samples were tested using a rapid immunochromatographic detection of Clostridiodes difficile antigen (glutamate dehydrogenase antigen), and toxins A and B.
The use of third-generation cephalosporins, piperacillin-tazobactam, glycopeptides, tigecycline, parenteral fluoroquinolones, aminoglycosides, and carbapenems has been restricted to the approval of ID physicians since 2003. ID consultation and Infectious disease approval are available for 7 days/24 h. ID team regularly visits all patients who are on antibiotics with restricted use until clinical stabilization is achieved.
Patient population
All inpatients ≥18 years of age who received a carbapenem for at least 24 hours during the study periods were enrolled. Patients receiving empiric therapy required at least one set of blood cultures taken before initiation of antibiotic therapy and samples from suspected sites of infection. Only the first episode of carbapenem treatment per patient was included. Exclusion criteria were: use of carbapenems based on culture results (pre-GP n = 38, post-GP n = 58), no culture tests before treatment (pre-GP n = 22, post-GP n = 43), death within 24 hours of treatment (pre-GP n = 7, post-GP n = 16), initiation of treatment in another center (pre-GP n = 3, post-GP n = 45), and recurrent episodes of carbapenem use (pre-GP n = 13, post-GP n = 35).
Outcome
The primary outcome was the appropriateness of carbapenem treatment defined according to the presence of risk factors for MDR bacteria infection. Duration of carbapenem use, transfer to the ICU, length of hospital stay, and mortality were assessed as secondary outcome measures.
Data collection
Data for this study were collected retrospectively for the pre-GP (Jan 1st, 2018 to Dec 31st, 2018), and post-GP (Jan 1st, 2020 to Dec 31st, 2020) from electronic records in the hospital information system. Hospital-specific treatment guidelines were introduced in April 2019.
Data including colonization by Candida spp., carbapenem-resistant Enterobacterales (CRE), C. difficile infection, and new infection episodes in 30 days were recorded. Infection density rates for nosocomial bacteremia per 10,000 patient days caused by ESBL-producing E. coli (ESBL-EC) and K. pneumoniae (ESBL-KP), carbapenem-resistant K. pneumoniae (CRKP), Acinetobacter baumannii (CRAB), and Pseudomonas aeruginosa (CRPA), methicillin-resistant Staphylococcus aureus (MRSA), and Vancomycin-resistant Enterococcus faecium (VRE) in 2018 and 2020 were extracted from Hospital Infection Control Committee reports. The consumption rates of carbapenems, piperacillin-tazobactam, ampicillin-sulbactam, 3rd and 4th generation cephalosporins, fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin), amikacin, tigecycline, polymyxins, and glycopeptides were measured in days of therapy (DOT) per 1,000 patient days.
Definitions
Carbapenem use was considered as appropriate if at least one of the following conditions was met:
-
1. History of a fluoroquinolone, 3rd or 4th generation cephalosporin, piperacillin-tazobactam, tigecycline, or carbapenem use for ≥7 days in the last 1 month
-
2. Sepsis in the ICU after ≥72 hours of ICU admission
-
3. Ventilator-associated pneumonia after ≥ 96 hours of ICU admission
-
4. Infection or colonization by an MDR gram-negative bacteria (GNB) resistant to non-carbapenem antibiotics in the last 3 months
-
5. Neutropenic patient with fever under levofloxacin prophylaxis
-
6. Patient with septic shock with a history of unknown recent antibiotic use.
In both study periods, the dosage of carbapenems was adjusted according to the renal function of the patient after the first day doses. Institutional guidelines recommend a three-hour prolonged infusion after the first dose of meropenem for patients treated in the ICU.
The patient was considered to be eligible for de-escalation from carbapenem treatment if the causative pathogen had a susceptibility profile that favored de-escalation, the patient was clinically stable and afebrile for ≥24 hours. Escalation was defined as broadening the empirical carbapenem regimen based on antimicrobial susceptibility results or clinical response.
Successful outcome was defined as the resolution of symptoms and signs of infection and improvement of relevant laboratory parameters. Death was attributed to the infectious process if it occurred within 7 days of carbapenem treatment. Crude all-cause mortality was defined in the 30-day follow-up period.
The source of the infection was established through the implementation of diagnostic criteria outlined by the Centers for Disease Control and Prevention (CDC). 9,10 Sepsis was defined according to the Sepsis-3 criteria. Reference Deutschman11
Sample size
The sample size was calculated with the Open epi program using the sample size for cohort-rct when the case/control ratio was calculated as 1/1, and delta value of 0.24 (percent of unexposed with outcome 0.39, percent of exposed with outcome 0.63) was used resulting in a minimum required number of 95 intervention group and 95 control group was achieved with 90% power, and all patients were included in the study to avoid loss of data.
Statistical analysis
Descriptive statistics were presented as n (%) for categorical variables and as median (25–75 percentile) for continuous variables, as the continuous variables did not exhibit a normal distribution. The distribution of continuous data was assessed using the Kolmogorov–Smirnov test and histogram. The χ2or Fisher’s exact test was utilized for the comparison of categorical variables. Mann–Whitney test was used to compare continous data in two groups. Binary logistic regression was used for multivariate analysis of the effect of guideline implementation on appropriate carbapenem use, while adjusting for confounders. Charlson Co-morbidity Index (CCI), Coronavirus diseases 2019 (COVID-19) history, ICU stay and blood culture growth were considered as confounders when examining the effect of guideline implementation on appropriate carbapenem use. The presence of effect modification was also examined in the multivariate analysis; no effect modifier was identified. Because there was no COVID-19 in the pre-guideline period, the effect-modifying role of COVID-19 could not be evaluated. Cox regression analysis was used for multivariate analysis of the effect of guideline implementation on mortality, while adjusting for confounders. COVID-19, CCI, ICU, blood culture growth, new infection episode within 30 days were evaluated as confounders in mortality analyses. The effect size was reported as a risk ratio, odds ratio or hazard ratio with 95% confidence intervals.
Nosocomial bloodstream infection density rates and antibiotic consumption rate per 100 days [95% confidence intervals (CI)] for each period were calculated by using OpenEpi (Open-Source Epidemiologic Statistics for Public Health) version 3.01 (https://www.OpenEpi.com). The Mid-P exact test was used to compare infection density rates and antibiotic consumption rates in pre-GP and post-GP.
A two-tailed type 1 error rate of 0.05 was considered for all analyses. The analyses were conducted using the Statistical Packages for the Social Sciences (IBM SPSS Corp, Armonk, New York, USA) version 29 software package.
Ethical issues
This study was approved by the Hacettepe University Clinical Research Ethics Board.
Results
A total of 326 patients were included in the pre-GP and 352 in the post-GP (Table 1). Rate of admission to ICU (P < .001), and CCI was higher (P = .011) in post-GP. Urinary tract infection was more common in the pre-GP (34.7% vs 22.7%, respectively P < .001), whereas pneumonia was the leading infection in the post-GP (27.3% in the pre-GP vs 40.3% in the post-GP, P < .001). A total of 74 out of 351 patients (21%) had a diagnosis of COVID-19 in the post-GP.
Table 1. Demographic characteristics of patients in the pre- and post-guideline periods

Note. Cardiovascular diseases: coronary artery disease, heart failure, and atrial fibrillation. Rheumatological diseases: rheumatoid arthritis, lupus, ankylosing spondylitis, scleroderma, psoriatic arthritis, polymyositis. Neurological diseases: Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis (ALS), epilepsy, and cerebrovascular stroke. Chronic lung diseases: comorbid asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, or bronchiectasis. Solid tumors: breast cancer, lung cancer, colon cancer, brain tumors, stomach cancer, liver cancer. Hematological malignancy: AML, ALL, myelodysplastic syndromes (MDS), CML, and CLL. SOT (solid organ transplantation): includes liver and kidney transplantation. Other diseases: benign prostatic hypertrophy, hypothyroidism, viral hepatitis, HIV, hemophilia, inflammatory bowel disease, psychiatric diseases, biliary tract diseases, dermatological diseases, and neurogenic bladder. Urinary system infections: urethritis, prostatitis, cystitis, and pyelonephritis. Intra-abdominal infections: appendicitis, peritonitis, intra-abdominal abscess, cholecystitis, cholangitis, infections following trauma to abdominal organs, and bowel perforations. CNS infections: meningitis, encephalitis, and cranial abscess. Bone and joint infections: septic arthritis and osteomyelitis. Pre-GP: pre-guideline period; post-GP: post-guideline period; COVID-19: coronavirus disease 2019.
Empirical carbapenem use was more frequent in the post-GP. The frequency of bacteremic patients and carbapenem-resistant GNB isolation from the clinical samples were comparable in both periods. The rate of appropriate use of carbapenems increased significantly in the post-GP overall (49.1% in pre-GP vs 71.9% in post GP, P < .001), and in the subgroup of bacteremic patients (56.4% in pre-GP vs 77.4% in post-GP, P = .021). In a sub-analysis of 286 patients admitted to the ICU with renal dysfunction, correct dosing on the first day and three-hour prolonged infusion increased (63.4% in pre-GP vs 27.4% in post-GP, P < .001) (Table 2). When CCI, COVID-19 history, ICU stay, and blood culture growth were controlled by multivariate analysis, appropriate carbapenem use increased in the post-GP period [OR (95% CI) = 1.8 (1.3–2.6), P < .001] (Table 3). De-escalation of carbapenem treatment was more frequent (12.4% in the pre-GP vs 22.7% in the post-GP, P = .002), and the median duration of carbapenem use was shorter in the post-GP (8 days in the post-GP vs 9 days in the pre-GP, P = .019).
Table 2. Comparison of carbapenem use and treatment outcomes in the pre- and post-guideline periods
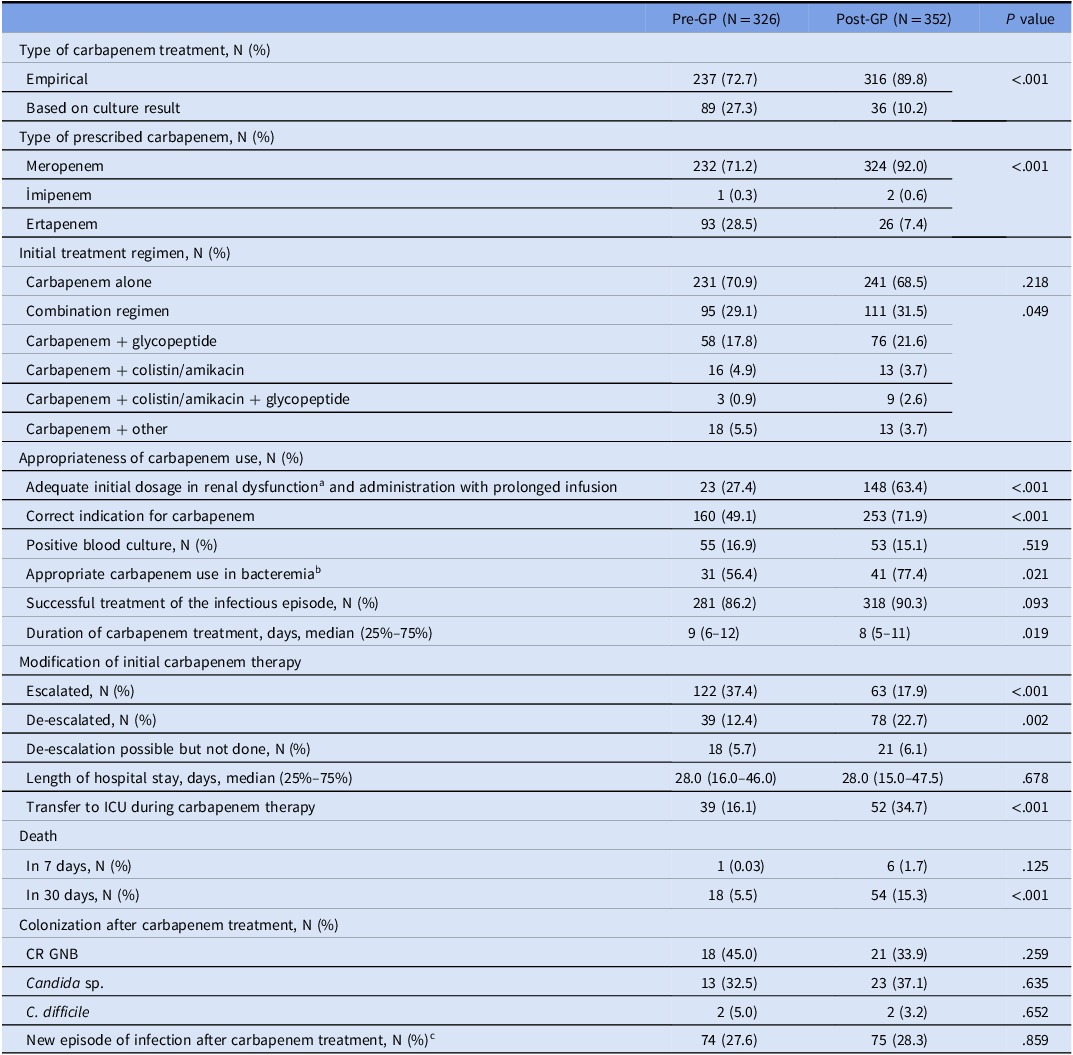
a Analyzed for 286 patients hospitalized in the ICU.
b Includes both correct indication, dosage and duration of infusion.
c Analysis was performed on 102 patients who had records of at least 30-day follow-up in the hospital system after carbapenem was discontinued.
Note. Pre-GP: pre-guideline period; post-GP: post-guideline period; GNB; gram-negative bacilli; CR GNB; carbapenem-resistant gram-negative bacteria.
Table 3. Multivariate analysis of the effect of guideline implementation on appropriate carbapenem use, binary logistic regression
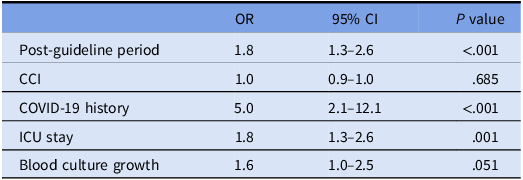
Note. Hosmer and Lemeshow test P value = .814. IQR = interquartile range; OR = odds ratio; COVID-19 = coronavirus disease 2019.
Empirical carbapenem treatment was escalated in 37.4% of cases in the pre-GP compared to 17.9% in the post-GP (P = .004). Patients with diabetes mellitus (P = .021), hematological malignancies (P = .006), and those infected with SARS-CoV-2 (P < .001) required escalation more frequently. A higher proportion of escalated cases were in the ICUs compared to the wards (77.8% vs 52.9%; P = .001). Escalation was also higher in patients in whom source control could not be achieved due to unremoved infected catheters, undrained intra-abdominal or pulmonary abscesses/collections (P = .008). Forty-six percent of the patients in whom treatment was escalated were infected by carbapenem-resistant (CR) GNB.
There was no significant difference in the infection-related mortality between periods. However, 30-day mortality rate was significantly higher in post-GP (15.3%) compared to that in pre-GP (5.5%) (P < .001). Group comparisons are shown in Table 4. When CCI, COVID-19 history, length of ICU stay, blood culture growth, appropriate carbapenem use, new infection episode within 30 days were controlled by cox regression analysis, the guideline was not associated with mortality. CCI, COVID-19 history, ICU stay, new infection episode in 30 days were found to be associated with mortality (P values <.001, .005, .003, <.001, respectively) (Table 5).
Table 4. Evaluation of mortality-related factors in 30 days of carbapenem treatment

Note. IQR: Interquartile range.
Table 5. Multivariate analysis of the effect of guideline implementation on 30-day mortality, cox regression
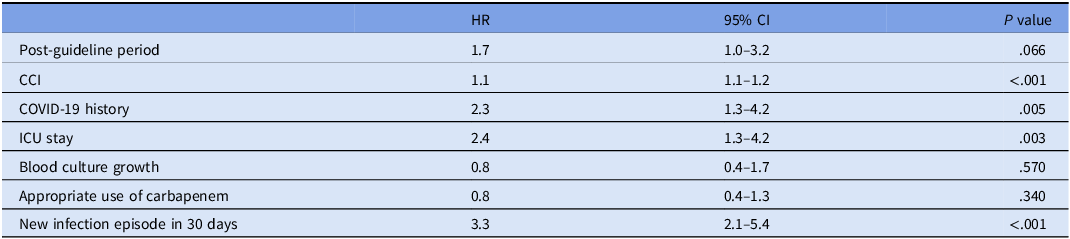
Note. Omnibus test P value <.001. COVID-19: coronavirus disease 2019; HR: hazard ratio; CI: confidence interval.
Nosocomial bloodstream infection density rates with MDR bacteria were similar in both periods except for an increase in CRAB (Table 6). Consumption rates of carbapenems, piperacillin-tazobactam, colistin, tigecycline, and glycopeptides increased in the post-GP (Table 7). In contrast, consumption rate of sulbactam-ampicillin, cephalosporins, and fluoroquinolones were significantly lower for the same period compared with pre-GP.
Table 6. Comparison of infection density rates of nosocomial bloodstream infections caused by multidrug-resistant bacteria in pre-guideline and post-guideline periods

a Per 10,000 patient days.
Note. Pre-GP: pre-guideline period; post-GP: post-guideline period; CI: confidence interval; ESBL-EC: ESBL-producing Escherichia coli; ESBL-KP: ESBL-producing Klebsiella pneumoniae; CRKP: carbapenem-resistant Klebsiella pneumoniae; CRPA: carbapenem-resistant Pseudomonas aeruginosa; CRAB: carbapenem-resistant Acinetobacter baumannii; VRE: vancomycin-resistant Enterococcus faecium.
Table 7. Consumption antibiotic consumption rated per 1,000 patient days in pre-guideline and post-guideline periods

Note. Third-and fourth-generation cephalosporins: cefotaxime, ceftriaxone, ceftazidime, cefepime; fluoroquinolones: ciprofloxacin, levofloxacin, moxifloxacin. Pre-GP: pre-guideline period; post-GP: post-guideline period; CI: confidence interval.
Discussion
In this study, we found that the implementation of hospital-specific guidelines alone had a positive effect on the appropriate use of carbapenems. This was also seen in patients with bacteraemia. The improvement in the use of carbapenems was not only limited to the choice of the appropriate indication, but also to the dosage strategies. There was a 2.3-fold increase in the use of prolonged infusion and correct initial dose of carbapenems in ICU patients with renal impairment after the hospital guidelines were introduced. De-escalation has been encouraged as important component of antimicrobial stewardship protocols and a statistically significant increase in the rate of de-escalation was observed after the introduction of the guidelines (Table 2). This is remarkable finding at an institution with a high endemicity for MDR bacteria.
Local guidelines for diagnosis, treatment, and follow-up of infectious conditions are essential for standardized patient management in accordance with current medical principles. This is especially important for both correct clinical approach of the junior residents during night calls and for training purposes. Construction of a local guideline should encompass evidence-based data from the literature and recommendations of widely accepted international societies or organizations. In addition, blending the existing data with local resistance profiles as well as available local resources is imperative. A local guideline also provides the basis for assessment of the quality of care provided for that specific condition, most frequently as audit and feedback.
Although carbapenem use was more appropriate after the local guidelines, this did not result in an increased success rate in the treatment of the infectious episode (89.0% vs 92.0%, P = .169). This lack of effect could be attributed to several factors, mainly the presence of unfavorable conditions in the post-GP such as the type of infection (urinary tract infection vs pneumonia), more patients in the ICU, and higher CCI values.
Carbapenem treatment was escalated more frequently in pre-GP compared to post-GP. This reflects the appropriateness of pre-defined criteria for carbapenem use in the guidelines. Yet, 17.9% escalation rate suggests some improvement may still be achieved in terms of better coverage in the initial treatment regimen. The variables associated with escalation were significant for COVID-19, ICU admission, and uncontrolled infection source. It has been shown that the SARS-CoV-2 pandemic had a negative effect on health care; more infections caused by MDR bacteria and more frequent use of antibiotics were observed. We reported similar findings in a recent study where we examined the impact of COVID-19 pandemic on bloodstream infections caused by MDR bacteria and antibiotic consumption between 2018 and 2020. Reference Metan, Çuha and Hazirolan12 Infection density rates of MDR bacteria were the lowest at the start of the pandemic (the first half of 2020), but then increased rapidly which triggered the increased rate of meropenem consumption, especially in the COVID-ICUs. Active surveillance was suspended between March 2020 and April 2021 due to the COVID-19 pandemic, an outbreak of MDR A. baumannii was detected in COVID ICUs through a laboratory-based analysis of bloodstream infection surveillance in November 2020, which may also contribute to escalation of carbapenem treatment.
Most carbapenem stewardship studies have reported no change in mortality despite an improvement in antimicrobial use. Reference Makina, Poulakou and Sympardi13–Reference Ronda, Padullés and Grau15 After adjustment for CCI, COVID-19 history, ICU stay and blood culture growth, appropriate use of carbapenems and new infection episode in 30 days, the guideline did not have an influence on mortality in our study, either. However, Spernovasilis et al. found a reduction in mortality when a carbapenem-focused antimicrobial stewardship program was implemented during the COVID-19 pandemic in a setting of high endemicity for MDR GNB. Reference Spernovasilis, Kritsotakis and Mathioudaki16 In contrast to this study, in our study antibiotic use was restricted with the approval of the ID physician, including the pre-GP period. Also, unlike the other study, the effect of the guideline alone was assessed without audit feedback. The difference between the studies may be due to differences in both the intervention and control groups.
Our study is unique in that it evaluates the effect of local guidelines with no further interventions on carbapenem use. Most early studies have shown a favorable influence of a guideline on patient care, such as in the treatment of community-acquired Reference Benenson, Magalski, Cavanaugh and Williams17–Reference Marrie, Lau and Wheeler20 or health-care-associated pneumonia, Reference Dellit, Chan, Skerrett and Nathens21–Reference Worrall, Anger, Simpson and Leon23 febrile neutropenia, Reference Porto24 skin and soft tissue infections, Reference Jenkins, Knepper and Sabel25 and staphylococcal bacteremia Reference Holland, Raad and Boucher26 with no specific focus on carbapenems. More recent studies have utilized various interventional approaches for optimal carbapenem use. Reference Makina, Poulakou and Sympardi13–Reference Spernovasilis, Kritsotakis and Mathioudaki16 Garcia-Rodriguez et al reported their experience with the introduction of local guidelines, auditing, and feedback. Reference Garcia-Rodriguez, Bardan-Garcia, Juiz-Gonzalez, Vilarino-Maneiro, Alvarez-Diaz and Marino-Callejo3 Their approach resulted in an increase in the eligibility of carbapenem prescription (from 49.7% in 2015 to 80.9% in 2019), and a subsequent decrease in carbapenem consumption while consumption of cefepime and piperacillin increased. This was accompanied by a decrease in the overall frequency of bacteremia caused by MDR bacteria. This latter finding is in contrast to the result of our study. It should be emphasized that the prevalence of MDR bacteria in a particular healthcare facility depends on several factors other than the availability of antimicrobial stewardship protocols and appropriate use of antimicrobials, such as effective infection prevention and control measures, availability of narrow-spectrum antibiotics in the hospital pharmacy, timely transfer of patients from the ICU to the ward, effective transfer of patients requiring palliative care to nursing homes and end-of-life care facilities, and finally the burden of a pandemic on healthcare workers.
Our study has several limitations. First, retrospective data collection relies on the hospital’s electronic system, which may introduce potential bias. Second, routine documentation of some confounding factors, such as infection control practices and information on clustering and mini-epidemics in the hospital may not optimal, especially during the COVID-19 pandemic. Due to the lack of randomization, the effect of unmeasured confounders cannot be completely excluded. As the level of hospital-wide compliance with the guideline is not known, the impact of the guideline may have been underestimated.
In conclusion, introduction of hospital-specific guidelines alone improves appropriate use of carbapenems even when antimicrobial treatment is mainly regulated by ID Department. A multifaceted carbapenem-focused ASP together with an effective infection control program may result in desired reductions in all-cause mortality rates, and prevalence of MDR pathogens.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2024.415.
Acknowledgments
None available.
Author contribution
Cemre Boşnak; Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Şeyda Betül Fındık; Investigation, Data curation. Muhammed Atay; Investigation, Data curation. Ward Fakhouri; Investigation, Data curation. Sada Babazade; Investigation, Data curation. Eda Karadoğan; Formal analysis, Writing—review and editing. Gökhan Metan; Methodology, Writing—review and editing. Ömrüm Uzun; Supervision, Methodology, Writing—review and editing.
Financial support
None available.
Competing interests
All authors report no conflicts of interest relevant to this article.
Research transparency and reproducibility
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to privacy and ethical considerations, access to the data will be granted on a case-by-case basis, ensuring that the request is aligned with the research objectives and ethical standards.









