Impact statement
Plastic pollution is a global environmental problem that is becoming increasingly important. Wastewater treatment plants (WWTPs) are a significant source of plastic pollution as they receive a large amount of plastic waste from households and businesses. However, the literature on plastics entering WWTPs and their fate is insufficient and largely unexplored. This review article attempts to describe the current state of knowledge on plastic pollution in WWTPs and identify future research areas.
Introduction
Environmental pollution through chemicals is a global concern, specifically for aquatic ecosystems, threatening the water supplies and food production as they pose significant environmental risks due to their novelty or lack of information about their fate (Bashir et al., Reference Bashir, Lone, Bhat, Mir, Dar, Dar, Hakeem, Bhat and Qadri2020). Even those chemicals that have been known for some time may only recently be identified as potentially hazardous to the environment (Bhat et al., Reference Bhat, Beigh, Mir, Lone and Khosrow-Pour2022). Microplastics (MPs) classify under this category, with recorded data showing increasing concern, particularly in terms of pollution levels in aquatic systems (Andrady, Reference Andrady2011; Twiss, Reference Twiss2016; Blettler et al., Reference Blettler, Abrial, Khan, Sivri and Espinola2018). These pollutants are chemicals with no legal status, and their effects on human health or the environment are unknown, according to government-related organizations (Deblonde et al., Reference Deblond, Cossu-Leguille and Hartemann2011; Yaşar et al., Reference Yaşar, Can Doğan and Arslan2013). Plastic debris poses a particular threat because it is resistant to degradation processes, leading to ubiquitous accumulation in the environment, and because it is resistant to corrosion and damage from various factors (Hu et al., Reference Hu, Gong, Wang and Bassi2019). Furthermore, earlier studies also show that as the volume of this waste in water bodies increases, the amount of solar heat energy trapped in the water per unit volume decreases, resulting in additional energy escaping to the nearby environment and affecting global warming (Ford et al., Reference Ford, Jones, Davies and Koldewey2022).
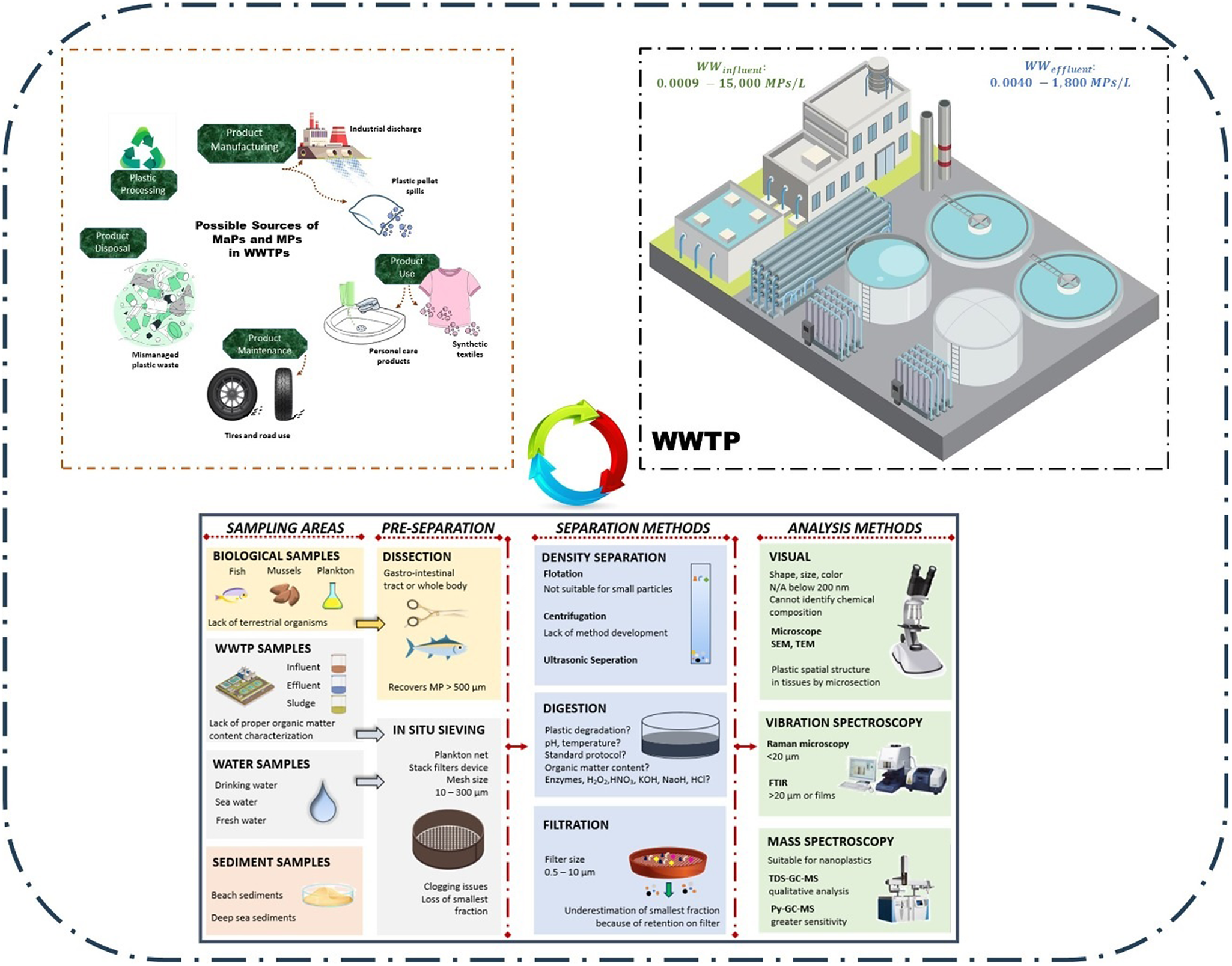
Plastic particles accumulate in wastewater treatment plants (WWTPs) from various sources such as domestic sewage, industrial effluents, rainwater and landfills (Okoffo et al., Reference Okoffo, O’Brien, O’Brien, Tscharke and Thomas2019). Therefore, they have been identified as major sources of plastic release into the environment. Due to primary MPs (PMPs) added to cosmetics and personal care products, and secondary MPs (SMPs) formed during the degradation of synthetic plastics after washing, MPs can be detected in wastewater (Lares et al., Reference Lares, Ncibi, Sillanpää and Sillanpää2018). During rainy weather, urban storm water, which may contain MPs from traffic, airborne and synthetic degradation, enters the sewer system either independently or together with street runoff (Sugiura et al., Reference Sugiura, Takada, Takada, Mizukawa, Tsuyuki and Furumai2021).
MPs pose the problems mentioned previously. Therefore, it is crucial to understand their likely sources and pathways in order to reduce their negative impacts on biotic and abiotic habitats. In addition to MPs, the presence of nanoplastics (NPs) resulting from fragmentation of MP in WWTPs also raises serious questions due to their physicochemical properties and potential damage to ecosystems (Twiss, Reference Twiss2016; Ali et al., Reference Ali, Ding, Peng and Liu2021). Despite the growing awareness of the scale, nature and impact of MP pollution, there is still not enough information on how plastics enter WWTPs and what happens to them (Rasmussen et al., Reference Rasmussen, Iordachescu, Tumlin and Vollertsen2021). Therefore, the purpose of this study is to identify research gaps and provide a thorough overview of the current state of knowledge on plastic pollution in WWTPs. The study draws attention to the lack of knowledge about the sources of plastic inputs into WWTPs and emphasizes the need for further thorough research in this area. Additionally, the study investigates the function of plastics as a potential source of MPs and the difficulties they pose in wastewater treatment, as well as the analytical methods used to assess the presence and properties of plastics in WWTPs. The variables that affect the fluctuation of plastic content in WWTPs are being studied in order to gain a better understanding of the complicated dynamics of plastic pollution in these systems. In addition, the need for policies to effectively manage plastic pollution in line with the United Nations Sustainable Development Goals (SDGs) was assessed.
WWTPs as a potential source of plastic pollution in the environment
WWTPs are designed to effectively remove essential contaminants and purify water by removing debris, organic compounds and inorganic pollutants. Maintaining quality standards for wastewater from urban areas has been a critical aspect of wastewater management. However, the current treatment plants may not provide an adequate solution for the complete removal of plastics from domestic wastewater.
WWTPs usually involves several treatment steps, including screening, sedimentation, flocculation and aeration, which together contribute to the removal of various pollutants, including macroplastics (MaPs) and MPs (Ziajahromi et al., Reference Ziajahromi, Neale, Telles Silveira, Chua and Leusch2021; Keerthana Devi et al., Reference Keerthana, Karmegam, Manikandan and Govarthanan2022). The initial step of the treatment process usually involves screening of the influent wastewater to remove larger plastic debris, primarily MaPs. Subsequently, in the secondary treatment stage, advanced oxidation tanks ensure that dissolved organic wastes, including MPs, are eliminated through various mechanisms. To ensure the highest possible water quality, tertiary treatment protocols are often used in WWTPs following secondary treatment. Advanced treatment methods, such as activated sand filters, biofilm reactors and membrane bioreactors, are often used as part of tertiary treatment to provide additional purification and removal of MPs before the treated wastewater is discharged into nearby rivers or water bodies (Mahon et al., Reference Mahon, O’Connell, Healy, O’Connor, Officer, Nash and Morrison2017). Typical processes of WWTP and indicated sources of plastics are schematically given in Figure 1.

Figure 1. Typical processes of a tertiary WWTP (indicated sources of plastics in WWTP, and primary, secondary and tertiary processes).
Nevertheless, it has been found that the efficacy of treatment varies depending on the specific design and operating parameters of each WWTP. In particular, the accumulation of plastics in treatment units such as sedimentation tanks and pumps poses operational challenges, leading to increased maintenance and reduced efficiency. In addition, the accumulation of plastics in aeration tanks hinders oxygen transport and thus affects the performance of treatment processes (Rasmussen et al., Reference Rasmussen, Iordachescu, Tumlin and Vollertsen2021).
According to Karapanagioti (Reference Karapanagioti2017), there are two main pathways by which plastics can enter a WWTP. The first is direct exposure, which occurs when people intentionally or accidentally flush solid waste down toilets or sinks (Mattsson et al., Reference Mattsson, Hedström, Ashley and Viklander2015). The second is indirect introduction into combined sewer systems, where the sewer system carries both stormwater and wastewater (Di Nunno et al., Reference Di Nunno, Granata, Parrino, Gargano and de Marinis2021). In addition, WWTPs serving smaller towns and suburban areas are more likely to contain certain types of MaPs such as cotton buds, as well as other wastes such as condoms, wet wipes, sanitary pads and baby wipes (Alda-Vidal et al., Reference Alda-Vidal, Hoolohan, Dyson, Danino and Browne2020; Besley and Cassidy, Reference Besley and Cassidy2022; Köklü et al., Reference Köklü, Ateş, Deveci and Sivri2023). This suggests that people in urban areas are more environmentally aware and active than people in suburban areas. This difference could be related to a greater awareness of the functions of urban WWTPs and a greater commitment, sensitivity and responsibility for environmental protection (Mourgkogiannis et al., Reference Mourgkogiannis, Kalavrouziotis and Karapanagioti2018). Items such as cotton buds, plastic caps and non-plastics such as condoms and baby wipes are common among the plastics found in various WWTPs (Besley and Cassidy, Reference Besley and Cassidy2022). Due to the difficulty in identifying tiny plastic waste, these smaller plastics can pollute the environment in overflow situations when wastewater is discharged into water bodies (Akarsu et al., Reference Akarsu2023).
Both MPs and MaPs can also enter wastewater directly, for example, when products containing plastics are washed into wastewater (e.g., textile fibers released during laundry, microbeads in consumer goods, sanitary products or cotton buds), or indirectly via the combined sewer system from street debris and litter (Nunno et al., Reference Di Nunno, Granata, Parrino, Gargano and de Marinis2021). Although an average of 70% of wastewater is treated in high-income countries, only 20% of wastewater generated is treated worldwide (Duis and Coors, Reference Duis and Coors2016). Furthermore, during periods of heavy rainfall or snowfall, overflows in combined sewers, together with the lack of functioning treatment plants, often result in MPs into the environment through the inefficient treatment of wastewater (Winton et al., Reference Winton, Anderson, Rocliffe and Loiselle2020).
Some WWTPs should be known for the fact that their effluents flow directly into water bodies (Sun et al., Reference Sun, Jia, Ye, Zhu, Song, Guo and Chen2023). The significant contribution of WWTP effluents to plastic pollution has been underlined by studies that estimate the daily release of millions or perhaps billions of plastic particles into receiving waters from WWTPs (Carr et al., Reference Carr, Liu and Tesoro2016; Kalčíková et al., Reference Kalčíková, Alič, Skalar, Bundschuh and Gotvajn2017; Leslie et al., Reference Leslie, Brandsma, van Velzen and Vethaak2017; Gündoğdu et al., Reference Gündoğdu, Çevik, Güzel and Kilercioğlu2018). For instance, one study found that a WWTP with a capacity of about 150,000 m3.d−1 discharges about 87.6 million MPs into the Mersin Bay (Akarsu et al., Reference Akarsu, Kumbur, Gökdağ, Kıdeyş and Sanchez-Vidal2020). According to another study, more than 80% of the 210 trillion microbeads that enter the waters of mainland China each year originate from WWTP effluent (Cheung and Fok, Reference Cheung and Fok2017). Similarly, it is estimated that between 50,000 and 15 million plastic particles per day enter freshwater from some sewage treatment plants in the United States (Mason et al., Reference Mason, Garneau, Sutton, Chu, Ehmann, Barnes, Fink, Papazissimos and Rogers2016).
The transfer of plastic pollutants from aquatic to terrestrial ecosystems is primarily attributed to the use and disposal of sewage sludge from wastewater treatment. Sewage sludge, which is known to contain high levels of organic matter, nutrients and contaminants such as plastics, has been found to carry an average of 14,750 particles per kilogram (Ragoobur et al., Reference Ragoobur, Huerta-Lwanga and Somaroo2021). Initial studies of plastic pollution from sewage sludge application have shown elevated concentration of MP in topsoil compared to non-fertilized soils (Corradini et al., Reference Corradini, Meza, Eguiluz, Casado, Huerta-Lwanga and Geissen2019; Tagg et al., Reference Tagg, Brandes, Fischer, Fischer, Brandt and Labrenz2022; Hassan et al., Reference Hassan, Prasetya, Hanun and Jiang2023).
Restrictions such as the German regulation limiting the application of sewage sludge to 5 tons per hectare every 3 years have led to a decline in agricultural use, a trend that is expected to continue if regulations are tightened. Nonetheless, significant amounts of sewage sludge and associated plastics have been intentionally applied to soils in the past. Notably, MPs and NPs pose a significant threat to soil biota by affecting plant growth, organism reproduction and soil biodiversity (Hale et al., Reference Hale, Seeley, La Guardia, Mai and Zeng2020). As soil is an important habitat for terrestrial organisms, its fauna is increasingly affected by the ecotoxicological impacts of MPs and NPs, which are ingested by soil invertebrates and poultry and potentially serve as entry points for humans and other animals (Cox et al., Reference Cox, Covernton, Davies, Dower, Juanes and Dudas2019). The leaching of additives such as bisphenol A and phthalates from MPs and NPs disrupts the endocrine system of vertebrates and has estrogenic effects (Zhang and Chen, Reference Zhang and Chen2020). Several factors, including sunlight, oxygen content, temperature, soil microorganisms and terrestrial biota, contribute to the rate of degradation of plastic waste in the upper soil layer (Wong et al., Reference Wong, Lee, KHD and Yap2020).
Sources and pathways of plastic waste in wastewater treatment plants
MPs can be divided into two categories depending on their origin: PMPs and SMPs. PMPs, which account for an estimated 19–31% of MPs in the oceans, are plastics that enter the environment directly in the form of small particles. Examples of PMPs are plastics from the manufacture, use or maintenance of MaPs objects such as personal care products (facial cleansers, toothpaste, etc.), tire wear from driving and synthetic textile products from washing (Boucher and Friot, Reference Boucher and Friot2017).
On the other hand, SMPs, which is estimated to account for 69–81% of MPs in the oceans (Evangeliou et al., Reference Evangeliou, Tichý, Eckhardt, Zwaaftink and Brahney2022), is formed by the degradation of MaP- and mesoplastic into smaller dimensions. SMPs can enter the marine environment from both terrestrial and marine sources. A major terrestrial source of SMPs is WWTPs, which receive a variety of primary plastic wastes, such as wet wipes, plastic gloves, bandages, diapers, feminine hygiene products and plastic medical consumables (Figure 2). These items are often improperly disposed of in toilets, where they can cause blockages, clogs and overflows and disrupt biological treatment processes in WWTPs. In addition, these items can cause unpleasant odors in sewage systems, pumping stations and treatment plant pipes. Approximately, 75–90% of plastic in the marine environment comes from land-based sources transported via rivers (1.4 Mt/year) and coastal areas (5.1 Mt/year), whereas 10–25% comes from activities such as commercial fishing, maritime transport and sea travel (Belzagui Elder, Reference Belzagui Elder2017). Improper disposal of plastic waste is a major contributor to the accumulation of SMPs in the marine environment (Pandey et al., Reference Pandey, Pathak, Singh, Kumar, Kumar, Kaushik and Thakur2022).

Figure 2. Visual data depicting the MaPs wastes found within a WWTP (with Prof. Dr. Mustafa Öztürk’s permission and consent).
The presence of MPs in the influent and effluent of WWTP has been reported in several countries, including the United States (Mason et al., Reference Mason, Garneau, Sutton, Chu, Ehmann, Barnes, Fink, Papazissimos and Rogers2016; Ridall et al., Reference Ridall, Farrar, Dansby and Ingels2023), the Netherlands (Leslie et al., Reference Leslie, Brandsma, van Velzen and Vethaak2017), Germany (Mintenig et al., Reference Mintenig, Int-Veen, MGJ, Primpke and Gerdts2017; Barkmann-Metaj et al., Reference Barkmann-Metaj, Weber, Bitter and Engelhart2023), England (Murphy et al., Reference Murphy, Ewins, Carbonnier and Quinn2016), Sweden (Magnusson and Noren, Reference Magnusson and Noren2014), Australia (Ziajahromi et al., Reference Ziajahromi, Neale, Rintoul and Leusch2017) and Turkey (Akarsu et al., Reference Akarsu, Kumbur, Gökdağ, Kıdeyş and Sanchez-Vidal2020; Vardar et al., Reference Vardar, Onay, Demirel and Kideys2021; Koyuncuoğlu and Erden, Reference Koyuncuoğlu and Erden2023). Despite the high efficiency of WWTPs in removing of MPs, significant amounts of MPs can still enter into receiving waters. For example, one WWTP serving 650,000 residents was found to have an MP removal efficiency of 98.41%, yet an estimated 65 million MPs were discharged daily (Murphy et al., Reference Murphy, Ewins, Carbonnier and Quinn2016). Similarly, a study conducted at 17 WWTPs in the United States reported releases of over 4 million MPs per plant per day (Mason et al., Reference Mason, Garneau, Sutton, Chu, Ehmann, Barnes, Fink, Papazissimos and Rogers2016). Another example is a WWTP in northern Italy with a population of about 1,200,000 that had a MP removal efficiency of 84%, but still released an estimated 160,000,000 MPs per day (Magni et al., Reference Magni, Binelli, Pittura and Regoli2019). In a study by Vardar et al., (Reference Vardar, Onay, Demirel and Kideys2021), it was predicted that Ambarlı Advanced Biological WWTP contributes approximately 2,934 × 106 MPs per day into the Sea of Marmara. These results highlight that despite their treatment efforts, WWTPs can be a significant source of MP pollution, as they continuously discharge large amounts of effluent into the aquatic environment (Bozdaş et al., Reference Bozdaş, Üstün and Aygün2020).
Sampling, sample pre-treatment and analytical methodologies
Sampling and description of WWTP
MPs in WWTPs show heterogeneous distribution in both effluent and sludge (Gao et al., Reference Gao, Chen, Cizdziel and Huang2023). Although there are no standardized methods for sampling MPs in WWTPs, several successful approaches have been used. These include non-discrete techniques such as continuous pumping combined with in situ filtration and discrete sampling methods such as manual sampling or the use of an auto-sampler (Üstün et al., Reference Üstün, Bozdaş and Can2022). These different sampling methods allow researchers to effectively detect MP and investigate its presence in different parts of the WWTP (Figure 3).

Figure 3. Classification, measurement method and some typical apparatuses (adapted from Ye et al., Reference Ye, Yu and Zhao2022).
Among them, grab sampling is the most common method for collecting water, sediment or other environmental matrices to quantify the presence and concentration of MPs (Sönmez et al., Reference Sönmez, Akarsu and Sivri2023). There are a number of different methods for conducting grab sampling, but the most common method is to use a container to collect a set amount of samples (Green et al., Reference Green, Kregting, Boots and Crowley2018). Various sample volumes have been reported for collecting samples from organically contaminated wastewater, ranging from 0.1 L to 50 L (Michielssen et al., Reference Michielssen, Michielssen, Ni and Duhaime2016; Leslie et al., Reference Leslie, Brandsma, van Velzen and Vethaak2017; Mintenig et al., Reference Mintenig, Int-Veen, MGJ, Primpke and Gerdts2017). These samples are usually taken from both the influent and the biological unit of the WWTP (Table 1).
Table 1. A summary of commonly applied sampling approaches (adapted from Gao et al., Reference Gao, Chen, Cizdziel and Huang2023)

Sample preparation
MPs found in the environmental matrix have the ability to absorb various pollutants and may also have altered density due to biofilm layers covering its surface (Tu et al., Reference Tu, Chen, Zhou, Liu, Wei, Waniek and Luo2020). Therefore, it is important to wash the collected particles several times with distilled water before determining the morphological characteristics and performing the chemical structure analysis. This washing procedure effectively removes potential contaminants and impurities that could interfere with the subsequent analysis. In some cases, the MaPs and MPs are analyzed directly without undergoing additional processes to detect contaminants or organisms that may be adsorbed on their surface. In the case of water matrices, the separation of MPs is usually done with a series of screens or filters with different mesh or pore sizes through which the collected wastewater is passed (Costa et al., Reference Costa, Cruz, Martins and Da Silva2021). For example, Ziajahromi et al. (Reference Ziajahromi, Neale, Rintoul and Leusch2017) developed a method using a large-volume sampling device with multiple mesh screens to effectively separate various size of MPs from wastewater. This method showed high efficiency with a retention efficiency of 92% for the 25 μm mesh screen and 99% for the 500 μm mesh screen. As an alternative, Besley et al. (Reference Besley, Vijver, Behrens and Bosker2017) proposed a slightly different approach using a fully saturated NaCl solution in combination with filtration for MP extraction. This method allows the quantification of MPs in the size range of 0.3–5 mm.
Filtration is also used to separate them from the aqueous environment to determine the abundance and characterize the morphological properties of MPs. Various filter paper pore sizes have been used in different studies, including 0.2 μm (alumina oxide), 0.45 μm (GF/C), 1.2 μm (GF/C) and 5 μm (silicone, silver) (Robertson, Reference Robertson2018). Among the filter options, glass fiber filters have been widely used in MP research (Hanvey et al., Reference Hanvey, Lewis, Lavers, Crosbie, Pozo and Clarke2017). These filters are commonly selected due to their suitability for capturing MPs and their compatibility with subsequent analysis techniques.
On the other hand, density fractionation methods are widely used to extract MPs from a complex soil matrix. The methods are based on the principle of combining the sample with a saturated salt solution of known density, followed by separation of the MP from its environment after a certain retention time (Sönmez et al., Reference Sönmez, Akarsu, Cumbul Altay, Sivri and Hashmi2022). Retention times for MPs can vary considerably, ranging from 5 min to 48 h (Fries et al., Reference Fries, Dekiff, Willmeyer, Nuelle, Ebert and Remy2013). After the specified retention time, the MPs are separated from the supernatant of the separating funnel so that they are available for subsequent analysis. Sodium chloride (NaCl), sodium iodide (NaI) and zinc chloride (ZnCl2) are commonly used salts in the density-based separation process (Nabi et al., Reference Nabi, AUR and Zhang2022). Among them, NaCl with a density of 1.2 g.L−1 is preferred because of its affordability and nontoxic properties (Li et al., Reference Li, Song and Cai2020). The saturated salt solution with NaCl creates a density gradient that floats the MP particles, which makes the separation process. Using the density separation method with NaCl provides a cost-effective and safe approach to isolate MP based on their density properties (Parashar and Hait, Reference Parashar and Hait2023).
Following the separation process, a clean-up procedure to remove microbes and various organic deposits is frequently used (Li et al., Reference Li, Song and Cai2020). In the literature, various approaches including peroxide digestion (H2O2), alkaline digestion (NaOH) and acid digestion (HNO3 and H2SO4) have been used to degrade organic matter in wastewater samples and sewage sludge (Sönmez et al., Reference Sönmez, Akarsu, Cumbul Altay, Sivri and Hashmi2022). Sewage sludge is a more challenging sample than other environmental matrices, especially sediment, as it contains a mixture of organic material from human waste, inorganic solids, food waste, trace chemicals, heavy metals, microbes, pharmaceuticals and other micropollutants (Zhang and Chen, Reference Zhang and Chen2020; Gao et al., Reference Gao, Chen, Cizdziel and Huang2023). Several extraction techniques including oxidative digestion (Bretas Alvim et al., Reference Bretas Alvim, Bes-Piá and Mendoza-Roca2020; Cunsolo et al., Reference Cunsolo, Williams, Hale, Read and Couceiro2021), alkaline treatment (Mintenig et al., Reference Mintenig, Int-Veen, MGJ, Primpke and Gerdts2017) and acid-based digestion (Hernández-Arenas et al., Reference Hernández-Arenas, Beltrán-Sanahuja, Navarro-Quirant and Sanz-Lazaro2021) have been proposed to remove organics efficiently with minimal impact on MPs. The applicability of Fenton’s reagent in the extraction of plastics from environmental matrices such as sludge and soil in combination with density separation has been confirmed (Hurley et al., Reference Hurley, Lusher, Olsen and Nizzetto2018). Oxidation with hydrogen peroxide coupled with density separation has been shown to reduce chemical consumption, sample pretreatment time and cost by adjusting the reaction temperature (Sujathan et al., Reference Sujathan, Kniggendorf, Kumar, Roth, Rosenwinkel and Nogueira2017; Zhang and Chen, Reference Zhang and Chen2020). For samples with high organic content, a mixture of 30% H2O2 and 0.05 M Fe(II), known as Fenton reagent, is commonly used. The temperature is usually increased to 50°C to promote decomposition of the organic compounds during the oxidation process (Hurley et al., Reference Hurley, Lusher, Olsen and Nizzetto2018).
Enzymatic treatments with enzymes such as amylase, lipase, chitinase, proteinase and fibers have also been used to remove organic matter from MPs (Cole et al., Reference Cole, Webb, Lindeque, Fileman, Halsband and Galloway2014; Parashar and Hait, Reference Parashar and Hait2023). However, it should be noted that enzymatic treatments can be costly and may have limitations in terms of MP processing efficiency (Zhu and Wang, Reference Zhu and Wang2020). In addition, acid and alkali treatments can be used to remove organic matter. However, care should be taken as they can potentially damage certain MP species that are sensitive to pH changes (Sönmez et al., Reference Sönmez, Akarsu, Cumbul Altay, Sivri and Hashmi2022). Among the various isolation methods, the peroxide oxidation is considered effective for MP extraction and offers advantages over other methods.
Analytical methods
Previous studies on MaPs and MPs have performed abundance, enumeration and identification using stereomicroscopy and visual identification based on their physical characterization of type, morphology and color (Derraik, Reference Derraik2002; Andrady, Reference Andrady2011) (Figure 3). However, with increasing number of studies, this method has been found to have more drawbacks. There is a risk of up to 70% in the visual identification of possible plastic particles under the stereomicroscope (Hidalgo-Ruz et al., Reference Hidalgo-Ruz, Gutow, Thompson and Thiel2012). To overcome this problem, the use of selected dyes to determine the abundance and types of MPs has become popular (Hurley et al., Reference Hurley, Lusher, Olsen and Nizzetto2018). However, it is not feasible to find a single dye that is suitable for all types of polymers. While staining remains an effective technique for quantifying and distinguishing different types of MPs, it is not sufficient to determine the colors of MPs, as shown by the research of Tu et al. (Reference Tu, Chen, Zhou, Liu, Wei, Waniek and Luo2020). When a thorough knowledge of the surface structure of MPs is required, for example, in a study of weathering mechanisms or confirmation of plastisphere potential, the limitations of optical microscopy become apparent. Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) is a useful technique in such situations (Huang et al., Reference Huang, Hu and Wang2023). SEM is a very useful tool for the morphological analysis of MPs, as shown by Wagner et al. (Reference Wagner, Wang, Ghosal, Rochman, Gassel and Wall2017).
By comparing the characteristic chemical fingerprints of novel particles with a database of spectra of known materials, vibrational spectroscopy is a useful method for detecting unknown particles (Vianello et al., Reference Vianello, Boldrin, Guerriero, Moschino, Rella, Sturaro and da Ros2013). To identify possible MPs, this method first examines the particles visually. If MP are found, this is then confirmed using methods such as Raman spectroscopy or attenuated total internal reflection Fourier transform infrared spectroscopy (ATR-FTIR) (Yang et al., Reference Yang, Zhang, Kang, Wang and Wu2021; De Frond et al., Reference De Frond, Cowger, Renick and Christiansen2023). According to Chalmers (Reference Chalmers and Meyers2000), the spectrum of a polymer in ATR-FTIR represents the link between the observed infrared intensity and the wavelength of light. However, it is important to note that FT-IR can identify polar groups more accurately (Silva et al., Reference Silva, Bastos, CIL, da Costa, Duarte and Rocha-Santos2018). While manual processing of particles larger than 500 μm is relatively easy, this method becomes increasingly impractical as particle size decreases (Sönmez et al., Reference Sönmez, Akarsu, Cumbul Altay, Sivri and Hashmi2022).
On the other hand, Raman spectroscopy offers several advantages over FTIR, such as better resolution and response to nonpolar symmetric bonds, by exposing the sample to a monochromatic light source, typically a laser (Imhof et al., Reference Imhof, Laforsch, Wiesheu and Ivleva2016; Ivleva et al., Reference Ivleva, Wiesheu and Niessner2017; Yang et al., Reference Yang, Zhang, Kang, Wang and Wu2021). By exciting the molecules with a laser of a single wavelength, the interaction between the radiation and the sample is detected (Li et al., Reference Li, Liu and Paul Chen2018a). The spatial resolution of the Raman microscope improves as the excitation wavelength of the laser decreases (Anger et al., Reference Anger, von der Esch, Baumann, Elsner, Niessner and Ivleva2018). As another vibrational spectroscopic technique in the analysis of MPs, Raman spectroscopy decodes the molecular vibrations of MPs, provides their vibrational spectrum and gives information about the different components present in the sample (Ribeiro-Claro et al., Reference Ribeiro-Claro, Nolasco, Araújo, Rocha-Santos and Duarte2017). Raman spectroscopy allows the characterization of MPs ranging in size from 1 to 20 μm, with no limitations on sample size and thickness (Li et al., Reference Li, Liu and Paul Chen2018a). However, interference from the presence of microorganisms or organic or inorganic contaminants can cause interference with the fluorescence signal (Li et al., Reference Li, Liu and Paul Chen2018a). Despite its advantages, Raman spectroscopy has some known disadvantages, including a long processing time, potential polymer degradation and interference from fluorescence (Parashar and Hait, Reference Parashar and Hait2023).
Gas chromatography is widely recognized as one of the most popular and efficient chromatographic methods for the characterization of MPs, despite its destructive nature (Gniadek and Dąbrowska, Reference Gniadek and Dąbrowska2019). Gas chromatography allows the separation and identification of volatile organic compounds (VOCs) released from MPs. Pyrolysis gas chromatography–mass spectrometry (Pyr-GC–MS) and thermal extraction–desorption gas chromatography–mass spectrometry (TED-GC–MS) are two promising approaches to obtain accurate information on polymers, additives and contaminants (Pipkin et al., Reference Pipkin, Belganeh, Robberson, Allen, Cook and Watanabe2021). Pyr-GC–MS can analyze the chemical composition and structural properties of high molecular weight polymers. This method provides a detailed description of the sample as well as an accurate assessment of its chemical properties (Tianniam et al., Reference Tianniam, Bamba and Fukusaki2010). TED-GC–MS, on the other hand, is a more advanced thermal analysis method that combines thermogravimetric analytical solid-phase extraction (TGA-SPE) with thermal desorption gas chromatography–mass spectrometry (TDS-GC–MS) (Dümichen et al., Reference Dümichen, Barthel, Braun and Senz2015).
Occurrence and characteristics of plastics in WWTPs influents
In numerous investigations, plastics have been found in both influent and effluent samples from WWTPs. Reported concentrations of plastic particles in influent samples ranged from 0.0009 to 15,000 particles/L, while in effluent samples they ranged from 0.004 to 1,800 particles/L (Table 2). The use of different sampling methods, sample pretreatment techniques and analytical methods could be responsible for these differences in particle counts (Sönmez et al., Reference Sönmez, Akarsu, Cumbul Altay, Sivri and Hashmi2022). Most of the plastic particles found were larger than 500 μm in the influent samples, while most of them were smaller than 500 μm in the effluent samples (Bayo et al., Reference Bayo, Olmos and López-Castellanos2020; Üstün et al., Reference Üstün, Bozdaş and Can2022). However, in some studies, plastic particles smaller than 100 μm were also found in effluent samples (Jiang et al., Reference Jiang, Wang, Ding, Cao and Sun2022). In particular, with regard to samples with a mesh size of 10 μm, which mainly contained millimeter-sized debris, there are not many studies specifically address the presence of nano-sized plastics in wastewater samples (Okoffo et al., Reference Okoffo, O’Brien, O’Brien, Tscharke and Thomas2019). This discrepancy can be explained by a lack of information or evidence on the presence of nanoscale plastics in wastewater, as well as inadequate sampling procedures at WWTPs (Lehner et al., Reference Lehner, Weder, Petri-Fink and Rothen-Rutishauser2019). However, due to the environmental impacts associated with nano-sized plastics, it is essential to consider them in future studies focusing on WWTPs.
Table 2. MPs sampling, sample processing, occurrence and identification methods for wastewater and sludge in various WWTPs

It has been shown that when a storm sewer is connected to a WWTP, the amount of plastics in the influent of the treatment plant generally increases (Sun et al., Reference Sun, Dai, Wang, van Loosdrecht and Ni2019). This leads to an increase in the amount of plastics associated with the wastewater system due to the release of plastics from brake and tire wear, which eventually enter the sewer system via road runoff (Mason et al., Reference Mason, Garneau, Sutton, Chu, Ehmann, Barnes, Fink, Papazissimos and Rogers2016; Michielssen et al., Reference Michielssen, Michielssen, Ni and Duhaime2016; Wagner et al., Reference Wagner, Hüffer, Klöckner, Wehrhahn, Hofmann and Reemtsma2018). Plastics in the influent not only affect the technologies and processes used in the WWTP, but also the amount of plastics that end up in the effluent (Sun et al., Reference Sun, Dai, Wang, van Loosdrecht and Ni2019). It should be mentioned that some wastewater pipes are made of PVC polymers, the abrasion of which can increase the total amount of plastics in the WWTP (Xu et al., Reference Xu, He, Liu and Huangfu2019a).
Plastic particles have been identified in the influent and effluent of WWTP as spherical beads, microbeads, pellets, fibers, particles, flakes, films, fragments, foams, paint chips, nurdles, foils, spheres, sheets, granules, lines and irregular shapes (Hamidian et al., Reference Hamidian, Ozumchelouei, Feizi, Wu, Zhang and Yang2021). Fibers have been found to be the most common form with an average of 65.4% of the wastewater samples. Irregular pieces came second with an average of 42.6% (Lv et al., Reference Lv, Dong, Zuo, Liu, Huang and Wu2019). According to the study by Mason et al. (Reference Mason, Garneau, Sutton, Chu, Ehmann, Barnes, Fink, Papazissimos and Rogers2016), the most common plastic particles in 17 wastewater samples from effluent treatment plants were microfibers (59%), followed by fragments (33%), films (5%), foams (2%) and pellets (1%). These results indicate that the main sources of plastics entering WWTPs are secondary plastics that have been degraded and synthetic fibers (Kang et al., Reference Kang, Park, Kwon and Kwon2018). There is a possibility that natural fibers such as cotton have been misrecorded or classified as synthetic fibers during identification and quantification, and it is important to consider that current sampling and analytical techniques may not be sufficient to fully capture and identify plastics in effluents (Talvitie et al., Reference Talvitie, Mikola, Setälä, Heinonen and Koistinen2017b; Sun et al., Reference Sun, Dai, Wang, van Loosdrecht and Ni2019).
The predominant polymer types in the influent and effluent of the WWTP were polyethersulfone (PES, ca. 30–90%), polyethylene (PE, ca. 6–60%), polyethylene terephthalate (PET, ca. 5–40%), polyamide (PA, ca. 5–35%), acrylate (ca. 4–31%), polypropylene (PP, ca. 4–27%), alkyds (ca. 4–25%), polystyrene (PS, ca. 4–25%), polyurethane (PU, ca. 3–25%), polyvinyl acetate (PVA, ca. 3–20%), polylactic acid (PLA, ca. 2–18%) and polytetrafluoroethylene (PTFE, ca. 2–8%) (Hurley et al., Reference Hurley, Lusher, Olsen and Nizzetto2018; Long et al., Reference Long, Pan, Wang and Jin2019; Wolff et al., Reference Wolff, Kerpen, Prediger, Barkmann and Müller2019). Since PES, PET and PA are commonly used in synthetic garments, laundry effluents could be the reason for their presence in wastewater. PE, on the other hand, the most commonly produced plastic in the world, is used in personal care products such as toothpaste, body and facial cleansers, water bottles and food packaging films (Alavian Petroody et al., Reference Alavian Petroody, Hashemi and van Gestel2020). A recent study in a Finnish WWTP found that MP dominate in the following order: PET > PE > PAR > PVC > PS > PP (Talvitie et al., Reference Talvitie, Mikola, Koistinen and Setälä2017a). Another study conducted in three Australian WWTPs found that the concentrations of different types of polymers (PET, PE, PVC, PP, PS and nylon) varied, with PET and PE being the predominant polymers (Ziajahromi et al., Reference Ziajahromi, Neale, Rintoul and Leusch2017). It is crucial to focus research efforts on the identification and quantification of these polymers in influent and effluent samples, as they are known to be generated by routine human activities, even though the exact sources and pathways by which plastics enter WWTPs are not yet fully known.
Fate of plastics in WWTPs
Plastics not only accumulate in the natural environment, but also enter WWTPs via domestic and industrial wastewater discharges (Hajji et al., Reference Hajji, Ben-Haddad, Abelouah, De-la-Torre and Alla2023; Ruffell et al., Reference Ruffell, Pantos, Northcott and Gaw2023) (Figure 4). Concentrations of MP in these environments have been shown to range from 0.0009 to 15 MPs/L. It is estimated that nearly 520,000 tons of MPs are discharged from WWTPs into freshwater rivers in Europe (Hajji et al., Reference Hajji, Ben-Haddad, Abelouah, De-la-Torre and Alla2023). For these reasons, the numerous studies that have been conducted to investigate the fate of plastics in WWTPs have mainly focused on three key aspects: removal efficiency, fate of MPs and impacts on treatment processes (Carr et al., Reference Carr, Liu and Tesoro2016; Ziajahromi et al., Reference Ziajahromi, Neale, Rintoul and Leusch2017). But the main focus, the fate of plastics in WWTPs is a complex issue that is influenced by various factors such as size, composition and treatment processes. MPs can take different pathways after their release into the environment and enter the three main environmental compartments: water, air and soil. There are many variables that influence the behavior of MP in the different environments. The physico-chemical properties of MP, such as size and density, as well as environmental conditions, such as temperature and solar radiation, play a crucial role. According to Gkika et al. (Reference Gkika, Tolkou, Evgenidou and Kyzas2023), the unique properties of MPs polymers have a significant impact on their durability, fate, degradation and ability to absorb or release organic pollutants.

Figure 4. MPs removal efficiency in different treatment stages.
Several studies have investigated the removal efficiency of MP at different treatment stages in different WWTPs and identified their removal mechanisms. In a study by Iyare et al. (Reference Iyare, Ouki and Bond2020), it was found that although WWTPs are not designed to remove MPs, an average removal value of 88% was achieved at secondary treatment and 94% removal efficiency at tertiary treatment. The authors also reported that most of the MPs, 72% on average, were removed during the pre- and primary treatment steps. Similarly, Parashar and Hait (Reference Parashar and Hait2023) reported that the overall removal efficiency of MPs during primary, secondary and tertiary treatment in WWTPs ranged from 57% to 99%, 78.1% to 99.4% and 90% to 99.2%, respectively. Furthermore, Gkika et al. (Reference Gkika, Tolkou, Evgenidou and Kyzas2023) found that the efficiency of MP removal in primary treatment may depend on several variables. A key factor is the presence of an aerated grit chamber, which plays a role in improving removal efficiency. The exact categories of polymers present in the influent also influence removal rates. Studies in the literature have shown that removal efficiencies vary widely, ranging from 40.7% to 91.7%. The study also highlighted the influence of the different treatment steps on the relative size distribution of the MPs. Pre-treatments were found to have a significant impact on the removal of larger particles. For the secondary treatments, such as activated sludge and sedimentation, their configuration and retention time played a role in determining the removal efficiency, which ranged from 28.1% to 66.7%. These results show the importance of considering different treatment strategies and their specific configurations in order to effectively reduce MP pollution during wastewater treatment.
Numerous studies have reported that the conventional treatment process are not always effective in removing MPs larger than 500 μm resulting discharge of MPs into aquatic environments along with the effluent (Bilgin et al., Reference Bilgin, Yurtsever and Karadagli2020; Schmidt et al., Reference Schmidt, Kumar, Yang and Büttner2020; Xu et al., Reference Xu, Zhang, Jian, Xue, Gao, Peng, Jiang and Zhang2021).
While typical WWTPs are capable of removing up to 90% of MPs from wastewater, it is important to consider that smaller MPs, such as the microbeads in facial cleansers and synthetic textile fibers, may escape from these treatment processes (Ziajahromi et al., Reference Ziajahromi, Neale, Rintoul and Leusch2017; Raju et al., Reference Raju, Carbery, Kuttykattil, Senthirajah, Lundmark, Rogers, SCB, Evans and Palanisami2020; Funck et al., Reference Funck, Al-Azzawi, Yildirim and Tuerk2021). This highlights the need for improved treatment steps and the application of state-of-the-art technologies to address the problem of tiny MPs escaping conventional treatment processes. Advanced wastewater treatment methods, including membrane bioreactors, are mentioned in the literature as possible options to increase effectiveness in removing small size MPs (less than 100 μm). However, research on the fate of MPs in WWTPs has shown that they can interact with the solid fraction of wastewater treatment, causing them to accumulate in the sludge (Mahon et al., Reference Mahon, O’Connell, Healy, O’Connor, Officer, Nash and Morrison2017). If the sludge is subsequently used in agriculture or disposed of in landfills, MPs can enter terrestrial habitats (Zettler et al., Reference Zettler, Mincer and Amaral Zettler2013). However, little is known about the fate of MPs when used in agriculture (Tu et al., Reference Tu, Chen, Zhou, Liu, Wei, Waniek and Luo2020; Casella et al., Reference Casella, Sol, Laca and Díaz2023). The use of sewage sludge as an additive in agriculture is considered an important source of MP in soil matrices. Similarly, MP can serve as colonization sites for specific microorganisms in both terrestrial and aquatic ecosystems (Zettler et al., Reference Zettler, Mincer and Amaral Zettler2013). Recent research has shown that MP in soil matrices have the potential to disrupt fungal community diversity (Zhang et al., Reference Zhang, Wu, Su and Xie2021b). Moreover, the favorable growth of Actinobacteria and Bacteroidetes was found on polyethylene (PE) surfaces (Zhang et al., Reference Zhang, Wang, Halden and Kannan2019, Reference Zhang, Wu, Su and Xie2021b; Ren et al., Reference Ren, Sun, Wang, Barceló, Wang, Zhang and Zhang2020). Indeed, the potential transfer of MPs through the application of sewage sludge in agriculture still raises questions about the long-term consequences for soil health and crop safety (Zhang et al., Reference Zhang, Xie, Liu, Zhong, Qian and Gao2020; Weber et al., Reference Weber, Santowski and Chifflard2022).
Possible strategies for robust management plan
The global campaign against MP pollution has gained significant momentum in recent years. Calls have been made to strengthen the capacity to manage plastic waste, particularly through increased recycling initiatives (Diggle and Walker, Reference Diggle and Walker2022). While effective recycling can help address the broader problem of plastic pollution, its impact on reducing MP pollution remains rather limited (Kumar et al., Reference Kumar, Verma, Shome, Sinha, Sinha, Jha, Kumar, Kumar and Shubham Das2021). This study highlights the critical shortcomings of the current waste management system that allow MP to escape. Given the inability of the existing waste management system to adequately address the scale and severity of MP pollution, it is unrealistic to expect significant improvements without fundamental changes. It is unlikely that simply intensifying efforts under the existing framework will lead to significantly better results. There is therefore an urgent need to re-evaluate and adapt current waste management practices to effectively block the pathways through which MP escapes into the receiving environment.
In order to prevent ecosystems degradation, the implementation of strong legislative measures to monitor and regulate the overuse of plastics is essential (Prata et al., Reference Prata, Silva, da Costa, Mouneyrac, Walker, Duarte and Rocha-Santos2019). Effective management, recycling practices and the establishment of environmentally friendly disposal systems are crucial in the pursuit of a plastic-free environment. Developing countries have introduced extensive measures to combat the proliferation of plastics, including comprehensive bans on plastic bags and bottles and the imposition of fines for plastic violations (Gopinath et al., Reference Gopinath, Nagarajan, Krishnan and Malolan2020). Over the past three decades, global efforts have led to the formulation of laws aimed at addressing the hazards and impacts of increasing plastic consumption and waste (Table 3) (Bhardwaj et al., Reference Bhardwaj, Kumar and Verma2020; Wen et al., Reference Wen, Xie, Chen and Dinga2021; Usman et al., Reference Usman, Abdull Razis, Shaari, Azmai, Saad, Mat Isa and Nazarudin2022). As can be seen from the previous discussion and highlighted in Table 3, many international laws lack a comprehensive framework and the necessary global mechanisms to effectively monitor and evaluate progress toward the established goals. Although these legal frameworks often include sound governance strategies, they generally rely on individual countries to design and develop their own strategies for implementation. The effectiveness of these strategies in turn depends on a country’s political will and allocation of resources to address the problem. The United Nations (UN) has recognized the problem of plastic pollution by including it in 11 SDGs alongside SDG-14 (Walker, Reference Walker2021). However, the fact that only one indicator out of a total of 247 is dedicated to the impact of plastic in the ocean is woefully inadequate given the alarming rate at which plastic pollution is increasing globally. This allocation urgently needs to be reconsidered. Moreover, the European Parliament and the Council have failed to include MPs in their directives, while the measures proposed by WHO to mitigate the problem remain out of reach in many countries (Usman et al., Reference Usman, Abdull Razis, Shaari, Azmai, Saad, Mat Isa and Nazarudin2022). This underline the need for a concerted commitment to provide resources that can support research efforts and the development of practical, measurable tools to comprehensively address this pressing global problem.
Table 3. Different governance strategies to control MP pollution (adapted from Usman et al., Reference Usman, Abdull Razis, Shaari, Azmai, Saad, Mat Isa and Nazarudin2022)

A comprehensive global assessment of national laws and regulations introduced by countries to restrict the production, import, use and disposal of single-use plastics and MPs, which contribute significantly to the spread of marine pollution, found that in 2018, about 60% (127 out of 192) of countries had enacted various forms of legislation concerning plastic bags (Xanthos and Walker, Reference Xanthos and Walker2017). These laws specifically aim to regulate aspects such as production, distribution, use, trade, taxation, levies and disposal. While landfills remain the primary method of disposing of plastic waste, the gradual release of MPs and toxic additives poses a significant threat to the environment, inevitably leading to a phase-out of traditional landfill practices (Shen et al., Reference Shen, Xiong, Song, Zhou, Almatrafi, Zeng and Zhang2022). Although incineration is an option for the disposal of plastic waste, the significant release of greenhouse gases remains a critical issue. With approximately, 79% combustible carbon per ton, this method generates an estimated 2.9 tons of CO2 emissions, highlighting the environmental impacts associated with this approach (Hamilton et al., Reference Hamilton, Feit, Muffett, Kelso, Rubright, Bernhardt, Schaeffer, Moon, Morris and Labbe-Bellas2019).
In this context, recycling is widely recognized as the optimal long-term solution to address the current MP problem and ensure sustainable plastic use (Kassab et al., Reference Kassab, Al Nabhani, Mohanty, Pannier and Ayoub2023). Reusing 1 ton of plastic waste by recycling instead of producing new materials can save approximately 130 million kilojoules of energy (Ramirez and George, Reference Ramirez and George2019). However, the recycling rate for plastic waste, especially for secondary (recycled) plastics, remains low. Therefore, promoting environmentally friendly and cost-effective alternatives to plastics is essential in the long term. Researchers also need to explore methods to break down the basic components of plastics so they can be converted into new materials. Researchers have discovered a mutant enzyme capable of breaking down plastic bottles in days –a significant improvement over the centuries it takes in the oceans (Lamichhane, Reference Lamichhane, Acharya, Marahatha, Modi, Paudel, Adhikari, Raut, Aryal and Parajuli2023).
In recent years, several studies have underlined the effectiveness of plastic-degrading bacteria. In particular, Ideonella sakaiensis, a member of the Ideonella genus and Comamonadaceae family, has shown that it is able to consume plastic polyethylene terephthalate (PET) as a sole source of carbon and energy, effectively contributing to plastic recycling (Yoshida et al., Reference Yoshida, Hiraga, Takehana, Taniguchi, Yamaji, Maeda, Toyohara, Miyamoto, Kimura and Oda2016). With the help of two successive enzymes, these bacteria can break down PET into terephthalic acid and ethylene glycol, both environmentally friendly substances (Bornscheuer, Reference Bornscheuer2016). Similarly, research by Gao and Sun showed that a mixed culture of Exiguobacterium sp., Halomonas sp. and Ochrobactrum sp. degrades both PET and PE films better than individual isolates, as evidenced by observations with SEM (Gao and Sun, Reference Gao and Sun2021).
Given the ubiquitous presence of plastic, there is an urgent need for more than one alternative solution that is renewable, environmentally sound and biodegradable. Unregulated and poorly managed bioplastics could potentially lead to environmental damage comparable to that of conventional plastics. It is therefore crucial that legislators set strict criteria with high standards for the classification of bioplastics to promote consumer and business confidence (Bhagwat et al., Reference Bhagwat, Gray, Wilson, Muniyasamy, SGT, Bush and Palanisami2020). Biodegradable plastics based on cellulose and polyolefins should be actively promoted due to their cost-effectiveness, high mechanical strength and ease of decomposition in the environment (Ammala et al., Reference Ammala, Bateman, Dean, Petinakis, Sangwan, Wong, Yuan, Yu, Patrick and Leong2011). Recent advances have led to the development of plant-based materials capable of replacing single-use plastics in various consumer products and a polymer film that mimics the properties of spider silk. This innovative material has comparable durability to conventional plastics and can be produced on an industrial scale from sustainable components thanks to an energy-efficient technology that fuses plant proteins into silk-like materials (Kamada et al., Reference Kamada, Rodriguez-Garcia, Ruggeri, Shen, Levin and TPJ2021). Most importantly, this substance is compostable at home, so no special industrial composting facilities are required. Furthermore, due to its natural composition, the material can be safely biodegraded in most natural environments, providing a promising alternative to single-use plastics and MPs on the commercial market.
Future perspectives and conclusions
Aquatic ecosystems play a crucial role in the transport and deposition of plastic waste from terrestrial storage to surface waters (Margenat et al., Reference Margenat, Nel, Stonedahl, Krause, Sabater and Drummond2021). All recent studies consistently identify urban areas, transportation, infrastructure and WWTPs as major sources of micro-, meso- and macroplastics (van Emmerik, Reference Van Emmerik2021; Cowger et al., Reference Cowger, Gray, Hapich and Ajami2022). Recent scientific articles have shown that the movement of plastics over land and rivers is influenced by human activities, flood and storm events, hydrodynamics and their combinations. Interestingly, the majority of plastics do not reach the open sea but end up on beaches, float in coastal waters or accumulate on land and in river systems (Köklü et al., Reference Köklü, Ateş, Deveci and Sivri2023; Sönmez et al., Reference Sönmez, Akarsu and Sivri2023). To address this problem, several innovative technologies have been developed and deployed to reduce pollution from MaPs. These technologies mainly include innovative devices placed along rivers and streams to effectively collect MaPs and other litter. Prominent examples include the Ocean Cleanup Foundation’s Interceptor Project, which has been successfully implemented in Indonesia, Malaysia and the Dominican Republic (The Ocean Cleanup, 2021). The Bubble Barrier, which Waternet has installed in a number of Amsterdam canals, is another notable tool (Waternet Annual Report, 2021). This bubble barrier diverts floating litter, such as MaPs, so that it can be cleaned up on the riverbank.
Further research needs to focus on the pragmatic use of area-specific expertise on rivers, with particular attention to the collection and examination of extensive and original datasets. In addition, it is essential to identify the origins and entry points of plastic pollution and to understand the basic mechanisms of transport. This priority issue not only offers new perspectives on the production, distribution, fate and impact of plastics, but also highlights the urgent need for a thorough investigation of plastic pollution in the aquatic environment. Furthermore, there is a notable lack of comparative research between technology-based enhanced treatment strategies and conventional treatment methods (Iyare et al., Reference Iyare, Ouki and Bond2020).
In addition, the accumulation of plastics in WWTPs can lead to operational challenges and potential environmental impacts. Further research and the development of innovative treatment technologies are needed to effectively address the challenges posed by plastics in WWTPs effectively. Understanding the fate of plastics in WWTPs is critical to developing strategies to contain their presence and minimize their potential negative impacts on the environment. The United Nations Environment Programme considers plastic pollution to be a major environmental problem that, along with climate change, is becoming a threat to biodiversity and human health. Metrics such as models or ecological footprints can be useful tools for decision-making, public engagement and policy development. Nevertheless, contingency plans should be continuously adapted and developed to consider the future of waste management and plastics (Klemeš et al., Reference Klemeš, Fan, Van Tan and Jiang2020). For example, to achieve the best environmental performance through recycling, it is important to improve pre-treatment methods in line with the most appropriate recycling technology for a given polymer. Some of the studies emphasize the importance of polymer quality (e.g., mixed origin and mixed materials), which affects the overall environmental performance of a technology, but does not change the performance rating of the technology (Schwarzer et al., Reference Schwarzer, Brehm, Vollmer, Jasinski, Xu, Zainuddin, Fröhlich, Schott, Greiner, Scheibel and Laforsch2022).
Effective waste management is critical to achieving the SDGs that aim to address environmental, social and economic challenges. Governments and organizations around the world have adopted waste management plans that are aligned with the SDGs and emphasize the need for sustainable waste management practices. Under the SDGs, waste management plans and regulatory improvements focus on several key areas. These include reducing waste generation through waste prevention and promoting sustainable consumption patterns. Recycling and recovery efforts aim to increase waste prevention, conserve resources and reduce greenhouse gas emissions. Above all, improving regulation plays a crucial role in creating a regulatory framework that supports sustainable waste management. By adopting comprehensive waste management policies that include waste prevention, recycling and safe disposal, countries can minimize environmental impacts, conserve resources and contribute to the achievement of the SDGs. These regulations cover the classification, collection, treatment and disposal of waste, as well as extended producer responsibility (EPR). EPR requires producers to take responsibility for the entire life cycle of their products, including the disposal of post-consumer waste.
To further advance waste management in line with the SDGs, cooperation and knowledge sharing between countries and stakeholders is crucial. Sharing best practices, innovative technologies and scientific research can improve waste management systems and promote sustainable solutions worldwide. In addition, financial incentives, capacity building and public awareness campaigns are integral components to drive behavioral change and ensure the successful implementation of waste management plans.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2023.23.
Acknowledgments
The authors would like to thank the Scientific and Technological Research Council of Turkey (TÜBİTAK) for their support in this publication, which was prepared using the outputs of the preliminary work packages of the 118C556 project.










Comments
14.06.2023
Professor Steve Fletcher,
University of Portsmouth
Editor-in-Chief of Cambridge Prisms: Plastics
We are very pleased to submit our manuscript entitled “An overview of the occurrence and distribution of plastics in wastewater treatment plants and the necessity of developing up-to-date management strategies” by Ceyhun Akarsu, Vildan Zülal Sönmez, Nüket Sivri for consideration in Cambridge Prisms: Plastics. I believe that this manuscript aligns well with the aims and scope of the journal, specifically in addressing the plastic pollution in wastewater treatment plants and emphasising the need to develop comprehensive management strategies in line with the United Nations Sustainable Development Goals.
The article discusses the sources of plastics entering wastewater treatment plants, the analytical techniques used to study their occurrence and properties, and the role of these plastics as potential sources of microplastics. We also address the challenges posed by plastics in wastewater treatment plants and explore the factors that influence variations in the abundance of plastics. In addition, the manuscript assesses the policy implications for dealing with plastic pollution and emphasises its consistency with the United Nations Sustainable Development Goals.
We believe that our article will make a valuable contribution to the field by consolidating existing knowledge, identifying research gaps and highlighting the importance of developing effective management strategies. The comprehensive analysis presented in this manuscript will provide readers with a deeper understanding of the complexity of plastic pollution in wastewater treatment plants and stimulate further research in this critical area.
We confirm that this manuscript has not been published previously and is not under consideration elsewhere. We have carefully read and followed the guidelines for manuscript preparation provided by Cambridge Prisms: Plastics.
Thank you once again for inviting me to write an overview review for Cambridge Prisms: Plastics.
We look forward to hearing from you in due course.
Yours sincerely,
Dr. Ceyhun AKARSU
(On the behalf of all the authors)