It has been considered that retinal damage induced by light occurs through three general mechanisms involving thermal, mechanical and photochemical effects; the third is particularly prevalentReference Noell, Walker, Kang and Berman1, Reference Ham, Mueller and Sliney2, Reference Glickman3. Reduced visual function and disintegration of the retinal outer and inner segment occurs in the early phase of photochemical stress, then the photoreceptors degenerate, the outer nuclear layer (ONL) becomes thinner, and eventually retinal function may be totally lost, leading to blindnessReference Noell, Walker, Kang and Berman1, Reference Li, Cao and Anderson4. Photochemical damage is correlated with properties of the light source such as wavelength, intensity, temperature and other factorsReference Mainster, Ham and Delori5–Reference Kaldi, Martin, Huang, Brush, Morrison and Anderson7. During daily life, visible light, UV light, IR light and lasers can lead to retinal photochemical damageReference Istock8–Reference Specht, Leffak, Darrow and Organisciak10. It is thus important to investigate protection against retinal damage induced by photochemical stress.
The mechanisms of retinal damage induced by photochemical stress are unclear at present. The classical view is that apoptosis leads to the retinal damage produced by photochemical stressReference Aonuma, Yamazaki and Watanabe6, Reference Hafezi, Steinbach, Marti, Munz, Wang, Wagner, Aguzzi and Reme11, Reference Libman12. After photons are absorbed by chromophores (melanin and lipofuscin), rhodopsin and retinoids, lipid peroxidation and reactive oxygen intermediates might trigger photochemical stressReference Grimm, Wenzel, Hafezi and Reme13–Reference Wenzel, Grimm, Samardzija and Reme17. Then, activator protein-1 (AP-1) and NF-κB transduce the death signalReference Kaltschmidt, Uherek, Wellmann, Volk and Kaltschmidt18–Reference Wu, Chiang, Chau and Tso22. Finally, DNA fragmentation mainly depends on the caspase (caspase-1) apoptotic pathwayReference Grimm, Wenzel, Hafezi and Reme13, Reference Grimm, Wenzel, Hafezi, Yu, Redmond and Reme14, Reference Krishnamoorthy, Crawford, Chaturvedi, Jain, Aggarwal, Al-Ubaidi and Agarwal19, Reference Wu, Chiang, Chau and Tso22 and non-caspase apoptotic pathways such as the (LEI)/L-DNase II pathwayReference Chahory, Padron, Courtois and Torriglia23. Antioxidants such as vitamin C, vitamin E, dimethylthiourea and Ginkgo biloba extract have been shown to protect against retinal damage from photochemical stressReference Organisciak, Bicknell and Darrow24–Reference Ranchon, Gorrand, Cluzel, Droy-Lefaix and Doly26.
Taurine is abundant in the retina, especially in photoreceptor cells and Müller cellsReference Pasantes-Morales and Cruz27, Reference Schuller-Levis and Park28. It not only acts as a neuromodulator inhibitor, Ca modulator and osmoregulator, but also interferes with the metabolism of lipid synthesis and stabilises the membrane systemReference Pasantes-Morales and Cruz27–Reference Chen, Pan, Liu and Han29. It is documented that taurine can inhibit lipid peroxidation, thereby protecting the retina from oxidative damageReference Obrosova, Fathallah and Stevens30, Reference Di Leo, Santini and Cercone31. Some studies have also indicated an anti-apoptotic effect of taurineReference Schuller-Levis and Park28, Reference Foos and Wu32, Reference Marucci, Alpini and Glaser33. It is widely accepted that taurine plays a pivotal role in the visual system, but little is known about its protection of the retina from photochemical stress. Thus, we conducted the present study to investigate the effect of dietary taurine on retinal damage produced by photochemical stress in Sprague–Dawley rats and to further explore the possible molecular mechanisms of this action.
Materials and methods
Animals and diets
Sprague–Dawley rats (n 60) of age 14 weeks and weight 150 ± 20 g were housed in standard stainless steel cages at 25°C. All animal procedures were followed in accordance with the approved protocol for use of experimental animals set by the standing committee on animal care at The Third Military Medical University. After consuming a purified diet based on the AIN-93 formulationReference Reeves, Nielsen and Fahey34 for 1 week, forty rats were randomly divided into two groups and then treated with taurine (4 g taurine/100 g diet, n 20) or without taurine (n 20) for 15 d respectively. After treatment, these two groups were exposed to light for 1, 3, 6, 9, 12 or 24 h in an illumination chamber (Chongqing City, China) that transmitted a fluorescent light at an illuminance level of 3000 ± 200 lux. Sixteen fluorescent lamps were mounted vertically and evenly along the four sides of the chamber, and the chamber temperature during the light exposure was 25°C. Twenty rats fed the AIN-93 formulation and maintained in the dark for 48 h without light exposure were used as controls (n 20). After treatment all rats were weighed and then killed by decapitation under anaesthesia. One eye was harvested for biochemical measurements and the other was collected for morphology, Western blot, immunohistochemistry, quantitative real-time PCR, or terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay.
Morphology and morphometry
The eyes were marked for orientation and chilled immediately in pre-cooled isopentane with liquid N2. They were then embedded and sectioned in the sagittal plane. For light microscopy, the sections were cut to 16 μm, fixed in 2 % paraformaldehyde, and stained with haematoxylin and eosin. To quantify photoreceptor survival, the thickness of the ONL was measured by morphometry according to a method used previouslyReference Michon, Li, Shioura, Anderson and Tso35.
Observation of retinal ultrastructural organisation
The eyes were enucleated and fixed. After dehydration in a series of graded ethanol, the specimens were embedded in Luveak 812 Ultrathin (Nacalai Tesque, Inc., Kyoto, Japan). Sections were made with a Porter–Blum MT2 microtome (Sorvall, Norwalk, CT, USA) and examined with a Hitachi H300 (Tokyo, Japan) electron microscope.
Dark-adapted electroretinogram examination
Retinal physiological function was assessed by dark-adapted electroretinography as described previouslyReference Geller, Sutton, Marshall, Hunter, Madden and Peiffer36. Electroretinograms (ERG) were recorded with a system developed at the US Environmental Protection Agency. The amplitude and implicit time of the a- and b-waves of ERG were analysed.
Taurine assay in retina
The retinas were homogenised in 300 μl 0·4 m-potassium borate buffer and 20 % sulfosalicylic acid (50 μl). A sample was kept for protein analysis. Centrifugation of the other tissue homogenate was carried out at 35 000 g for 20 min at 4°C. The supernatant fraction (25 μl) was used for HPLC analysisReference Nusetti, Obregon, Quintal, Benzo and Lima37. The concentration of taurine was quantified by the method of the external standard and expressed as μmol/g protein.
Determination of malondialdehyde, superoxide dismutase and glutathione peroxidase
The level of malondialdehyde (MDA) was estimated by the double-heating methodReference Draper and Hadley38. The concentration of MDA was expressed as mmol/g wet tissue. Total (Cu-Zn and Mn) superoxide dismutase (SOD) activity was determined as described previouslyReference Yamamoto, Lidia, Gong, Onitsuka, Kotani and Ohira39. One unit of SOD activity was defined as the amount of enzyme causing 50 % inhibition in the nitroblue tetrazolium reduction rate. SOD activity was expressed as units/mg protein. Glutathione peroxidase (GSH-Px) activity was measured as described previouslyReference Siu, Reiter and To40. One unit of activity was equal to the number of mmol of reduced NADPH oxidised by 1 mg protein in 1 min. GSH-Px activity was also given in units/mg protein.
Apoptosis study
The frozen sections of retinas were used for apoptosis assay with a TUNEL assay kit (Apoptag; Oncor, Gaithersburg, MD, USA). The apoptotic index, expressed as a percentage, was calculated by dividing the number of TUNEL-positive photoreceptor cells by the total number of photoreceptor cells in the section as seen under the light microscope.
Immunohistochemistry
Frozen sections of retinas were fixed in acetone, quenched in 0·3 % H2O2 in methanol and incubated in a mouse IgG blocking solution for 1 h. Antibodies specific for c-fos and caspase-1 (Sigma, St Louis, MO, USA) were applied (1:200 dilution), and sections were incubated at 4°C overnight. Immunoreactivity was detected with a biotinylated secondary antibody and 3,3′-diaminobenzidine as the chromogen.
Western blot analysis
Total proteins of retinas were denatured and resolved by 12 % SDS-PAGE. After the transfer of the protein from the gel to a polyvinylidene difluoride membrane, the membrane was saturated in PBS–Tween plus 5 % milk and incubated with anti-c-Jun or anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight, followed by a horseradish peroxidase-linked secondary antibody (1:1000 dilution). Specific protein bands were revealed by enhanced chemiluminescence and scanned using a gel imaging analytical system (Bio-Rad Laboratories, Hercules, CA, USA).
Gene expression analysed with quantitative real-time polymerase chain reaction
For analysis of gene expression, a quantitative real-time PCR method was used as in our previous procedureReference Xia, Hou and Zhu41. Oligonucleotide primers and TaqMan probes were designed by using Primer Express software 2.0 (PE Applied Biosystems, Foster City, CA, USA) and were synthesised by Takara Biotechnology Inc. (Dalian, China). Sequences of primers are listed in Table 1. Total RNA was extracted from the retinas using TRIzol reagent according to the protocol provided by the manufacturer (Invitrogen Corp., Carlsbad, CA, USA). Real-time quantitative TaqMan PCR analysis was performed according to the manufacturer's instructions (TaqMan Gold RT-PCR protocol; PE Applied Biosystems) with an ABI Prism 7000 TaqMan real-time fluorescent thermal cycler (PerkinElmer Life Sciences, Waltham, MA, USA). The thermal cycling conditions included 2 min at 93°C, 1 min at 93°C and 1 min at 55°C. Thermal cycling proceeded with forty cycles. Levels of the different mRNA were subsequently normalised to glyceraldehyde-3-phosphate dehydrogenase mRNA levels.
Table 1 Nucleotide sequences of the polymerase chain reaction primers used to assay gene expression by quantitative real-time polymerase chain reaction
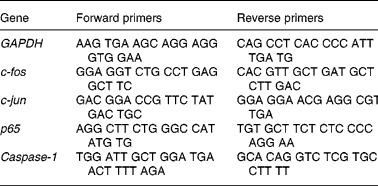
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Results are given as means and standard deviations. The weight, levels of MDA, SOD and GSH-Px, apoptotic index, mRNA expressions and relative protein expression between rats treated with or without taurine and exposed to light were analysed by Student's t test. Comparisons of the ERG components, thickness of ONL, taurine concentration among normal rats, and rats treated with or without taurine and exposed to light were evaluated by the Ryan's multiple-range test when significant differences were detected by one-way ANOVA, and the differences of the same dietary rats exposed to different light time were also analysed by the Ryan's multiple-range test (Stat-Light; Yukmus Co., Tokyo, Japan). All differences were considered statistically significantly different at P < 0·05.
Results
Dietary taurine protected retinal morphological integrity
During the experiment there were no abnormalities. All rats gained weight normally but the weight of rats treated with 4 % taurine was higher than that of rats not treated with taurine (P = 0·035). Fig. 1 (A) shows that the retinas of the normal rats were highly organised, with intact layers. The severity of damage varied among the individuals exposed to light for 24 h without taurine. Maximal loss of photoreceptor nuclei was observed. The pigment epithelium was injured and not discernible in severely damaged regions (Fig. 1 (B)). The outer and inner segments of the photoreceptors were disorganised and disrupted to varying degrees in rats exposed to light for 24 h (Fig. 1 (E) and (H)). The mitochondria had swelled and the mitochondrial cristae were disorganised (Fig. 1 (K)). However, there were no destructive changes in the retinas of rats treated with taurine visualised microscopically. These retinas were relatively organised. Comparison of the morphology of the retinas of rats treated with taurine (Fig. 1 (C), (F), (I) and (L)) and those of normal rats (Fig. 1 (A), (D), (G) and (J)) revealed no significant differences.

Fig. 1 Examples of retinas in rats fed AIN-93 formulationReference Reeves, Nielsen and Fahey34 and without light exposure (A, D, G, J), rats treated without (B, E, H, K) or with (C, F, I, L) 4 % taurine for 15 d and exposed to light for 24 h showing morphological structure (bar = 100 μm) stained with haematoxylin and eosin (A, B, C), and ultrastructural organisation (bar = 2·5 μm) of retina outer segment (ROS) (D, E, F), retina inner segment (RIS) (G, H, I) and mitochondria (J, K, L) with the electron microscope. Images are representative fields from three experiments. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Fig. 2 shows the mean values of ONL thickness were 33·5 (sd 8·3), 18·8 (sd 6·7) and 29·6 (sd 7·4) μm in the retinas of normal rats, rats exposed to light, and rats exposed to light and treated with taurine, respectively. Light exposure resulted in a 47·1 % loss of ONL thickness in rats without taurine and 16·7 % loss in rats with taurine. The reduction in ONL thickness was statistically different between the retinas of rats treated with and those without taurine (P < 0·05).
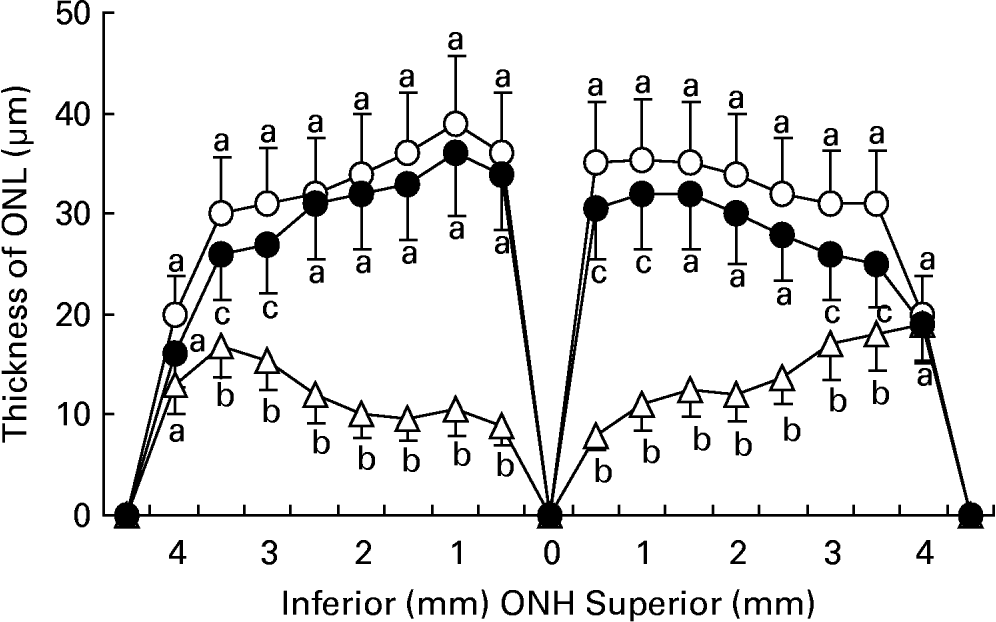
Fig. 2 The thickness of the outer nuclear layer (ONL) from optic nerve heads (ONH) in retinas of rats fed AIN-93 formulationReference Reeves, Nielsen and Fahey34 and without light exposure (-○-) and rats treated with (-●-) or without (-Δ-) 4 % taurine for 15 d and exposed to light for 24 h. Values are means for five determinations, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters at the same distance from ONH were significantly different (P < 0·05; Ryan's multiple-range test).
Taurine ameliorated retinal function
There were untypical a- and b-waves, a decrease in amplitude even to quench, and an increase in implicit time in the dark-adapted ERG of rats exposed to light. In contrast, the a- and b-waves of ERG in rats treated with taurine were typical. Table 2 shows the amplitudes of the a- and b-wave were increased (P = 0·016), and the implicit time decreased relative to those of rats exposed to light without taurine (P = 0·015). There were no differences in the components of ERG between normal rats and rats treated with taurine (P = 0·41), except a lower a-wave amplitude (P = 0·03).
Table 2 The changes of electroretinograph components in Sprague–Dawley rats after dietary supplementation with or without 4 % taurine for 15 d and exposed to light for 24 h (Mean values and standard deviations)

AIN-93, American Institute of Nutrition-93 purified diets for laboratory rodentsReference Reeves, Nielsen and Fahey34.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01; Ryan's multiple range test).
Dietary taurine elevated taurine concentration in retina
HPLC results indicated that the mean concentrations of taurine were 60·4 (sd 17·1), 23·3 (sd 3·6), and 66·1 (sd 16·7) μmol/g protein in the retinas of control rats, rats exposed to light for 24 h, and rats exposed to light for 24 h and treated with taurine, respectively. There was a decrease in the concentration of taurine in the retina after rats were exposed to light (P = 0·017), but dietary taurine elevated the decreased concentration (P = 0·008).
Antioxidative ability increased by taurine
Fig. 3 shows the level of MDA in retinas increased gradually with light exposure time, especially after exposure for 3 h (P < 0·01), but dietary taurine markedly decreased the higher levels of MDA stimulated by light (P < 0·01). The activities were higher in retinas of rats treated with taurine only after 6 h for SOD (P < 0·01) and after 3 h for GSH-Px (P < 0·01) than those of rats without taurine treatment. In many cases, the activities of the two enzymes in the rats without taurine treatment were also greater than the control level (P < 0·01).
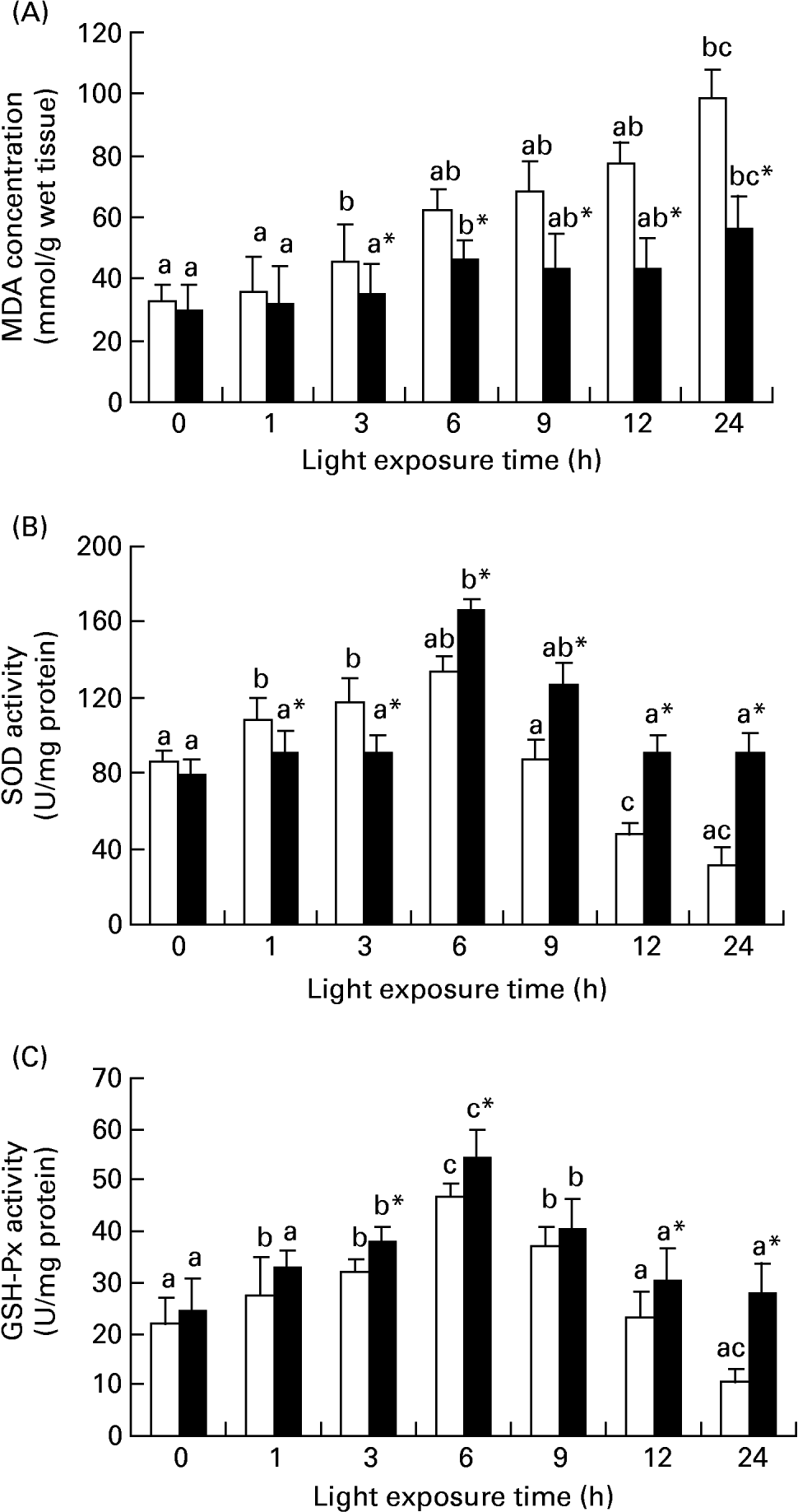
Fig. 3 Diet-related variation in malondialdehyde (MDA) (A), superoxide dismutase (SOD) (B) and glutathione peroxidase (GSH-Px) (C) levels in the retinas of rats treated with (■) or without (□) 4 % taurine for 15 d and exposed to light for 0–24 h. Values are means for eight determinations on twenty specimens for each time point, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters among the same diet group at different exposure times were significantly different (P < 0·05; Ryan's multiple-range test). * Mean value was significantly different from that for rats at the same light exposure time treated without taurine (P < 0·05; Student's t test).
Taurine reduced photoreceptor apoptosis
We investigated whether taurine decreased light-induced apoptosis in the retina. Nuclei labelled by the TUNEL assay were observed only occasionally in the retinas of normal rats, but more apoptotic cells were found in the retinas of rats exposed to light. Sporadic apoptotic cells were found in the retinas of rats treated with taurine. Fig. 4 shows the apoptotic index increased gradually with exposure time (P < 0·05). The apoptotic index was lower in the retinas of rats treated with taurine than in rats without taurine after exposure for 6 h (P < 0·05).
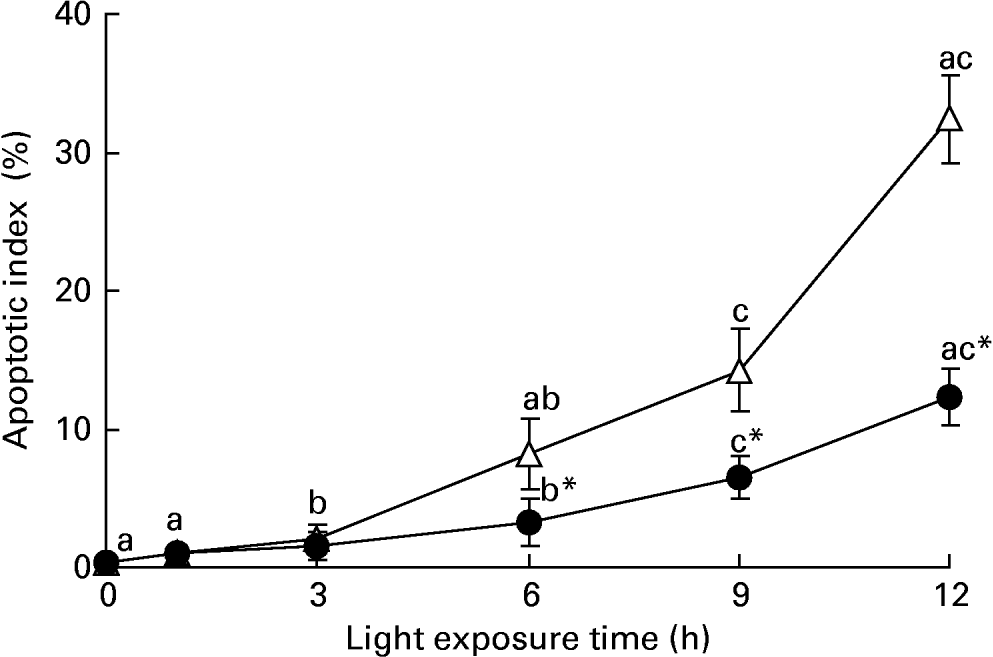
Fig. 4 Apoptotic index in the retinas of rats treated with (-●-) or without (-○-) 4 % taurine for 15 d and exposed to light for 0–12 h. Values are means for six rats for each time point, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters on the same curve at different exposure times were significantly different (P < 0·05; Ryan's multiple-range test). * Mean value was significantly different from that for rats at the same light exposure time treated without taurine (P < 0·05; Student's t test).
Taurine inhibited activator protein-1 expression
Fig. 5 (A) shows that there was an increase of c-fos mRNA expression in the retinas of rats exposed to light only at 1 h (P = 0·017). c-fos mRNA expression was lower in the retinas of rats treated with taurine than in rats without taurine at this time (P < 0·05). Fig. 6 (A–C) shows the analogous result of c-fos protein expression detected by immunohistochemistry. Fig. 5 (B) shows that the expression of c-jun mRNA was increased in the retinas of rats after exposure for 1 h (P = 0·006). There was a decrease in the expression of c-jun mRNA in the retinas of rats treated with taurine compared with the rats without taurine after exposure for 6 h (P < 0·05). Fig. 7 (A) shows that the c-jun protein expression was lower in the retinas of rats treated with taurine than in rats without taurine (P < 0·05). Dietary taurine could partially decrease c-fos and c-jun expression in the retinas of rats exposed to light.
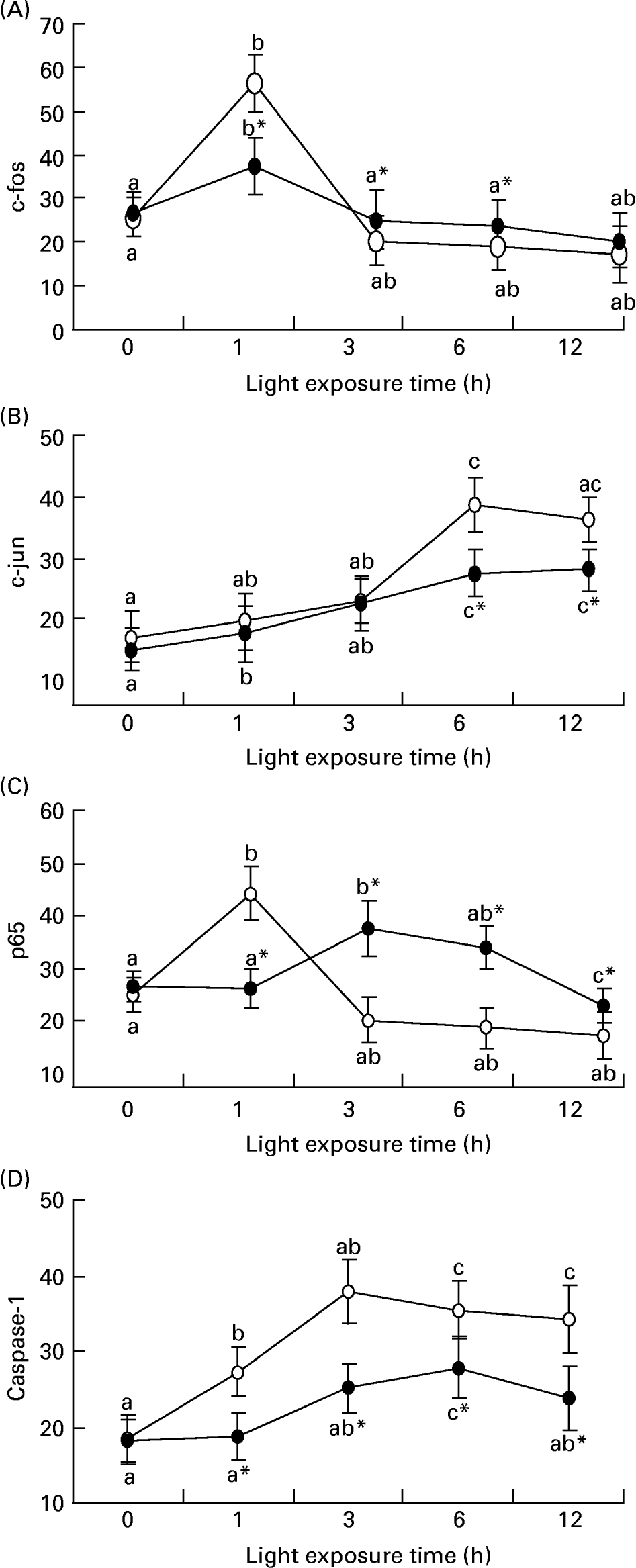
Fig. 5 The relative c-fos (A), c-jun (B), p65 (C) and caspase-1 (D) mRNA expressions normalised for corresponding glyceraldehyde-3-phosphate dehydrogenase levels in retinas of rats treated with (-●-) or without (-○-) 4 % taurine for 15 d and exposed to light for 0–12 h. Values are means for three determinations for each time point, with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters on the same curve at different exposure time were significantly different (P < 0·05; Ryan's multiple-range test). * Mean value was significantly different from that for rats at the same light exposure time treated without taurine (P < 0·05; Student's t test).

Fig. 6 The protein expressions of c-fos (A, B, C) and caspase-1 (D, E, F) in retinas of rats fed AIN-93 formulationReference Reeves, Nielsen and Fahey34 and without light exposure (A, D), rats treated with (B, E) or without (C, F) 4 % taurine for 15 d and exposed to light for 24 h detected by immunohistochemistry and afterstained with (A, B, C) or without (D, E, F) haematoxylin. Images are representative fields from three experiments. ↑ , Respective antibody-labelled positive cells. Bar = 100 μm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RIS, retina inner segment; ROS, retina outer segment.
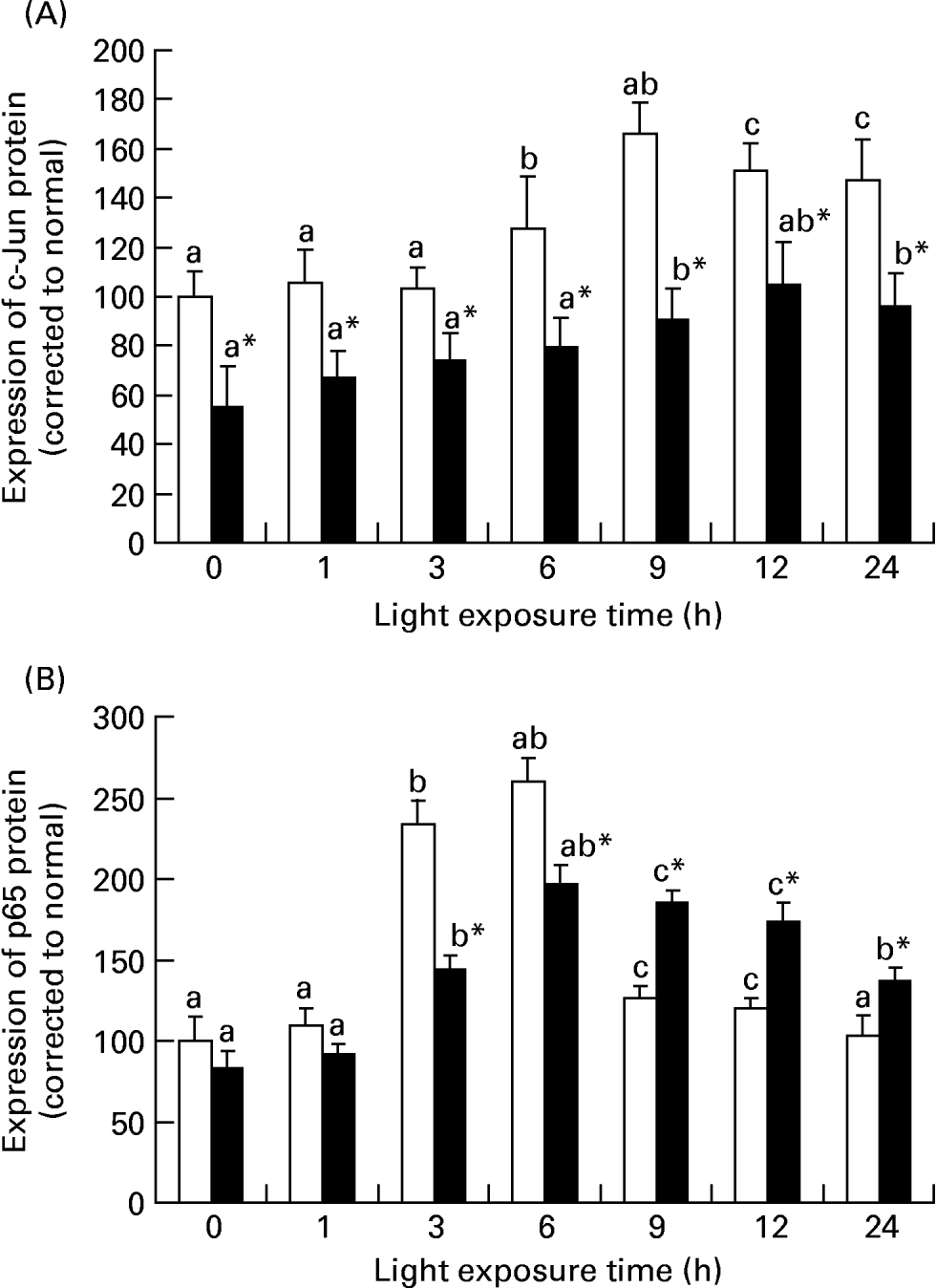
Fig. 7 The relative c-fos (A) and caspase-1 (B) protein expressions normalised for 0 h light levels (set as 100) in rats treated with (■) or without (□) 4 % taurine for 15 d and exposed to light for 0–24 h. Values are means for three determinations for each time point, with their standard deviations represented by vertical bars. a,b,c Mean values with unlike letters among the same diet group at different exposure times were significantly different (P < 0·05; Ryan's multiple-range test). * Mean value was significantly different from that for rats at the same light exposure time treated without taurine (P < 0·05; Student's t test).
Taurine stimulated nuclear factor-κB expression
Fig. 5 (C) shows that the expression of p65 mRNA increased transiently after exposure for 1 h (P = 0·034) and subsequently decreased in the retinas of rats exposed to light (P < 0·05). However, p65 mRNA expression was initially lower (1 h; P = 0·018) and then was higher (P < 0·05) in the retinas of rats treated with taurine than in rats without taurine. Fig. 7 (B) shows that the expression of p65 protein in the retinas of rats without taurine was raised after exposure for 3 h (P = 0·0023), reached its highest level at 6 h (P = 0·0005), then declined. It was obviously lower (3 h; 6 h; P < 0·05) and then higher (after 9 h; P < 0·05) in the retinas of rats treated with taurine than in rats without taurine.
Decreased caspase-1 expression by taurine
Fig. 5 (D) shows that there was an increase of caspase-1 mRNA expression in the retinas of rats exposed to light (P < 0·05), and a lower caspase-1 mRNA expression in the retinas of rats treated with taurine than in rats without taurine (P < 0·05). Fig. 6 (D–F) shows that the positive cells stained with caspase-1 antibody were mainly distributed in the ONL of rats exposed to light, but less in the retinas of rats treated with taurine than in rats without taurine. Dietary taurine down regulated the increased expression of caspase-1 induced by light.
Discussion
Taurine (2-aminoethane sulfonic acid) is present at high levels in the retina of many vertebratesReference Militante and Lombardini42. This amino acid is known to possess neuroprotective and neurotrophic properties in the central nervous system during development and regenerationReference Pasantes-Morales and Cruz27, Reference Foos and Wu32, Reference Nusetti, Obregon, Quintal, Benzo and Lima37, Reference Huxtable43–Reference Lima45. Mammals synthesise taurine from sulfur precursors, but the ability of different species to do so varies greatlyReference Huxtable43. Dietary sources of taurine are thus necessary for those animals that cannot synthesise sufficient taurine, for example, the cat and man. Dietary taurine is absorbed via the digestive system and then is transported by the Na+-dependent taurine transporter into the retina through the blood–retinal barrierReference Heller-Stilb, van Roeyen, Rascher, Hartwig, Huth, Seeliger, Warskulat and Haussinger46. A role taurine may play in the retina is the promotion of retinal cell differentiation during rod photoreceptor developmentReference Militante and Lombardini47. In the present study, we found that dietary taurine reduces the retinal damage produced by photochemical stress, and further confirmed that the protective role of taurine on the retina from photochemical stress is mediated by antioxidants and anti-AP-1–NF-κB–caspase-1 apoptotic mechanisms, which are novel findings for the biological function of taurine. The phenomenon that light provoked a significant decrease of taurine in the retinas of rats without taurine treatment may be explained by the loss or degeneration of photoreceptors. However, dietary taurine elevates the decreased concentration of taurine caused by light exposure in the retina. These results further confirm the theory that taurine is an essential component during the development and maintenance of retinal structure and function in the ratReference Altshuler, Lo Turco, Rush and Cepko44, Reference Imaki, Neuringer and Sturman48, Reference Ishikawa, Shiono, Ishiguro and Tamai49.
Apoptosis not only participates in the morphogenesis and tissue reconstruction of the retina, but is also involved in retinopathyReference Mainster, Ham and Delori5, Reference Donovan, Carmody and Cotter15, Reference Kaltschmidt, Uherek, Wellmann, Volk and Kaltschmidt18. Buchi & SzczesnyReference Buchi and Szczesny50 presumed that loss of photoreceptors is due to necrosis, but some researchers considered that apoptosis is the only form of photoreceptor loss caused by 2500–3500 lux high-intensity lightReference Krishnamoorthy, Crawford, Chaturvedi, Jain, Aggarwal, Al-Ubaidi and Agarwal19, Reference Wenzel, Grimm, Marti, Kueng-Hitz, Hafezi, Niemeyer and Reme20, Reference Wu, Chiang, Chau and Tso22, Reference Hafezi, Grimm, Wenzel, Abegg, Yaniv and Reme51. The latter hypothesis is supported by our observations with TUNEL technology. In addition, we found that taurine reduces the apoptosis of photoreceptors induced by photochemical stress, which supports the view that taurine has an anti-apoptotic effectReference Foos and Wu32, Reference Marucci, Alpini and Glaser33, Reference Cetiner, Sener and Sehirli52, Reference Oriyanhan, Yamazaki, Miwa, Takaba, Ikeda and Komeda53.
Many reports have been published concerning the mechanism of retinal photochemical damage. The free radicals arising from light absorption in the retina play a pivotal role in photochemical stressReference Krishnamoorthy, Crawford, Chaturvedi, Jain, Aggarwal, Al-Ubaidi and Agarwal19, Reference Stoyanovsky, Goldman, Darrow, Organisciak and Kagan25, Reference Siu, Reiter and To40, Reference Eppler and Dawson54. Application of substances possessing an antioxidative action can reduce retinal light-induced injury to some extentReference Organisciak, Bicknell and Darrow24–Reference Ranchon, Gorrand, Cluzel, Droy-Lefaix and Doly26. In the present study, we found that the antioxidant taurine not only decreases the concentration of MDA caused by light, but also elevates the activities of SOD and GSH-Px in the retina. These results indicate that the anti-apoptotic effect of taurine is correlated with its antioxidative activity.
Expression of AP-1 is involved in the apoptosis of photoreceptors induced by photochemical stressReference Wu, Chiang, Chau and Tso22, Reference Hafezi, Grimm, Wenzel, Abegg, Yaniv and Reme51, Reference Fleischmann, Hafezi, Elliott, Reme, Ruther and Wagner55–Reference Roca, Shin, Liu, Simon and Chen58. However, the theory behind this action has yet to be fully elucidated. In the present experiment, we found that c-fos/c-jun mRNA and protein expression is up regulated in the retinas of rats exposed to light. Dietary taurine down regulates the transcription and expression of c-fos/c-jun. Based on these results, we deduced that taurine reduces the AP-1 complex formation, thereby blocking the photoreceptor apoptotic pathway.
The transcription factor NF-κB acts as a master regulator of stress responses by exerting a strong modulatory effect on apoptosisReference Barkett and Gilmore59–Reference Mattson and Camandola61. The present results implied that continuous light exposure leads to an early increase of NF-κB (p65) expression because of its constitutive expression, but a later decrease because of photochemical oxidative stress, which is supported by previous findingsReference Wu, Chen, Chiang and Tso62. In contrast to light-induced activation of NF-κB in vivo, NF-κB activity in 661W cells exposed to light is down regulatedReference Krishnamoorthy, Crawford, Chaturvedi, Jain, Aggarwal, Al-Ubaidi and Agarwal19. The difference between the modulation of NF-κB in response to light in vivo and in vitro may be due to the different cellular context and environment. We also found that dietary taurine up regulates NF-κB expression, which may be because of its antioxidative ability.
The caspases are a family of cysteine proteases that are indispensable to mammalian apoptosis. Caspase-1 has been demonstrated as a central player in neuronal cell apoptosisReference Wenzel, Grimm, Marti, Kueng-Hitz, Hafezi, Niemeyer and Reme20, Reference Strasser, O'Connor and Dixit63. Caspase-1 is regulated by NF-κB, and its over-expression can induce apoptosis in vivo and in vitro Reference Krishnamoorthy, Crawford, Chaturvedi, Jain, Aggarwal, Al-Ubaidi and Agarwal19, Reference Wu, Chiang, Chau and Tso22. Furthermore, caspase-1 activation is related to retinal photoreceptor apoptosis caused by lightReference Grimm, Wenzel, Hafezi and Reme13, Reference Grimm, Wenzel, Hafezi, Yu, Redmond and Reme14. Progressively increased caspase-1 mRNA and protein expression is observed in the present study, which agrees with the findings of others researchers using different experimental protocolsReference Wu, Chiang, Chau and Tso22, Reference Hafezi, Grimm, Wenzel, Abegg, Yaniv and Reme51. However, we observed that dietary taurine inhibits the increased expression of caspase-1 protein and mRNA induced by light in the retina. Meanwhile, the results of caspase-1 expression detected by immunohistochemistry are supported by the results with TUNEL technology. The present findings strongly suggest that taurine inhibits the expression of caspase-1, which is involved in photoreceptor apoptosis produced by photochemical stress.
However, there is a different result of taurine for light-injury protection. Voaden et al. Reference Voaden, Hussain and Lalji64 found that 5 % taurine used in drinking water for 6 d has no effect on the rate of DNA and protein loss. The different findings of taurine on the photochemical damage of the retina in the rat may be due to the different methods applied and the period of exposure to taurine. Another question is that the eye of the Sprague–Dawley rat is biochemically very different from the human eye, and more susceptible to light damage. Whether humans or albino humans will benefit from dietary taurine is unclear. These questions need to be researched further.
In summary, we have provided experimental evidence that dietary taurine partially protects against retinal morphological and functional photochemical damage in vivo, which suggests that taurine might have therapeutic implications in the treatment of retinal photochemical stress. Though the present study confirms the mechanism by which dietary taurine decreases oxidative stress and influences the AP-1–NF-κB–caspase-1 apoptosis signal pathway, the theory behind the protective effect of taurine against retinal damage induced by photochemical stress remains to be fully elucidated.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant 30200 228). We thank Professor Zhu Jundong and Xu Hongxia for their expert technical assistance and gratefully thank Professor Ling Wenhua (Sun Yat-sen University) for most valuable comments on an earlier draft of this paper.











