The incidence of diabetes has been increasing globally, with an estimated world prevalence reaching 7·7 % by 2030(Reference Shaw, Sicree and Zimmet1). In Japan, the prevalence of diabetes has increased markedly over the last few decades(Reference Iso2), and it has been argued that the increase is primarily due to the Westernisation of diet, including the increased intake of animal products(Reference Nakanishi, Okubo and Yoneda3). Data from prospective studies have indicated that total meat intake is consistently associated with the increased risk of type 2 diabetes among men(Reference Brand, van der Schouw and Onland-Moret4–Reference van Dam, Willett and Rimm6), whereas inconsistent findings have been observed in women(Reference Brand, van der Schouw and Onland-Moret4, Reference Villegas, Shu and Gao7). The results of recent meta-analyses of prospective studies support that a high red meat(Reference Aune, Ursin and Veierod8, Reference Pan9) and processed meat(Reference Aune, Ursin and Veierod8–Reference Micha10) intake increases the risk of type 2 diabetes in both men and women. Fe, which is contained in red meat, may play a role as a mediator of the adverse effect of red meat intake on glucose metabolism(Reference Jiang, Manson and Meigs11, Reference Tuomainen, Nyyssonen and Salonen12). Additionally, SFA in meat may increase inflammatory response and secondarily enhance the risk of type 2 diabetes(Reference Salas-Salvado, Martinez-Gonzalez and Bullo13). In terms of poultry intake, cohort studies have reported no association among men(Reference Brand, van der Schouw and Onland-Moret4–Reference van Dam, Willett and Rimm6, Reference Ericson, Sonestedt and Gullberg14, Reference Steinbrecher, Erber and Grandinetti15), but data among women are conflicting(Reference Brand, van der Schouw and Onland-Moret4, Reference Villegas, Shu and Gao7, Reference Ericson, Sonestedt and Gullberg14–Reference Schulze, Manson and Willett16).
Epidemiological evidence on this issue is limited among Asian populations, in which meat consumption is much lower than that in Western populations(17). In a study of Chinese women, the only prospective investigation in Asia, intake of total meat (red meat and poultry), was inversely associated with the risk of type 2 diabetes(Reference Villegas, Shu and Gao7), a finding that conflicts with the findings of Western studies(Reference Brand, van der Schouw and Onland-Moret4–Reference van Dam, Willett and Rimm6, Reference Vang, Singh and Lee18), although the association between processed meat intake and the risk of type 2 diabetes in the Chinese study(Reference Villegas, Shu and Gao7) is consistent with those in Western studies(Reference Brand, van der Schouw and Onland-Moret4, Reference Mannisto, Kontto and Kataja-Tuomola5, Reference Ericson, Sonestedt and Gullberg14–Reference Schulze, Manson and Willett16, Reference van Woudenbergh19–Reference Song, Manson and Buring23). Japanese patients with type 2 diabetes are on average leaner than their Western counterparts(Reference Sone, Ito and Ohashi24), and Japanese-Americans have lower β-cell function than do non-Hispanic whites(Reference Jensen, Cnop and Hull25). Thus, the effect of meat consumption on type 2 diabetes in the Japanese population may differ from the effect in Western populations. Here, we prospectively investigated the association between meat intake (total red meat, unprocessed red meat, processed red meat and poultry) and the risk of type 2 diabetes in a large-scale, population-based cohort of Japanese men and women. Further, we examined the association by BMI, which is a major predictor of type 2 diabetes risk, and by menopausal status (women only), which is a determinant of body Fe storage that may increase with the intake of Fe-containing foods including meat, and which has been linked to type 2 diabetes risk(Reference Szmuilowicz26).
Materials and methods
Study design
Cohort I of the Japan Public Health Center-based Prospective (JPHC) Study was established in 1990, and cohort II was established in 1993(Reference Tsugane and Sobue27). The study protocol was approved by the institutional review board of the National Cancer Center, Tokyo, Japan. The participants of cohort I included residents aged 40–59 years in five Japanese public health centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo). The participants of cohort II included residents aged 40–69 years in six public health centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka). Although we did not require written informed consent, the study participants were informed of the objectives of the study, and participants who responded to the questionnaire survey were considered to have consented to participate in the survey.
Among the baseline subjects (n 140 420), 113 403 subjects responded to the questionnaire survey at baseline. Of these, 89 947 (79·3 %) subjects responded to the 5-year follow-up survey (second survey). Of these subjects, 76 901 (67·8 %) responded to the 10-year follow-up survey (third survey). We excluded 12 462 subjects who reported a history of type 2 diabetes, cancer, stroke, IHD and chronic liver disease at baseline or at the second survey, as well as those who reported kidney disease at the baseline survey. Individuals who were missing information regarding their meat intake were excluded. We also excluded 590 subjects who reported extreme total energy intakes (outside of the mean ± 3 sd according to sex). Finally, a total of 63 849 subjects (27 425 men and 36 424 women) remained in the present analysis.
FFQ
Participants completed a self-administered FFQ at the baseline, second and third surveys. The data included 147 food and beverage items and nine frequency categories(Reference Sasaki, Kobayashi and Ishihara28). For the present analysis, we used data from the second survey as the baseline data because the questionnaire used for the second survey more comprehensively inquired about food intakes than the one used for the baseline survey. At the second survey, we asked about the usual consumption of sixteen meat items over the past year(Reference Sasaki, Kobayashi and Ishihara28). The red meat items included three beef dishes (steak, grilled beef and stewed beef), six pork dishes (stir-fried pork, deep-fried pork, stewed pork in the Western style, stewed pork in the Japanese style, pork in soup and pork liver), four processed meat products (ham, sausage or Wiener sausage, bacon and luncheon meat) and chicken liver. Poultry items included two chicken meals (grilled chicken and deep-fried chicken). For most food items, nine response options were available to describe consumption frequency, ranging from rarely ( < 1 time/month) to ≥ 7 times/d. A standard portion size was specified for each food, and respondents were asked to choose their usual portion size from three options ( ≤ 0·5 times, standard or ≥ 1·5 times). The daily intake of meat and meat products was calculated by multiplying the daily consumption frequency by the typical portion size, and was expressed as g/d. These individual items were categorised into five main groups of total meat, total red meat (unprocessed and processed red meat items), unprocessed red meat, processed red meat and poultry.
Referring to the Standard Tables of Food Composition in Japan(29), dietary intakes for energy and selected nutrients were estimated. The validity and reproducibility of the FFQ was examined in a subsample of the participants in the JPHC Study cohort I and cohort II. Details of the validation study have been described elsewhere(Reference Ishihara, Sobue and Yamamoto30–Reference Sasaki, Kobayashi and Tsugane32). For the validity of the FFQ, energy-adjusted Spearman's correlation coefficients between intake values for meat derived from the FFQ and those derived from 28 or 14 d dietary records were 0·50 for men and 0·45 for women, respectively, for cohort I(Reference Sasaki, Kobayashi and Tsugane32), and 0·48 for men and 0·44 for women, respectively, for cohort II(Reference Ishihara, Sobue and Yamamoto30). With regard to the reproducibility of the FFQ, energy-adjusted Spearman's correlation coefficients for intake of meat derived from the two FFQ administered 1 year apart were 0·52 for both men and women for cohort I(Reference Sasaki, Ishihara and Tsugane31) and 0·52 for men and 0·41 for women, respectively, for cohort II(Reference Ishihara, Sobue and Yamamoto30).
Ascertainment of type 2 diabetes
Type 2 diabetes was ascertained using a self-administered questionnaire. At the third survey, study participants were asked about their history of major diseases including diabetes and, if it was present, the timing of the initial diagnosis in relation to the first and second surveys. Because the 5-year survey was used as the baseline measure in the present study, only participants who were subsequently diagnosed were regarded as incident cases during the follow-up. Details regarding the assessment of the validity of self-reported diabetes have been described elsewhere(Reference Kato, Noda and Inoue33). Previously, we showed that 94 % of self-reported diabetes cases were confirmed by medical records.
Statistical analyses
Analyses of men and women were performed separately. Participants were divided into intake quartiles. The confounding variables considered were as follows: age (years, continuous); study area (eleven areas); BMI ( < 21, 21–22·9, 23–24·9, 25–26·9 or ≥ 27 kg/m2); smoking status (lifetime non-smoker, former smoker or current smoker with a consumption of either < 20 or ≥ 20 cigarettes/d); alcohol consumption (non-drinker, occasional drinker or drinker with a consumption of < 150, 150–299, 300–499 or ≥ 450 g ethanol/week for men and < 150 or ≥ 150 g ethanol/week for women); total physical activity level (metabolic equivalent-h/d, quartiles); history of hypertension (yes or no); family history of diabetes mellitus (yes or no); coffee consumption (almost never, < 1, 1 or ≥ 2 cups/d); total energy intake (kJ/d, continuous); Ca intake (mg/d, continuous); Mg intake (mg/d, continuous); rice intake (g/d, continuous); fish intake (g/d, continuous); vegetable intake (g/d, continuous); soft drink intake (g/d, continuous). The dietary factors considered here have been shown to be associated with the risk of type 2 diabetes both in previous studies and in the present cohort. An indicator variable for missing data was created for each covariate. Trend associations between confounding factors and meat intakes were examined using the Mantel–Haenszel χ2 test for categorical variables and linear regression analysis for continuous variables.
The association between the intakes of energy-adjusted total meat, total red meat, unprocessed red meat, processed red meat or poultry and the risk of diabetes was assessed by OR, which were estimated using a multiple logistic regression. A 95 % CI of the OR was estimated using the Wald method. The first model was adjusted for age and study area, and the second was further adjusted for BMI, smoking status, alcohol consumption, the family history of diabetes mellitus, the history of hypertension, total physical activity level, total energy intake, coffee consumption and the intakes of Ca, Mg, rice, fish, vegetables and soft drinks. An additional model was further adjusted for Fe intake (g/d, continuous) or saturated fat intake (g/d, continuous). The trend association was assessed by assigning the ordinal numbers 0–3 to the four categories of each meat or specific groups of meat consumption. We also analysed data by BMI ( < 25 or ≥ 25 kg/m2) in both men and women and menopausal status (pre-menopausal or postmenopausal) in women. An interaction term of dietary intake (continuous) and the above stratifying variables (dichotomous) was created and added to the model to assess statistical interactions. Statistical significance was declared if the two-sided P value was less than 0·05. All analyses were performed using SAS software (version 9.2; SAS Institute).
Results
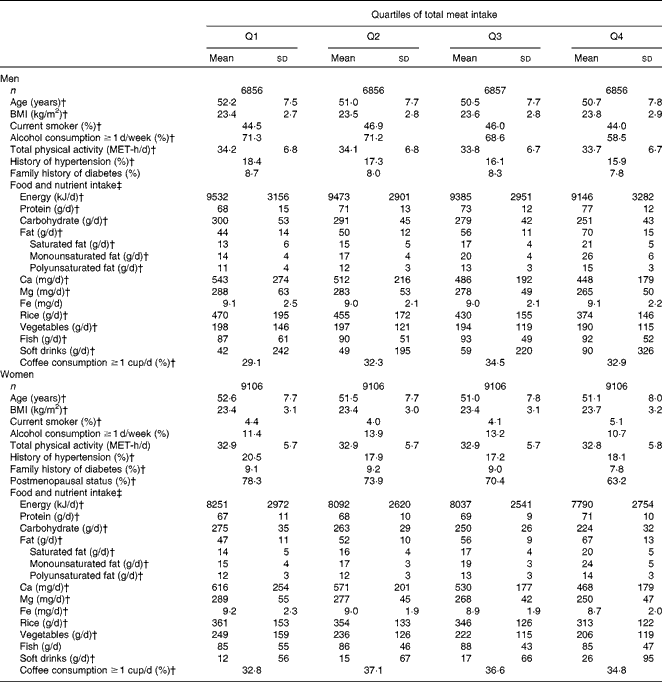
During the 5-year period, 1178 participants (681 men and 497 women) were newly self-reported as having type 2 diabetes. At baseline (the time of the second survey), both men and women with higher intakes of total meat were more likely to be young and to have a high BMI. They also had higher intakes of protein, fat, soft drinks and coffee, and lower intakes of carbohydrates, Ca, Mg, rice and vegetable than those with lower total meat intakes (Table 1). Men who consumed greater amounts of total meat were less likely to report higher levels of physical activity at work or during leisure time and to consume alcohol.
Table 1 Baseline characteristics of the subjects according to categories of energy-adjusted meat intake* (Mean values and standard deviations; percentages)

MET, metabolic equivalents.
* On the basis of the Mantel–Haenszel χ2 test for categorical variables and linear regression analysis for continuous variables with the assignment of ordinal numbers 0–3 to the categories of total meat intake.
† P for trend < 0.05.
‡ Energy adjusted by the residual method except for energy intake and coffee consumption.
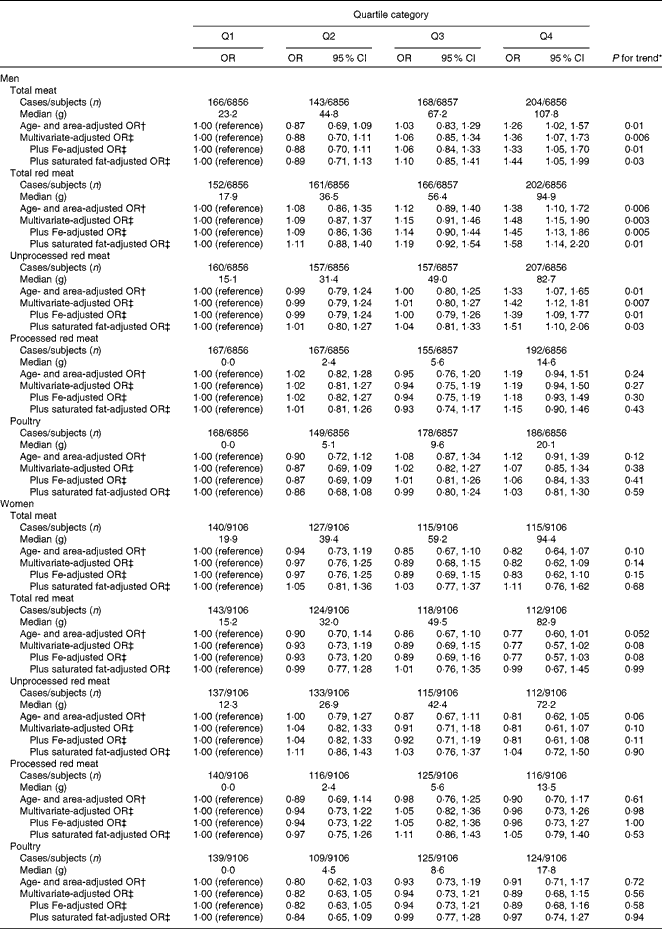
Total meat was positively associated with the risk of type 2 diabetes in men (Table 2). The OR in the highest quartile of total meat intake compared with those in the lowest was 1·36 (95 % CI 1·07, 1·73; P for trend = 0·006) in the multivariate-adjusted model. The median intake of total red meat was 45·6 and 40·1 g/d in men and women, respectively. High intakes of total red meat and unprocessed red meat were statistically significantly associated with an increased risk of type 2 diabetes in men. The multivariate-adjusted OR of type 2 diabetes for the lowest to the highest quartile category of intake were 1·00 (reference), 1·09 (95 % CI 0·87, 1·37), 1·15 (95 % CI 0·91, 1·46) and 1·48 (95 % CI 1·15, 1·90) (P for trend = 0·003) and 1·00 (reference), 0·99 (95 % CI 0·79, 1·24), 1·01 (95 % CI 0·80, 1·27) and 1·42 (95 % CI 1·12, 1·80) (P for trend = 0·007) for total red meat and unprocessed red meat, respectively. These associations were only slightly attenuated after additional adjustment for Fe intake. We did not observe significant associations of total red meat and unprocessed red meat with the risk of type 2 diabetes in women. Poultry consumption was not associated with the risk of type 2 diabetes among either men or women. In stratified analyses, there was no significant interaction by BMI or menopausal status (women only).
Table 2 Type 2 diabetes according to the quartile categories of energy-adjusted meat intakes (Odds ratios and 95 % confidence intervals)

* Trend association was assessed by assigning the ordinal numbers 0–3 to the four categories of each meat or specific groups of meat consumption.
† Adjusted for age and public health centre area.
‡ Additionally adjusted for BMI, smoking status, alcohol consumption, total physical activity, the history of hypertension, coffee consumption, the family history of diabetes, Mg intake, Ca intake, rice intake, fish intake, vegetable intake, soft drink consumption and energy intake.
Discussion
In the present large-scale population-based prospective study in Japanese adults, a high consumption of total meat, total red meat and unprocessed red meat were associated with the increased risk of type 2 diabetes in men but not in women. Processed red meat and poultry intakes were not associated with an increased risk of diabetes in men or women. Thus, the associations with total meat and total red meat are largely accounted for by the association with unprocessed red meat.
The present finding of a positive association of total meat intake with the risk of type 2 diabetes in men is consistent with a recent meta-analysis of three cohort studies from the USA; the summary relative risk of type 2 diabetes per 100 g/d of total meat was 1·12 (95 % CI 1·05, 1·19)(Reference Micha10). Although another meta-analysis of five prospective studies from the USA, Australia, Japan and China reported no clear association of total meat intake with the risk of type 2 diabetes, a statistically significantly increased risk associated with a high intake of total meat emerged after excluding a Chinese study(Reference Villegas, Shu and Gao7); the summary relative risk of type 2 diabetes comparing a high v. low intake of total meat was 1·31 (95 % CI 1·12, 1·52)(Reference Aune, Ursin and Veierod8). In contrast, the present study found no association in women, a finding that is inconsistent with those found for women in a Western study(Reference Brand, van der Schouw and Onland-Moret4). The above-mentioned study in Chinese women reported a decreased, rather than increased, risk of type 2 diabetes associated with a higher intake of total meat. Although caution needs to be exercised when interpreting the results of the Chinese study, as their measure of total meat included unprocessed red meat and poultry but not processed meat(Reference Villegas, Shu and Gao7), available evidence from Japan and China did not support the hypothesis that total meat intake is associated with the increased risk of type 2 diabetes among female Asian populations.
The present finding of a positive association with the consumption of total red meat, which is mainly derived from the association with unprocessed red meat, is consistent with the result of two meta-analyses of data from prospective studies that were mainly conducted in Western populations (pooled hazard ratio 1·21, 95 % CI 1·07, 1·38(Reference Aune, Ursin and Veierod8) and pooled hazard ratio 1·19, 95 % CI 1·04, 1·37 for 100 g/d(Reference Pan9)). However, the absolute amount of total red meat intake in the present study population (median 45·6 and 40·1 g/d in men and women, respectively) was lower than that in the US population (mean 69·8 g/d)(Reference Daniel, Cross and Koebnick34), but was similar to that in the Chinese study population (median 42·6 g/d)(Reference Villegas, Shu and Gao7). Additionally, the type of unprocessed red meat consumed was considerably different among studies: pork intake was almost twice as high as beef intake in the USA(Reference Bachman, Reedy and Subar35) and in the present study population, while in the Chinese study population, pork was consumed much more often than beef (9:1)(Reference Villegas, Shu and Gao7). Given that the Chinese study showed no increased risk of type 2 diabetes(Reference Villegas, Shu and Gao7), beef intake might have a stronger influence on the risk of type 2 diabetes than pork intake. In fact, a randomised controlled trial has suggested that a regular intake of pork in place of other meats improves body composition(Reference Murphy, Thomson and Coates36).
A positive association between processed meat and type 2 diabetes has been observed in three meta-analyses of cohort studies(Reference Aune, Ursin and Veierod8–Reference Micha10) and in prospective studies(Reference Brand, van der Schouw and Onland-Moret4, Reference Mannisto, Kontto and Kataja-Tuomola5, Reference Ericson, Sonestedt and Gullberg14, Reference Steinbrecher, Erber and Grandinetti15, Reference Lajous, Tondeur and Fagherazzi20) that were not included in those meta-analyses. The magnitudes of increases in risk associated with processed meat intake were greater than those associated with red meat intake(Reference Aune, Ursin and Veierod8–Reference Micha10). For example, there were 41 and 21 % increased risks for the highest v. lowest intake of processed meat and red meat, respectively(Reference Aune, Ursin and Veierod8). In the present study, however, men in the highest quartile of processed meat intake had a statistically insignificant 15 % increased risk of type 2 diabetes compared with those in the lowest quartile. One explanation for this discrepancy is that processed meat consumption in Japan is much lower than in the USA (mean 12·4 g/d in Japan and 23·2 g/d in the USA)(Reference Daniel, Cross and Koebnick34, Reference Kenko Eiyo Joho37). In the present study population, the median in the highest quartile of processed red meat consumption was 13·1 g/8368 kJ (2000 kcal) per d among men and 14·1 g/8368 kJ (2000 kcal) per d among women. Similarly, in the Multiethnic Cohort in Hawaii, Japanese-American men and women in the highest category of processed red meat intake (17·1 g/8368 kJ (2000 kcal) per d in men and 13·9 g/8368 kJ (2000 kcal)/d in women) showed only modest increases in the risk of type 2 diabetes (24 and 23 % for men and women, respectively) compared with those with the lowest category of meat intake(Reference Steinbrecher, Erber and Grandinetti15). Few Japanese are likely to consume processed red meat above the level that is apparently associated with the increased risk of type 2 diabetes.
Poultry intake was not appreciably associated with the risk of type 2 diabetes among either men or women in the present study. Previously, no association between poultry intake and the risk of type 2 diabetes has been consistently reported in men(Reference Brand, van der Schouw and Onland-Moret4–Reference van Dam, Willett and Rimm6, Reference Ericson, Sonestedt and Gullberg14, Reference Steinbrecher, Erber and Grandinetti15). In women, however, data have been mixed(Reference Brand, van der Schouw and Onland-Moret4, Reference Villegas, Shu and Gao7, Reference Ericson, Sonestedt and Gullberg14–Reference Schulze, Manson and Willett16), with two studies showing a decrease(Reference Villegas, Shu and Gao7, Reference Schulze, Manson and Willett16), one study showing no association(Reference Steinbrecher, Erber and Grandinetti15) and two studies showing an increase in the risk of type 2 diabetes(Reference Brand, van der Schouw and Onland-Moret4, Reference Ericson, Sonestedt and Gullberg14). Given that the majority of these studies did not detect an increased risk of type 2 diabetes associated with poultry intake, poultry intake may not impair glucose metabolism. There are some possible explanations. As poultry contains a lower amount of haem Fe than red meat, a higher intake of poultry may not significantly increase Fe storage, which has been linked to the risk of type 2 diabetes(Reference Rajpathak, Crandall and Wylie-Rosett38). Moreover, PUFA in poultry can improve insulin sensitivity(Reference Lichtenstein39).
In the present study, intakes of total meat, red meat and unprocessed red meat were associated with the risk of type 2 diabetes in men only. This could be ascribed, at least in part, to sex difference in Fe storage, which increases with the intake of Fe-rich foods including red meat. Circulating ferritin concentrations (a marker of Fe storage) in pre-menopausal women are much lower than those in men(Reference Rajpathak, Crandall and Wylie-Rosett38), and showed no association with insulin resistance(Reference Lee, Kim and Kim40, Reference Jehn, Clark and Guallar41). In postmenopausal women, whose blood ferritin levels are also lower than those in men(Reference Rajpathak, Crandall and Wylie-Rosett38), studies on insulin resistance have yielded mixed results, with one US study reporting a positive association with ferritin concentrations(Reference Jehn, Clark and Guallar41), whereas a Korean study reported no association(Reference Lee, Kim and Kim40). In the present study, no increase in the risk of type 2 diabetes with a high intake of meat was observed among either pre- or postmenopausal women. The present finding may be supported by a Japanese study, in which serum ferritin concentrations were significantly associated with an insulin resistance marker in men but not in women(Reference Pham, Nanri and Yi42). For Asian women, meat intake may not increase Fe storage to the level above which glucose metabolism is impaired.
The mechanism underlying the association between meat intake and the risk of type 2 diabetes is unclear. Haem Fe in red meat has been suggested to play an unfavourable role in glucose metabolism(Reference Rajpathak, Crandall and Wylie-Rosett38). Fe is a strong pro-oxidant and pro-inflammatory factor that catalyses several reactions leading to the formation of reactive oxygen species and resulting in elevated oxidative stress and inflammation(Reference Wagener43), which may decrease insulin sensitivity(Reference Rajpathak, Crandall and Wylie-Rosett38, Reference Haffner44). Increased Fe stores in the liver may induce insulin resistance by impeding the capacity for insulin extraction(Reference Rajpathak, Crandall and Wylie-Rosett38). Fe impairs insulin action and interferes with glucose uptake in adipocytes(Reference Rajpathak, Crandall and Wylie-Rosett38). In addition, increased muscle Fe stores enhance NEFA oxidation and cause them to interfere with glucose disposal(Reference Rajpathak, Crandall and Wylie-Rosett38). Excess body Fe causes Fe deposition in pancreatic β-cells, resulting in impaired insulin secretion(Reference Rajpathak, Crandall and Wylie-Rosett38). In epidemiological studies, intake of haem Fe has been consistently associated with an increased risk of type 2 diabetes(Reference Rajpathak, Crandall and Wylie-Rosett38). In the present study, however, adjustment for Fe intake only slightly attenuated the association between total meat and total red meat and the risk of type 2 diabetes, indicating that mechanisms other than the latter may exist. Meat is also a major source of saturated fat, which is increased in cell membranes and leads to decreased membrane fluidity and decreased insulin receptor affinity(Reference Ginsberg, Brown and Simon45). Saturated fat increases inflammatory responses and secondarily enhances the risk of type 2 diabetes(Reference Salas-Salvado, Martinez-Gonzalez and Bullo13). However, the adjustment for saturated fat intake did not appreciably change the result in the present study. Alternatively, advanced glycation end products and heterocyclic amines, which are formed in meat through heating and processing(Reference Sinha, Rothman and Salmon46, Reference Hofmann, Dong and Li47), have been shown to increase oxidative stress and inflammation, leading to the progression of insulin resistance in mice(Reference Cai, He and Zhu48). In a study of patients with type 2 diabetes, restriction of dietary advanced glycation end products improved insulin sensitivity(Reference Uribarri, Cai and Ramdas49).
The major strengths of the present study include a large number of male and female participants, the population-based prospective design, the use of a validated FFQ and adjustment for or stratification by potentially important confounding variables. Limitations of the present study also deserve mention. First, the diagnosis of type 2 diabetes was ascertained via self-report. We confirmed that 94 % of self-reported diabetes cases were correctly documented in medical records in a sample of a validation study(Reference Kato, Noda and Inoue33), but undiagnosed cases may exist. It is unlikely, however, that the probability of under-diagnosis differs according to meat intake, and the use of self-reported data might not have substantially influenced the risk estimate. Second, we used only 5-year survey data for the assessment of dietary intake. Due to random variation, this would lead to a non-differential misclassification of meat intake and would thus distort the OR towards the null. In addition, one-time dietary measurement at baseline may not capture long-term intake, which is relevant to the development of type 2 diabetes. Repeated assessment of the diet over a long period of time before disease onset will probably provide a better estimate of exposure status. Third, although the validity of the FFQ for meat intake was relatively high (r 0·44–0·50), the measurement error in the FFQ might result in biased associations between meat intake and the risk of type 2 diabetes, which would drive the results towards the null. Fourth, we adjusted for dietary Fe intake but not for blood ferritin concentration, which is a better marker of body Fe status. Fifth, the follow-up period was relatively short (5 years). Finally, we could not rule out the possibility of unmeasured and residual confounding.
In conclusion, we found that total red meat and unprocessed red meat intakes were associated with the increased risk of type 2 diabetes after adjustment for other risk factors for type 2 diabetes among Japanese men, whose meat consumption is lower than that of Westerners. The present study adds to the evidence showing the adverse effect of high meat consumption on glucose metabolism.
Acknowledgements
The present study was supported by Grants-in-Aid for Cancer Research (19shi-2) and a Health Sciences Research Grant (Research on Comprehensive Research on Cardiovascular Diseases H19-016) from the Ministry of Health, Labour and Welfare of Japan.
The authors' contributions are as follows: S. T. was involved in the design of the study as the principal investigator; S. T. and M. I. conducted the survey; K. K., A. N., A. G., T. M., M. N., S. O., M. K. and Y. M. drafted the plan for the data analyses; K. K. conducted the data analyses; T. M. provided statistical expertise; K. K. drafted the manuscript; K. K. and T. M. had primary responsibility for the final content. All authors were involved in the interpretation of the results and the revision of the manuscript and approved the final version of the manuscript. None of the authors had a conflict of interest.
Members of the JPHC Study (principal investigator: S. T.) Group are as follows: S. T., M. I., T. Sobue and T. Hanaoka, National Cancer Center, Tokyo, Japan; J. Ogata, S. Baba, T. Mannami, A. Okayama and Y. Kokubo, National Cardiovascular Center, Osaka, Japan; K. Miyakawa, F. Saito, A. Koizumi, Y. Sano, I. Hashimoto, T. Ikuta and Y. Tanaba, Iwate Prefectural Ninohe Public Health Center, Iwate, Japan; Y. Miyajima, N. Suzuki, S. Nagasawa, Y. Furusugi and N. Nagai, Akita Prefectural Yokote Public Health Center, Akita, Japan; H. Sanada, Y. Hatayama, F. Kobayashi, H. Uchino, Y. Shirai, T. Kondo, R. Sasaki, Y. Watanabe, Y. Miyagawa and Y. Kobayashi, Nagano Prefectural Saku Public Health Center, Nagano, Japan; Y. Kishimoto, E. Takara, T. Fukuyama, M. Kinjo, M. Irei and H. Sakiyama, Okinawa Prefectural Chubu Public Health Center, Okinawa, Japan; K. Imoto, H. Yazawa, T. Seo, A. Seiko, F. Ito, F. Shoji and R. Saito, Katsushika Public Health Center, Tokyo, Japan; A. Murata, K. Minato, K. Motegi and T. Fujieda, Ibaraki Prefectural Mito Public Health Center, Ibaraki, Japan; K. Matsui, T. Abe, M. Katagiri and M. Suzuki, Niigata Prefectural Kashiwazaki and Nagaoka Public Health Center, Niigata, Japan; M. Doi, A. Terao, Y. Ishikawa and T. Tagami, Kochi Prefectural Chuo-higashi Public Health Center, Kochi, Japan; H. Sueta, H. Doi, M. Urata, N. Okamoto and F. Ide, Nagasaki Prefectural Kamigoto Public Health Center, Nagasaki, Japan; H. Sakiyama, N. Onga, H. Takaesu and M. Uehara, Okinawa Prefectural Miyako Public Health Center, Okinawa, Japan; F. Horii, I. Asano, H. Yamaguchi, K. Aoki, S. Maruyama, M. Ichii and M. Takano, Osaka Prefectural Suita Public Health Center, Osaka, Japan; Y. Tsubono, Tohoku University, Miyagi, Japan; K. Suzuki, Research Institute for Brain and Blood Vessels Akita, Akita, Japan; Y. Honda, K. Yamagishi, S. Sakurai and N. Tsuchiya, Tsukuba University, Ibaraki, Japan; M. Kabuto, National Institute for Environmental Studies, Ibaraki, Japan; M. Yamaguchi, Y. Matsumura, S. Sasaki and S. Watanabe, National Institute of Health and Nutrition, Tokyo, Japan; M. Akabane, Tokyo University of Agriculture, Tokyo, Japan; T. Kadowaki, Tokyo University, Tokyo, Japan; M. N. and T. M., National Center for Global Health and Medicine, Tokyo, Japan; Y. Kawaguchi, Tokyo Medical and Dental University, Tokyo, Japan; Y. Takashima and M. Yoshida, Kyorin University, Tokyo, Japan; K. Nakamura, Niigata University, Niigata, Japan; S. Matsushima and S. Natsukawa, Saku General Hospital, Nagano, Japan; H. Shimizu, Sakihae Institute, Gifu, Japan; H. Sugimura, Hamamatsu University, Shizuoka, Japan; S. Tominaga, Aichi Cancer Center Research Institute, Aichi, Japan; H. Iso, Osaka University, Osaka, Japan; M. Iida, W. Ajiki and A. Ioka, Osaka Medical Center for Cancer and CVD, Osaka, Japan; S. Sato, Chiba Prefectural Institute of Public Health, Chiba, Japan; E. Maruyama, Kobe University, Hyogo, Japan; M. Konishi, K. Okada and I. Saito, Ehime University, Ehime, Japan; N. Yasuda, Kochi University, Kochi, Japan; S. Kono, Kyushu University, Fukuoka, Japan.