PUFA are key components involved in a variety of physiological functions. They are also precursors of eicosanoids, such as PG and leukotrienes( Reference Spector 1 – Reference Chapkin, McMurray and Davidson 3 ), and docosanoids, such as protectins or resolvins( Reference Serhan, Gotlinger and Hong 4 ). Some of them, belonging to the n-6 or n-3 family, have to be provided by diet or are derived from the biosynthetic pathways resulting in the conversion of essential precursors to their respective elongated and desaturated products. The two essential precursors, linoleic acid (LA; 18 : 2n-6) and α-linolenic acid (ALA; 18 : 3n-3), are exclusively required in the diet, because a deficiency of either of them leads to well-documented symptoms( Reference Burr and Burr 5 – Reference Alessandri, Guesnet and Vancassel 8 ). First studies and several scientific committees have recommended the dietary intake of 2 % of energy intake (en%) from LA and 0·5 en% from ALA to prevent the biochemical and physiological symptoms of deficiency( Reference Holman 9 – 11 ).
The first dose–response studies of LA requirements in rats have been corrupted by an unintentional exclusion of ALA( Reference Holman 12 ), leading to a significant overestimation of the dietary requirement for dietary LA( Reference Cunnane 13 ). The n-3 and n-6 families have been known to be nutritionally different, with different biological functions; nevertheless, dietary ALA deficiency could affect physiological parameters similar to LA deficiency. For instance, several studies on rats have shown that the dietary intake of 0·5 en% from ALA could prevent the biological (growth, reproduction and early development) and biochemical functions with a LA intake as low as 0·3 en%( Reference Greenberg and Deuel 14 , Reference Bourre, Piciotti and Dumont 15 ).
Because LA is the most abundant PUFA in the Western diet (14 g/2000 kcal (8368 kJ) in the US diet)( Reference Whelan 16 ) due to changes in agricultural practices, and because the potential inflammatory properties are still controversial in human CVD( Reference Choque, Catheline and Rioux 17 , Reference Simopoulos 18 ), a precise estimation of the dietary LA requirements in healthy humans has to be carried out( Reference Hansen, Wiese and Boelsche 19 – Reference Wene, Connor and DenBesten 22 ). Since the historic value for humans has been estimated in rats, several studies use this animal model for nutritional experiments. Recently, one study( Reference Guesnet, Lallemand and Alessandri 23 ) including an adequate ALA intake in the experimental diets did not succeed to determine precisely the LA requirement in rats. Indeed, the animals used in the Guesnet et al.'s study( Reference Guesnet, Lallemand and Alessandri 23 ) were not totally LA deficient during the experiments due to excess large LA body stores at the beginning of the experiments, explaining the absence of scaly marks on the tail. For the first time, the present study was especially designed to create a specific and strong LA deficiency including deprivation of LA in the maternal diet. Thus, the main purpose of the present study was to carry out a dose–response study on a male rat model in order to determine the minimal intake of dietary LA required to avoid n-6 deficiency symptoms, when ALA is included in the diet. We measured and followed the physiological parameters of the deficiency (growth and scaliness on the tail), and biochemical markers of essential fatty acid deficiency 20 : 3n-9 and the 20 : 3n-9/20 : 4n-6 ratio( Reference Chu and Hegsted 24 ). The present study was divided into two steps in order to achieve a specific n-6 fatty acid deficiency. This first step determined whether the n-6, n-3 and n-9 fatty acid compositions in the tissue of the growing male rats could be affected by the nutritional status of their mothers, and it helped to obtain a powerful deficiency model. Moreover, we studied whether an adequate intake of 0·5 en% from ALA in growing male rats could partly prevent or not growth and other markers of LA deficiency in the present model. The second step was performed using the optimised conditions of deficiency, in order to determine the dietary LA requirement in the presence of ALA in growing male rats.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich, and solvents used in lipid analyses were purchased from Fisher Scientific.
Ethyl linoleate and ethyl linolenate purification
First, methyl linoleate (Sigma-Aldrich) and linseed oil (commercial vegetable oil) were refluxed with an excess of NaOH (1 m in methanol) for 1 h to separate the methyl extremity from NEFA (linoleate and α-linolenate). After cooling and acidification (3 m-HCl), fatty acids were extracted twice with diethyl ether. The organic layer was washed with water, dried over Na2SO4, then filtered and the solvent was removed under reduced pressure. Fatty acids were converted to ethyl esters by refluxing with a mixture of ethanol–H2SO4 (10:1, v/v) for 2 h. After hydrolysis, ethyl esters were extracted twice with pentane. The organic layer was washed with water, dried over Na2SO4, then filtered and the solvent was removed under reduced pressure.
On the one hand, ethyl linoleate was used without further purification because of its high purity in LA (90 %). On the other hand, ethyl linolenate was purified by preparative chromatography (Spot Liquid Chromatography Flash® (Armen Instrument) with a Chromabond® column (RS 200 C18 ec, diameter 60 mm, length 150 mm; Macherey-Nagel). An elution was performed at room temperature in an isocratic manner with a constant flow (100 ml/min). Fractions were collected every minute, evaporated and analysed by GC. Fractions with more than 95 % purity were collected and pooled. This pool enriched in ethyl linolenate was rinsed with NaCl 0·9 ‰ and dried over Na2SO4.
Animals and diets
All animal experiments in the present study were performed before January 2013 and before the implementation of the new European animal experimentation guidelines. The present study was carried out in accordance with the European Union Guideline for animal care and use (Agreement number 2003/35/CEE) and the ARRIVE guidelines for animal research. For each experiment, male and female Wistar rats, commonly used in physiological and nutritional studies, were reared in an air-conditioned room (20°C) with a humidity of 50 % and illuminated for 12 h (08.00–20.00 hours).
Two steps with two no-blinded fashion different experiments were performed.
Step 1: Achievement of a specific n-6 fatty acid deficiency in growing male rats (experimental procedure shown in Fig. 1)
To obtain a specific n-6 fatty acid deficiency, we performed a first experiment: 2 weeks before conception and throughout pregnancy and lactation periods, ten female Wistar rats (age 8 weeks old) were separated into two equal groups. Of the ten rats, five consumed a semi-purified diet containing 11 en% from fat, with an adequate amount of n-6 and n-3 PUFA, i.e. 2 en% from LA and 0·5 en% from ALA, and the other five females were fed a n-6-deficient diet containing 11 en% from fat and 0 en% from LA and 0·5 en% from ALA. All the diets were based on the AIN-93G formulation( Reference Reeves, Nielsen and Fahey 25 ).

Fig. 1 Expt 1 procedure. Female rats received two diets (0 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) or 2LA/0·5ALA) during pregnancy and lactation periods, and offspring were fed, for 63 d, on three different post-weaning diets (0LA/0ALA, 0LA/0·5ALA and 2LA/0·5ALA).
Then, the experimented male weanling (age 21 d old) rats were housed two per cages into six different experimental groups for 63 d (n 6 per group). For studying whether an adequate intake of 0·5 en% from ALA could partly prevent or not growth and other markers of LA deficiency, rats from each group were randomised to create three subgroups, either (1) 0 en% from LA, 0 en% from ALA (0LA/0ALA), (2) 0LA/0·5ALA or (3) 2LA/0·5ALA. All the diets had the same macronutrient and micronutrient content and contained 50 g of lipid/kg diet (Table 1). Totally hydrogenated sunflower oil was the sole source of dietary lipids in the 0LA/0ALA diet, and contained a residual trace of LA ( < 0·1 % of total fatty acids) and traces of ALA ( < 0·1 % of total fatty acids). Different blends of hydrogenated sunflower oil, ethyl linoleate (containing 95 % LA) and ethyl linolenate (containing 90·5 % ALA) were used to supply the intended contents of LA and ALA (Table 1).
Table 1 Fatty acid components and composition of the diets* determined during the first experiment
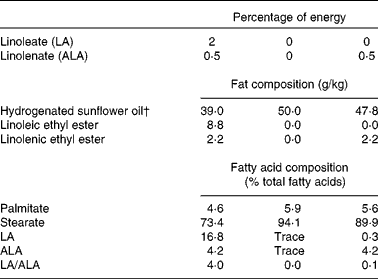
LA, linoleic acid; ALA, α-linolenic acid.
* The experimental diets were composed of the following ingredients: 50 g/kg lipids; 200 g/kg casein; 2 g/kg dl-methionine; 1 g/kg cellulose; 444 g/kg starch; 243 g/kg saccharose; 10 g/kg vitamin mixture; 36 g/kg mineral mixture. The energy density was 16 878 kJ/kg (4034 kcal/kg).
† The main fatty acids of this oil were as follows (% of total fatty acids): 12 : 0 (0·3); 14 : 0 (0·2); 16 : 0 (6·5); 18 : 0 (92); 20 : 0 (0·4); 22 : 0 (0·6).
Step 2: Estimation of linoleic acid requirement in the presence of α-linolenic acid in growing male rats (experimental procedure shown in Fig. 2)
To estimate precisely the LA requirement, we performed a second experiment: 2 weeks before mating, five female Wistar rats (age 8 weeks old) were fed with the n-6-deficient diet (0LA/0·5ALA) already described in the first experiment. They all consumed this diet during pregnancy and lactation periods. The experimented male weanling (age 21 d old) rats were randomly chosen and housed two per cages into experimental groups (n 6 per group). For the next 98 d, rats consumed either (1) 0LA/0ALA, (2) 0·5 en% from ALA plus increasing levels of LA (0·5, 1 and 1·5 en% from LA (0·5LA/0·5ALA; 1LA/0·5ALA; 1·5LA/0·5ALA)), (3) 1 en% from ALA plus increasing levels of LA (0·5 and 1 en% from LA (0·5LA/1ALA; 1LA/1ALA)). All the diets had the same macronutrient and micronutrient content and contained 50 g of lipid/kg diet (Table 2). As already described above, total hydrogenated sunflower oil was the sole source of dietary lipids in the 0LA/0ALA diet and contained a residual trace of LA ( < 0·1 % of total fatty acid) and traces of ALA ( < 0·1 % of total fatty acid). Different blends of hydrogenated sunflower oil, ethyl linoleate (containing 95 % LA), ethyl linolenate (containing 90·5 % ALA) were used to supply the intended contents of LA and ALA (Table 2).

Fig. 2 Expt 2 procedure. Female rats received 0 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet during pregnancy and lactation periods, and offspring were fed, for 98 d, on six different post-weaning diets (0LA/0ALA, 0·5LA/0·5ALA, 1LA/0·5ALA, 1·5LA/0·5ALA, 0·5LA/1ALA and 1LA/1LA).
Table 2 Fatty acid components and composition of the diets* determined during the second experiment
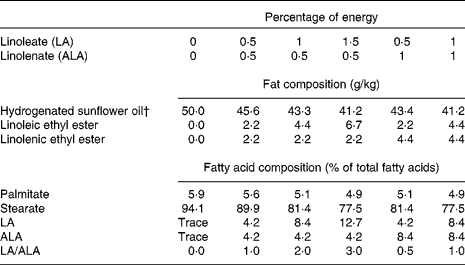
LA, linoleic acid; ALA, α-linolenic acid.
* The experimental diets were composed of the following ingredients: 55 g/kg lipids; 200 g/kg casein; 2 g/kg dl-methionine; 1 g/kg cellulose; 444 g/kg starch; 243 g/kg saccharose; 10 g/kg vitamin mixture; 36 g/kg mineral mixture. The energy density was 16 878 kJ/kg (4034 kcal/kg).
† The main fatty acids of this oil were as follows (% of total fatty acids): 12 : 0 (0·3); 14 : 0 (0·2); 16 : 0 (6·5); 18 : 0 (92); 20 : 0 (0·4); 22 : 0 (0·6).
Diets were prepared for 1 week at a time and stored at room temperature in the dark. Rats were weighed three times a week, and their food intake was recorded every day to determine the total food intake per cage. At the end of the fatty acid balance period, all the rats were killed by intracardiac punction after an overnight fast. Samples of adipose tissue (epididymal fat pad), brain and liver were dissected, weighted, freeze-dried in liquid N2 and stored at − 80°C. Plasma was obtained by centrifugation of blood anti-coagulated with heparin.
Fatty acid extraction
Aliquots of total lipids of various organs of each animal were extracted into dimethoxymethane–methanol (4:1, v/v) and plasma with hexane–isopropanol (3:2, v/v) for 30 min( Reference Blanchard, Pedrono and Boulier-Monthean 26 ). Margaric acid (100 μg) was added as an internal standard. After centrifugation (15 min, 800 g ), supernatants were collected. Total lipid extracts were saponified with 1 ml of 0·5 m-NaOH in methanol for 30 min at 70°C and methylated with 1 ml of BF3 (14 % w/v in methanol) for 15 min at 70°C. Fatty acid methyl esters were extracted twice with pentane and analysed by GC using an Agilent Technologies 6890N gas chromatograph (Bios Analytic) with a split injector (260°C, 10:1, injection volume 2 μl); a bonded silica capillary column (BPX 70, 60 m × 0·25 mm; 0·25 μm film thickness; SGE) and a flame ionisation detector (260°C, air 450 ml/min, hydrogen 40 ml/min). Helium was used as a carrier gas (constant flow 1·5 ml/min, average velocity 24 cm/s). The column temperature programme started at 150°C, increased by 1·3°C/min to 220°C and held at 220°C for 10 min.
Identification of fatty acid methyl esters was based on retention times obtained for fatty acid methyl esters and prepared from fatty acid standards. The area under the peaks was determined using ChemStation software (Agilent), and results were expressed as % of total fatty acids or mg/g of tissue after calculation with reference to the internal standard.
Plasma phospholipids were obtained by TLC. Lipid species were separated with hexane–ether–acetic acid (85:15:1) and determined with Primuline (malvidin-3-O-galactoside; Fisher Scientific) 5 % in acetone–H2O (80:20). Fatty acid of phospholipids were extracted in the same way as fatty acid methyl esters and analysed as above.
Gross symptoms
The feet, tail and hair coat of each experimented growing male rat were visually inspected three times per week for dermal symptoms associated with essential fatty acid deficiency depending on the associated diet.
Tail scaliness was scored as follows: (1) 0: the tail does not present any mark; (2) 0·5: first mark of scaliness; (3) 1: several marks of scaliness; (4) 2: several and severe marks of scaliness.
Statistical analysis
All data are presented as means and standard deviations (n 6 per group). Statistical differences between dietary groups were tested using one-way ANOVA followed by comparative mean or associated F-tests. The statistical analysis was performed using the open-source R software (http://www.r-project.org/). Differences were considered significant when P-value < 0·05.
The sample size (n) was estimated using the following equation:
where n is the effect sample size, Z is the z score, α is the probability of a type I error occurring at 5 %, β is the probability of a type II error occurring at 20 %, r is the standard deviation and Δ is the minimal difference between the closest groups (e.g. the 1·5LA and 1·0LA diets). Thus, for Z= 1·96, α = 0·05, Z= 0·84, β = 0·20, expected deviation of r 15 and Δ = 20, the effect sample size needed to detect a significant main effect between groups is at least n 5 rats per group. To ensure the present results, increase the power of our statistical test and in accordance with animal care committee, we decided to use six animals per group.
Results
The response of growing male rats to a total essential fatty acid deficiency (linoleic acid and α-linolenic acid) can be modified by the nutritional status of their mothers
We first focused on young rats fed the 0LA/0ALA post-weaning diet from mother rats fed 2LA/0·5ALA or 0LA/0·5ALA diet obtained from the first step (see experimental procedure shown in Fig. 1).
Physiological parameters
The growth curves of rats from mother rats fed on a 0LA/0·5ALA diet showed a significant loss of weight gain compared with the pups from mothers fed on a 2LA/0·5ALA diet (Fig. 3(A)). At the end of 63 d of the 0LA/0ALA post-weaning diet, rats born from the 0LA/0·5ALA diet fed mother group had significantly gained 10 % less than rats born from the 2LA/0·5ALA diet fed mother group. Both groups developed scaliness on the tail (Fig. 3(B)).

Fig. 3 Effects of maternal diet on the offspring deficiency (2 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet v. 0LA/0·5ALA diet). Results presented are obtained from post-weaning male rats fed on the 0LA/0ALA diet. (A) Body weight gains of offspring. (- - -), 2LA/0·5ALA; (-·-·-), 0LA/0·5ALA. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the 2LA/0·5ALA diet-fed group (P< 0·05; repeated-measures ANOVA). Final body weight gains (% of weaning weight) are reported inside the figure. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the 2LA/0·5ALA diet-fed group (P< 0·05; ANOVA). (B) Scaliness on the tail after 63 d of feeding. Scaly marks are indicated with red arrows. (C) 20 : 3n-9 plasma percentage after 63 d of feeding (% of total fatty acids). * Mean value was significantly different from that of the 2LA/0·5ALA diet-fed group (P< 0·05; ANOVA). (D) Plasma ratio ((20 : 3n-9/20 : 4n-6)) after 63 d of feeding. ![]() , 2LA/0·5ALA;
, 2LA/0·5ALA; ![]() , 0LA/0·5ALA. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
, 0LA/0·5ALA. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Biochemical deficiency markers (20 : 3n-9 (mead acid) and the 20 : 3n-9/20 : 4n-6 ratio)
Fig. 3(C) shows a significant increase in the percentage of 20 : 3 n-9, characterised as a key marker of essential fatty acid deficiency, 1·4-fold higher in rats receiving a 0LA/0ALA post-weaning diet and born from female rats fed on a 0LA/0·5ALA diet, compared with those born from female rats fed a 2LA/0·5ALA diet. Fig. 3(D) shows the 20 : 3n-9/20 : 4n-6 ratio used to detect essential fatty acid deficiency particularly in the plasma. For this ratio, the slight increase between the two diets is not significant. These results demonstrate that the best LA deficiency model was obtained using mother rats fed on a 0LA/0·5ALA diet.
α-Linolenic acid prevents growth loss but not dermatological marks and biochemical status in n-6-deficient growing male rats
Still taken from Expt 1, the following results only concern the rats obtained from mothers fed on the 0LA/0·5ALA diet.
Physiological parameters
No significant difference in the total food intake over the 63-d study period among all the groups was observed (data not shown). Fig. 4 shows the comparison of growth curves of rats receiving different post-weaning diets: 0LA/0ALA; 0LA/0·5ALA; 2LA/0·5ALA. Rats consuming the 0LA/0ALA post-weaning diet exhibited the lowest weight gain from the beginning to the end of the study period (63 d). The final weight gain was significantly different with 6·5 % less than the rats fed on the 2LA/0·5ALA diet. Interestingly, the 0LA/0·5ALA post-weaning diet group did not significantly decrease the weight gain of rats consuming a 2LA/0·5ALA post-weaning diet (+13 % more than the 0LA/0ALA post-weaning diet group).

Fig. 4 (A) Body-weight gains (% of weaning weight) of rats fed on the 2 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet (–-), 0LA/0ALA diet (-·-·-) or 0LA/0·5ALA diet (—) and weaned from n-6-deficient mothers. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value of the 0LA/0ALA group was significantly different from that of the other groups (P< 0·05; repeated-measures ANOVA). (B) Final body-weight gain (% of weaning weight). Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA).
Scaliness apparition (score of 2) on the tail began on day 49 of the post-weaning diet for the deficient group (0LA/0ALA) and 1 week later with a score of 1 for the 0LA/0·5ALA group, indicating that a 0·5 en% from ALA addition in the absence of LA was not sufficient to correct these symptoms (Fig. 5). At the end of the diet period, 0LA/0ALA group exhibited a score of 1·7, 0LA/0·5ALA group had a score of 0·8 and 2LA/0·5ALA group did not show any marks with a score of 0 (Table 3). Moreover, we did not see any other dermatological symptoms in the rats (i.e. hair loss or alteration of the skin structure).

Fig. 5 Scaliness observations from day 49 to day 63 (Expt 1) on rats fed on the 2 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet, 0LA/0·5ALA diet or 0LA/0·5ALA diet and weaned from n-6-deficient mothers. Scaly marks are indicated with red arrows. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Table 3 Tail scaliness score (day 63 of the diet period) determined during the first experiment (Mean values and standard deviations)

2LA/0·5ALA, 2 % energy intake of linoleic acid (LA), 0·5 % energy intake of α-linolenic acid (ALA); 0LA/0ALA, 0 % energy intake of LA, 0 % energy intake of ALA; 0LA/0·5ALA, 0 % energy intake of LA, 0·5 % energy intake of ALA.
* Mean value was significantly different (P< 0·05; ANOVA).
Biochemical deficiency markers (20 : 3n-9 and the 20 : 3n-9/20 : 4n-6 ratio)
Fig. 6 depicts both the percentage of the 20 : 3n-9 and the ratio in the liver, plasma and brain total lipids, respectively. The SFA and MUFA percentages were not changed.

Fig. 6 20 : 3n-9 percentage (% of total fatty acid) and ratio (20 : 3n-9/20 : 4n-6) in rats fed 2 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet, 0LA/0ALA diet or 0LA/0·5ALA diet and weaned from n-6-deficient mothers (Expt 1). 20 : 3n-9 liver percentage (A), 20 : 3n-9 plasma percentage (B) and 20 : 3n-9 brain percentage (C). Ratio (20 : 3n-9/20 : 4n-6) in the liver (D), ratio (20 : 3n-9/20 : 4n-6) in the plasma (E) and ratio (20 : 3n-9/20 : 4n-6) in the brain (F). Values are means (n 6), and standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; ANOVA).
In the liver, rats consuming the 0LA/0ALA diet had a significantly higher percentage of 20 : 3n-9 than those consuming the 2LA/0·5ALA diet; however, supplementation with ALA had no effect (Fig. 6(A)).
In comparison to the 2LA/0·5ALA group, rats in the 0LA/0ALA group had higher and more significant 20 : 3n-9 percentage in the plasma (1·3 v. 7·1 %, P< 0·05; Fig. 6(B)). The 0LA/0·5ALA diet induced a significant decrease in the percentage of 20 : 3n-9 compared with the 0LA/0ALA diet. In the brain, similar effects were observed (Fig. 6(C)).
Concerning the 20 : 3n-9/20 : 4n-6 ratio, liver, plasma and brain exhibited the same profile. As described in Fig. 6(D)–(F), there was a significant increase in the ratio between 2LA/0·5ALA and 0LA/0ALA groups and no difference between the 0LA/0ALA and 0LA/0·5ALA groups.
We then performed a second experiment with optimised conditions of LA deficiency, in order to determine the LA requirement in the presence of ALA in growing male rats (Step 2).
Dietary linoleic acid requirement appears to be lower than the first historically determined value in growing male rats
Physiological parameters
During the second experiment (step 2, see experimental procedure shown in Fig. 2), no significant difference in the total food intake was detected over the 14 weeks of study period among all the groups (data not shown). Fig. 7 depicts the growth curves for each diet group. Rats consuming the 0LA/0ALA diet had lower weight gain after being fed for 98 d on this diet, and kept their body weight gain lower until the end of the diet. When analysing variance, the growth data for LA-supplemented groups and 1 en% from ALA groups presented no significant difference either during the experimentation or at the end of the diet. The final body-weight gain diminished by 10 % for the 0LA/0ALA group compared with all the other diet groups.

Fig. 7 (A) Body-weight gains (% of weaning weight) of rats fed the 0 % of energy intake (en%) from linoleic acid (LA)/0 α-linolenic acid (ALA) diet (-·-·-), 0·5LA/0·5ALA diet (![]() ), 1LA/0·5ALA diet (
), 1LA/0·5ALA diet (![]() ), 1·5LA/0·5ALA diet (–-), 0·5LA/1ALA diet (
), 1·5LA/0·5ALA diet (–-), 0·5LA/1ALA diet (![]() ), 1LA/1ALA diet (—) (Expt 2). Values are means (n 6), with standard deviations represented by vertical bars. * Mean value of the 0LA/0ALA group was significantly different from that of the other groups (P< 0·05; repeated-measures ANOVA). (B) Final body-weight gain (% of weaning weight). Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA).
), 1LA/1ALA diet (—) (Expt 2). Values are means (n 6), with standard deviations represented by vertical bars. * Mean value of the 0LA/0ALA group was significantly different from that of the other groups (P< 0·05; repeated-measures ANOVA). (B) Final body-weight gain (% of weaning weight). Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; ANOVA).
Concerning the marks of scaliness, Fig. 8 shows that the 0·5LA/0·5ALA diet was not sufficient to correct the symptoms observed in the 0LA/0ALA diet (score of 1 at the end of the dietary period). It is important to note that when LA was increased up to 1 en% with 0·5 en% from ALA, scaly marks were corrected. Interestingly, it was not the case with an increase in ALA to 1 en% and with LA kept at 0·5 en%, which were scored 1 at the end of the diet. Nevertheless, in this latter case, marks appeared later with an increase in the scaliness score from 0 to 1 (after 98 d on the diet) (Fig. 8). The different scores are summarised in Table 4.

Fig. 8 Scaliness on the tail from day 77 to day 98 (Expt 2). Scaly marks are indicated with red arrows. The 1·5 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) group is similar to 1LA/0·5ALA group (data not shown). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Table 4 Tail scaliness score (day 98 of the diet period) during the second experiment (Mean values and standard deviations)

0LA/0ALA, 0 % energy intake of linoleic acid (LA), 0 % energy intake of α-linolenic acid (ALA); 0·5LA/0·5ALA, 0·5 % energy intake of LA, 0·5 % energy intake of ALA; 0·5LA/1ALA, 0·5 % energy intake of LA, 1 % energy intake of ALA; 1LA/0·5ALA, 1 % energy intake of LA, 0·5 % energy intake of ALA; 1LA/1ALA, 1 % energy intake of LA, 1 % energy intake of ALA; 1·5LA/0·5ALA, 1·5 % energy intake of LA, 0·5 % energy intake of ALA.
* Mean value was significantly different (P< 0·05; ANOVA).
Finally, none of the diets resulted in any hair loss or alterations of skin structure (except the tail) as assessed by inspections during the 14 weeks of experimentation.
Biochemical deficiency markers (20 : 3n-9 and the 20 : 3n-9/20 : 4n-6 ratio)
During the second experiment (step 2), in the liver, the important decrease in 20 : 3n-9 was attenuated between the 0LA/0ALA diet and the other diets (5·6 v. 3 % in mean; P< 0·05) compared with the plasma situation. However, 20 : 3n-9 decreased significantly, by 88 % in the 1LA/0·5ALA and 1·5LA/0·5ALA diets compared with the deficient diet (Fig. 9(A)). Then, we analysed the phospholipid plasma fraction and found an accumulation of 20 : 3n-9 and a dose-progressive significant decrease observed with the 0·5LA/0·5ALA, 1LA/0·5ALA and 1·5LA/0·5ALA diets (Fig. 9(B)). In the brain, the percentage of 20 : 3n-9 was diminished with the increase in LA. The 0LA/0ALA diet exhibits 1·7 % of 20 : 3n-9 compared with 0·3 % in the 1·5LA/0·5ALA diet (Fig. 9(C)).

Fig. 9 20 : 3n-9 percentage (% of total fatty acid) and ratio (20 : 3n-9/20 : 4n-6) in rats fed the 2 % of energy intake (en%) from linoleic acid (LA)/0·5 α-linolenic acid (ALA) diet, 0LA/0ALA diet or 0LA/0·5ALA diet and weaned from n-6-deficient mothers. (Expt 2). 20 : 3n-9 liver percentage (A), 20 : 3n-9 plasma percentage (B) and 20 : 3n-9 brain percentage (C). Ratio (20 : 3n-9/20 : 4n-6) in the liver (D), ratio (20 : 3n-9/20 : 4n-6) in the plasma (E) and ratio (20 : 3n-9/20 : 4n-6) in the brain (F). Values are means (n 6), and standard deviations represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05; ANOVA).
The 20 : 3n-9/20 : 4n-6 ratio in the 0LA/0ALA diet was significantly higher in the liver (Fig. 9(D)), the plasma phospholipids (Fig. 9(E)) and the brain (Fig. 9(F)) than in the other diets. More precisely, in plasma phospholipids, Fig. 9(E) shows a decrease by 40 and 67 % between the deficient group and 0·5LA/0·5ALA and 1LA/0·5ALA, respectively (P< 0·05). Moreover, an increase in dietary ALA concomitant or not with an increase in dietary LA did not modify the ratio.
In the liver (Fig. 9(D)) and in the brain (Fig. 9(F)), a dose-dependent effect of increasing LA intake from 0 to 1·5 en% was also noted on the ratio with an attenuated effect in the brain. We also noticed that rats fed the 1LA/0·5ALA diet exhibited a ratio as low as the one obtained with the 1·5LA/0·5ALA diet in the brain.
Discussion
Although LA is described as an essential fatty acid in the literature, the deficiency symptoms (reduction of growth and scaliness)( Reference Burr and Burr 5 – Reference Aaes-Jorgensen, Leppik and Hayes 7 , Reference Holman 9 ) could not be completely and specifically attributed to a sole LA deficiency since the conditions in the previous studies included both LA and ALA deficiency in lipidoprive conditions. The present study aimed at evaluating the minimal dietary LA requirement to avoid deficiency symptoms. The starting point of the present study was to create a strong enough and specific n-6 deficiency to observe these deficiency symptoms and correct them with an optimal and non-excessive content of LA. To our knowledge, our work is the first study that is designed to observe specific physiological and biochemical signs of an LA deficiency with a high consideration of the ALA amount in the diet, leading to propose a new requirement range of 1–1·5 en% from LA.
To do so, we used groups of growing male rats receiving a 0LA/0ALA post-weaning diet and born from female rats fed on a 0LA/0·5ALA diet. The weight gain of growing male rats was 10 % lower than that of rats receiving the same post-weaning diet but born from mothers fed on a 2LA/0·5ALA diet. Both growing male rat groups developed scaliness on their tail; however, the percentage of 20 : 3n-9 was significantly higher in totally deficient rats from mothers fed on the 0LA/0·5ALA diet. Because LA is the most abundant PUFA in the diet( Reference Whelan 16 ), some LA from the maternal adipose tissue fat content could be transferred to their descendants during pregnancy and lactation periods. The present results are relevant to a recent study showing the effects of maternal LA-enriched diets on the LA content of offspring liver and plasma phospholipids( Reference Hoile, Irvine and Kelsall 27 ). In our first experiment, we obtained a deficiency similar to that in Burr studies( Reference Burr and Burr 5 , Reference Burr and Burr 6 ) using rats descending from females fed LA-deficient diets during pregnancy and lactation periods.
The second important result obtained from this first experiment was the correction of the growth deficit once ALA had been introduced without LA in the diet of the growing male rats. The present results are relevant to the literature: previous studies have sometimes suggested that a low intake of ALA could correct a part of the LA deficiency symptoms: (1) growth( Reference Guesnet, Lallemand and Alessandri 23 ); (2) skin scaliness( Reference Cunnane and Anderson 28 ); (3) fatty acid changes such as the high level of 20 : 3n-9 content( Reference Greenberg and Deuel 14 , Reference Bourre, Piciotti and Dumont 15 , Reference Cunnane and Anderson 28 ). More recently, Guesnet et al. ( Reference Guesnet, Lallemand and Alessandri 23 ) published a dose–response study in a rat model showing that 0·5 en% from ALA could be an adequate intake to diminish only the deficit of growth among the symptoms of LA deficiency. Considering the correction of other symptoms, it cannot be compared with Guesnet et al.'s study( Reference Guesnet, Lallemand and Alessandri 23 ) since the authors did not obtain marks of scaliness using their totally essential fatty acid-deficient diet. That may be explained by the design of the experimentation, which associated a shorter diet period in their work (7 weeks compared with the present study), preceded by an acclimatisation period of 2 weeks during which rats consumed a 2LA/0·5ALA diet. In this case, the incorporation of LA during the early age of the rats reduced the chance of creating a maximal LA deficiency. It thus appears logical that Guesnet et al.'s results( Reference Guesnet, Lallemand and Alessandri 23 ) on LA requirements were 2-fold lower compared with the results proposed in the present study. This hypothesis is supported by similar results previously reported in growing rats fed on 0·2 en% from LA intake for a longer period, i.e. 12–15 weeks( Reference Igarashi, Gao and Kim 29 ). In the present study, the methodological novelty was to design a specific and optimised experiment to obtain all of the physiological LA deficiency symptoms including marks of scaliness. Moreover, the present results suggest for the first time that the skin symptoms remain specifically associated with LA deficiency since we demonstrated that ALA does not cure the dermatological signs during a LA deficiency. Interestingly, ALA may delay the onset of scaly marks. It is known that ALA shares several functions with LA notably structural (cell membrane composition)( Reference Ikeda, Kihara and Igarashi 30 , Reference Stubbs and Smith 31 ) and physiological (growth) functions( Reference Guesnet, Lallemand and Alessandri 23 ). The present results also suggest a slight ALA sparing probably due to LA oxidation, explaining the indirect ALA impact on dermal signs. Consequently, the present results suggest that the determination of the requirements for LA cannot be established in the absence of the ALA and vice versa.
The second part of the present study was performed to determine the requirement of LA in the presence of ALA in the growing male rats. The 0LA/0ALA group again exhibited a reduction of growth, whereas increasing dietary LA concomitant with 0·5 en% from ALA did not alter body growth. It should be noted that the growth of the two groups fed on 1 en% from ALA were not significantly increased compared with rats fed on 0·5 en% from ALA. Scaliness was not cured with 0·5 en% from LA concomitant with both 0·5 and 1 en% from ALA. However, skin alterations were not present with the 1LA/0·5ALA diet. We could put forward the hypothesis that 0·5 en% dietary intake of LA with ALA (0·5 or 1 en%) is not enough to correct all of the physiological criteria entirely, especially scaliness (reaching a score of 0). Moreover, in the present study, we did not study all the characteristic symptoms of the n-6 deficiency reviewed by Holman( Reference Holman 9 ) (fertility, sterility and perinatal mortality). The physiological symptoms were accompanied by characteristically altered plasma or tissue 20 : 3n-9 percentage and the 20 : 3n-9/20 : 4n-6 ratio. Both of them progressively decreased in each organ when increasing dietary LA in the diets (Fig. 9). If an increase in 20 : 3n-9 is admitted as a marker of essential fatty acid deficiency, the plasmatic 20 : 3n-9/20 : 4n-6 ratio remains more controversial( Reference Chu and Hegsted 24 ). Indeed, Chu & Hegsted( Reference Chu and Hegsted 24 ) suggested that LA deficiency is characterised when 20 : 3n-9/20 : 4n-6 ratio is lower than 0·4 in plasma phospholipids. However, this ratio has been established without ALA in the diet, suggesting that this ratio might be reassessed as well. Comparing the two groups of rats fed on either 0·5LA/0·5ALA or 0·5LA/1ALA diet, the 20 : 3n-9/20 : 4n-6 ratio is high and similar for both diets with 0·5 en% from LA. Moreover, we obtained a lower and common ratio value (0·6) with the two other diets containing both 1 en% from LA (concomitant with 0·5 en% or 1 en% from ALA). This strongly suggests that this ratio varies more specifically with the LA values in the diet than with the ALA or the total PUFA value. Thus, we now provide a better justification for using the 20 : 3n-9/20 : 4n-6 ratio concerning precisely and specifically the n-6 fatty acid deficiency. However, the present results also showed that in the presence of ALA, the decrease in 20 : 4n-6 percentage results in an artificial increase in the ratio, showing that the old threshold value of 0·4 proposed by Chu & Hegsted( Reference Chu and Hegsted 24 ) is not relevant anymore.
So, to conclude this part of the present study, it appears that the two diets supplying 1 or 1·5 en% from LA deeply decreased the powerful 20 : 3n-9 marker and secondarily the 20 : 3n-9/20 : 4n-6 ratio in plasma phospholipids. Except the major scaliness signs, classification of the different criteria could be proposed by a comparison of the P values of the different effects. This statistic classification establishment (data not shown) puts on top 20 : 3n-9, then 20 : 3n-9/20 : 4n-6 ratio and finally the body weight gain. However, we have to keep in mind that curing the physiological LA deficiency symptoms such as scaliness signs and growth deficit remain the major importance and prevent from simple classification. Therefore, altogether, the present results suggest that the dietary LA requirement in the present study using growing male rats is between 1 and 1·5 en% in the presence of 0·5 en% from ALA (Table 5). These results might be refined using growing female rat to consider the oestrogen activation of the n-3 conversion related in few studies( Reference Burdge 32 , Reference Childs, Romeu-Nadal and Burdge 33 ). However, Pudelkewic et al. ( Reference Pudelkewicz, Seufert and Holman 34 ) did not show any difference between male and female fatty acid incorporation and utilisation.
Table 5 Summary of the different diet effects on physiological and biochemical criteria
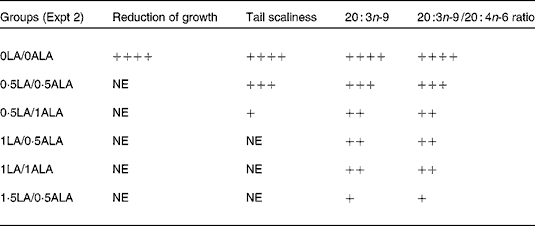
0LA/0ALA, 0 % energy intake of linoleic acid (LA), 0 % energy intake of α-linolenic acid (ALA); 0·5LA/0·5ALA, 0·5 % energy intake of LA, 0·5 % energy intake of ALA; 0·5LA/1ALA, 0·5 % energy intake of LA, 1 % energy intake of ALA; 1LA/0·5ALA, 1 % energy intake of LA, 0·5 % energy intake of ALA; 1LA/1ALA, 1 % energy intake of LA, 1 % energy intake of ALA; 1·5LA/0·5ALA, 1·5 % energy intake of LA, 0·5 % energy intake of ALA; +, proportionally indicates an effect; NE, no effect.
This update of the LA requirement for growing rats appears logically lower than the overestimated historic 2 en% value, and represents notably almost half of this established without ALA. The present study demonstrates and confirms that the absence of ALA in the diet during the first requirement experiments carried out by Holman( Reference Holman 9 ) has corrupted their results for LA. In other words, Holman study( Reference Holman 9 ) and the other studies( Reference Cunnane and Guesnet 35 ) that have tried to determine the LA requirement in healthy adults used exclusively LA to correct in fact an n-6 plus n-3 fatty acid deficiency. When considering now the study by Guesnet et al. ( Reference Guesnet, Lallemand and Alessandri 23 ), our requirement proposal appears this time 2-fold higher than their proposed values, due to their experimental conditions discussed above. Finally, our LA requirement proposed values were obtained using an optimised design and were intermediate between Holman's overestimation( Reference Holman 12 ) and Guesnet et al.'s underestimation( Reference Guesnet, Lallemand and Alessandri 23 ).
Here, the 1 en% value obtain in our rat model is not completely applicable to human nutrition. However, it has to be considered for reasoning new approaches and guidelines for LA in humans since the historic value for rats itself has been obviously used for humans to avoid LA deficiency, until now. Furthermore, the present study could help to complete the few studies carried out to determine the LA requirements in infancy (reviewed in Cuthbertson( Reference Cuthbertson 36 )) using milk formulas that provided no measurable intake of n-3 fatty acids. However, these important studies in infants could not be used to provide nutrient requirements for the adults. We are aware that beyond the necessity to redefine the dietary requirement of LA, many questions concerning the consequences of its excessive consumption on human health arise. Indeed, in human health, the precautionary principle has to be applied since the LA requirement was already over-evaluated. Although many authors claim that this minimal requirement could be overtaken without risk for health (The American Heart Association for example)( Reference Harris, Mozaffarian and Rimm 37 ), the literature points out the deleterious effects of a LA excess and of a high LA:ALA ratio as well. From this point of view, this redefined LA minimal requirement value between 1 and 1·5 en% could be considered as a good basis to discuss a benefit/risk compromise if an increase in ALA is supported. It is, thus, important to suggest and initiate researches to determine the optimal and relative (ratio LA:ALA) values considered as deleterious threshold.
Indeed, in vitro LA studies had already demonstrated the inflammatory properties of both LA oxidised metabolites and 20 : 4n-6 derivatives (i.e. PG and thromboxane)( Reference Ramsden, Ringel and Feldstein 38 , Reference Tilley, Coffman and Koller 39 ). In human studies, a first meta-analysis led by Ramsden et al. ( Reference Ramsden, Hibbeln and Majchrzak 40 ), including results from Oslo Diet Heart and Lyon Diet Heart Studies, detailed the specific n-6 PUFA effects in increasing CVD. Moreover, a very recent meta-analysis has completed the previous cited meta-analysis results and put forward the hypothesis that an excess of LA (by extension the n-6 fatty acid family) could play a non-negligible role in CHD. Actually this new approach of Sydney Diet Heart Study coupled with the use of a modern statistical analysis showed a higher total mortality in the group receiving the high LA diet. Moreover, the present study highlighted the increased risks of death from CHD and CVD with an increase in LA in the diet( Reference Ramsden, Zamora and Leelarthaepin 41 ). Obviously, more studies are needed to confirm these previous results.
In summary, we demonstrated that dietary intake of 1–1·5 en% from LA concomitant with an intake of 0·5 en% from ALA could be the appropriated requirement for LA, since it corrected the n-6 fatty acid deficiency in rats, using optimised design compared with the historic LA 2 en% value or the range of 0·5–1 en% values proposed by Holman( Reference Holman 12 ) and Guesnet et al. ( Reference Guesnet, Lallemand and Alessandri 23 ), respectively. The present results in the rat point out the actual overestimation of the physiological LA requirement, and this suggests that the requirements in humans should be revisited considering the presence of dietary ALA to set up the recommendation for LA and thus avoid excess LA since the literature also points out its deleterious effects.
Acknowledgements
The authors thank N. Monthean, F. Boissel, R. Marion and H. Ould Hamouda for animal care and technical assistance. They also thank Jane Wilson and Kevin Soulard for their English revision of the manuscript.
The present study received financial support from SIA (Lesieur)/SOFIPROTEOL (Paris, France).
None of the authors has any conflict of interest to declare.
The authors' contributions are as follows: B. C. and D. C. performed the experiments; B. C. wrote the manuscript; P. L., P. G. and B. D. reviewed the manuscript. All authors involved in the study design.

















