Current scientific evidence suggests a protective role for fruit and vegetables (FV) in the prevention of chronic degenerative diseases, such as CVD and cancerReference Van Duyn and Pivonka1. However, it is not yet clear what component or combination of components in FV is protective and what is the mechanism of action.
Among phytochemicals potentially responsible for beneficial effects on human health, salicylic acid (SA) is very interesting. SA is a phenolic compound present in plants, where it plays a central role in the development of local and systemic resistance to pathogen infectionReference Dempsey and Klessing2, Reference Dangl3. It is found in varying quantities in all fruits, vegetables, herbs and spices, but it is better known as the principal metabolite and active component of aspirin, an anti-inflammatory drug introduced into clinical practice more than 100 years agoReference Stanley and Hegedus4. It has been recently accepted that the chronic consumption of low-dose aspirin is an effective agent for the prevention of CVD and colorectal cancerReference Marcus5–Reference Gasche8. This supports the hypothesis that the beneficial effects in man attributed to FV habitual consumption could depend, at least in part, on the supply of SA.
To evaluate this hypothesis it is necessary to prove that human subjects not taking aspirin display circulating SA whose concentration is related to the consumption of FV, and to prove that the SA is present at concentrations capable of exerting biological effects. It was reported that in Scottish groups of the general population and vegetarian Buddhist monks, the vegetarians had higher circulating SA than the non-vegetarians, overlapping with those of subjects taking low-dose aspirin (75 mg/d)Reference Blacklock, Lawrence, Wiles, Malcolm, Gibson, Kelly and Paterson9. Beyond the study of Blacklock et al., data on the relationship between circulating SA and dietary habits are absent.
The aim of the present study was therefore to evaluate the concentrations of circulating SA in a group of subjects whose dietary habits and, specifically, FV intake were characterized. To this purpose, we developed an analytical method specific and sensitive enough to detect very low SA concentrations in human fluids, which has been reported separatelyReference Battezzati, Fiorillo, Spadafranca, Bertoli and Testolin10.
Subjects and methods
Subjects
Thirty-eight healthy subjects (twenty-two males and sixteen females; mean age 21·8 (sd 1·9) years; mean weight 64·7 (sd 11·5) kg; mean BMI 22·3 (sd 2·6)) were recruited from an Italian university campus. Exclusion criteria comprised diseases or serious medical condition, chronic or acute therapies with anti-inflammatory drugs and special diets (vegan, vegetarian or macrobiotic diet, gluten-free diet). Approval was obtained by the Institutional Ethical Committee and an informed consent was signed by all subjects.
Daily dietary record
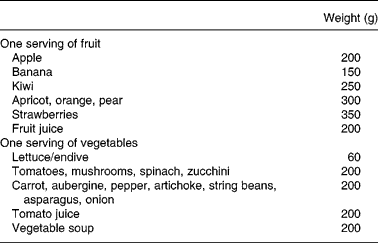
All subjects recorded their intake for seven consecutive days (from Monday to Sunday) including food and beverages consumed at home and outside as previously describedReference Bertoli, Battezzati, Merati, Margonato, Maggioni, Testolin and Veicsteinas11. Volunteers were trained by a dietitian in food-recording procedures. A list with different servings of common foods was used to assist in checking the estimated amounts of consumed foods. In particular, FV intake was indicated by volunteers as piece or weight and subsequently quantified by us as servings according to Table 1. Vegetable intake excluded potato and legume consumption because there are no reports that these are valuable SA sourcesReference Swain, Dutton and Truswell12, Reference Venema, Hollman, Janssen and Katan13. We categorized in quartiles the servings of FV consumed on the last day (seventh day). Energy and nutrient daily intakes were analysed using commercial software (Dieta Ragionata; Esi Stampa Medica srl, Milan, Italy) containing the food composition database of the Italian National Institute of Nutrition. Total energy intake (kJ) and intake of macronutrients (proteins, lipids and carbohydrates) were calculated and compared to the Italian Recommended Dietary Allowances14.
Table 1 Evaluation of fruit and vegetable (FV) intake as servings for FV consumed during the experimental period
Blood sampling
On the morning of the eighth day, a fasting blood sample was obtained from each subject. Serum aliquots were prepared and immediately stored at − 30°C for subsequent analysis.
Serum salicylic acid determination
SA serum concentration was measured using a specific and sensitive stable isotope dilution and GC–MS method as previously describedReference Battezzati, Fiorillo, Spadafranca, Bertoli and Testolin10.
Statistical analysis
Daily energy and macronutrient intakes were expressed as means and standard deviations. FV intake and serum SA concentrations were expressed as means and standard deviations and, where indicated, as median, minimum and maximum values. Pearson correlation coefficient analysis was used to examine the relationship of two variables.
Quartiles of FV intake of the seventh day were indicated by median values and range of the intake of FV servings. SA serum concentrations were compared between the extreme FV quartiles using an unpaired t test. The significance level for all statistical analyses was defined as P < 0·05. Statistical analyses were two-sided and performed using Stata Statistical Software, version 8.0 (Stata Corporation, College Station, TX, USA).
Results
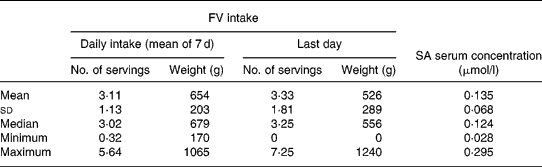
Two subjects were excluded from the study because they did not fill in their dietary diary completely. Thus the final sample comprised thirty-six individuals (twenty males and sixteen females; mean age 21·8 (sd 1·9) years; mean weight 64·5 (sd 11·5) kg; mean BMI 22·2 (sd 2·7)). Energy and macronutrient daily intakes are shown in Table 2. FV intake (mean daily intake of 7 d and intake of the last day) and SA serum concentrations are shown in Table 3. It should be noted that one subject did not eat FV on the last day. With correlation analysis we verified that the last day FV intake (expressed as weight) was related to the mean daily intake of the week (r 2 0·39, P < 0·05).
Table 2 Daily energy intake (EI) and macronutrient intake (n 36)
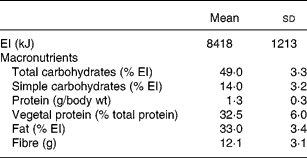
Table 3 Fruit and vegetable (FV) intake and salicylic acid (SA) serum concentration (n 36)
Relationship between servings of fruit and vegetables and circulating salicylic acid
Circulating SA was significantly related both to daily FV intake in the week (r 2 0·13, P = 0·03) and on the last day (r 2 0·16, P = 0·01) (Fig. 1).

Fig. 1 Relationship between intake of fruit and vegetables on the last (seventh) day and salicylic acid (SA) serum in thirty-six healthy subjects not taking aspirin.
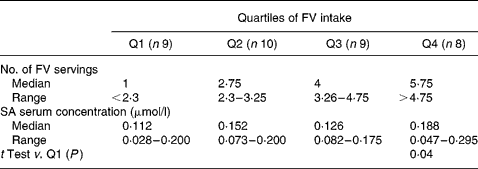
FV intakes on the last day were also categorized in quartiles and related to SA serum concentrations as shown in Table 4. The subjects in the highest FV intake quartile in the preceding day (>4·75 servings) had significantly higher SA concentrations than subjects in the lowest quartile ( < 2·3 servings).
Table 4 Quartiles of fruit and vegetable (FV) intake of the last day and salicylic acid (SA) serum concentrations
Discussion
The present study was carried out in order to evaluate the concentrations of circulating SA in a group of subjects whose dietary intake and, specifically, FV consumption were characterized by 7 d repeated dietary records.
This is the most practical approach for examining recent dietary intake and it is often regarded as the ‘gold standard’ against which other dietary assessment methods are compared15. However, dietary records have been associated with under-estimation of intakes compared to other survey methodsReference Thompson and Byers16. In the present study the reported absolute daily energy intake was about 15 % lower than the expected energy expenditure for Italian subjects with the same level of activity14. We suppose that this difference could be due to a probable simplification of reporting and to an imprecision in dietary recording. However, our main aim was to evaluate FV intake: considering the low energy density of FV it is improbable that the under-estimation of total energy intake depends on a wrong record of these foods. Moreover, the recall of FV servings is easier than the record of dishes, which are composed of different ingredients, for each of which it is often difficult to quantify the exact amount. Thus, we considered the repeated dietary records to be an acceptable method to perform in the present study.
On the basis of the ratio of macronutrient consumptions, the amount of FV servings and of the relative amount of complex carbohydrates, our subjects roughly followed a Mediterranean-type dietReference Willett, Sacks, Trichopoulou, Drescher, Ferro-Luzzi, Helsing and Trichopoulos17. Nonetheless the intake of total carbohydrates was slightly lower than recommended by LARN14, whereas fat intake was higher with a suboptimal SFA:MUFA:PUFA ratio. The protein consumption was adequate with 67 % of intake from animal source. The result confirms that the individuals were not vegetarians. Fibre intake was lower than recommended (30 g/d), but not very different from the mean intake of the Italian population (18 g/d)14. As expected, fibre intake was positively correlated with intake of complex carbohydrates (r 2 0·44, P < 0·001) and with FV intake (r 2 0·29, P < 0·001).
The mean daily FV intake resulted in agreement with recommendations of Guidelines for a Healthy Italian Nutrition18, for which it would be suitable to eat every day at least three servings. Specifically, 50 % of our experimental daily group ate more than three servings. FV intake of the seventh day was not significantly different from the mean daily intake over the week. Thus, we believe that FV intake of the seventh day is representative of the habitual intake of the subjects. Given the few hours duration of the SA half-life in serumReference Kees, Jehnich and Grobecker19, the recall of FV intake on the last day was probably the most accurate for detection of the influence of FV consumption on circulating SA concentration.
The analytical method used can detect circulating SA concentrations as low as 0·014 μmol/l. With this sensitivity, we quantified SA in all samples analysed, even when present at very low concentrations. The present results confirmed that it is possible to find circulating SA independently by preceding aspirin consumption. In addition, the most interesting result of the present study was that circulating SA in healthy subjects is related to FV consumption, proving that FV contribute to the presence of SA in vivo.
We found a large variation in the serum SA concentrations with a partial overlap among all quartiles of the last day FV intake. However, concentrations greater than 0·200 μmol/l were found only in the subjects belonging to the highest quartile of consumption. Few data in the literature are available about SA serum in subjects not taking aspirin. Blacklock et al. Reference Blacklock, Lawrence, Wiles, Malcolm, Gibson, Kelly and Paterson9, in non-vegetarian subjects, detected ranges of SA serum similar to those found in our subjects with a low intake of FV ( < 2·3 servings). The amounts of SA detected in our subjects with a high FV intake (>4·75 servings) partially overlaps that found by Blacklock et al. in vegetarian subjects. Moreover, half of our subjects in the highest consumption quartile had SA concentrations overlapping with the patients taking low-dose aspirin reported by Blacklock et al. Reference Blacklock, Lawrence, Wiles, Malcolm, Gibson, Kelly and Paterson9. The present results suggest that in the context of a Mediterranean diet rich in FV it is possible to achieve considerable SA serum concentrations, as in vegetarian and aspirin users.
Salicylates exert anti-inflammatory action in part by suppressing COX-2 induction, a key enzyme involved in inflammation and certain cancers, thereby reducing the synthesis of proinflammatory PGReference Xu, Sansores-Garcia, Chen, Matijevic-Aleksic, Du and Wu20. It is interesting that the inhibition of COX-2 transcription has been shown by Xu et al. Reference Xu, Sansores-Garcia, Chen, Matijevic-Aleksic, Du and Wu20 in human umbilical vein endothelial cells and foreskin fibroblasts at SA concentrations as low as 0·1 μmol/l, which were exceeded in the serum of 64 % of our subjects. The present results encourage further studies in order to evaluate whether circulating SA derived from a regular FV intake in the context of a Mediterranean diet could have in vivo anti-inflammatory effects such as those found in cell culture or attributed to chronic low doses of aspirinReference Ruffin, Krishnan, Rock, Normolle, Vaerten, Peters-Golden, Crowell, Kelloff, Boland and Brenner21. However, results in vivo will have to consider that the possible anti-inflammatory effects attributed to FV consumption could depend not exclusively on SA, but also by action of other FV typical compounds such as polyphenols that, modulating inflammatory signals such as p38 MAPK and NF-κB, could suppress proinflammatory cytokine productionReference Cho, Park and Kwon22, Reference Jiang and Dusting23.
The present study is the first to show positively the association between FV intake and circulating SA, however, a limitation is that the exact content of salicylates in FV consumed by the volunteers is not known. Thus we cannot exclude that the choice of specific fruits and vegetables might have affected SA serum concentrations more than the total amount of FV consumed. This could explain, at least in part, the large variability of circulating SA detected in each quartile of FV intake and the higher SA concentrations detected in quartile 2 with respect to quartile 3.
The presence of naturally occurring salicylates in fruits, vegetables, spices, confectionaries and beverages has been confirmed by several research groupsReference Venema, Hollman, Janssen and Katan13, Reference Robertson and Kermade24, but the determined amounts do not always agree. Swain et al. Reference Swain, Dutton and Truswell12 suggested that a normal mixed diet contains total salicylates in the range of 10–200 mg/d, although other groups have suggested that this may be overestimated owing to a lack of analytical specificityReference Herrmann25, Reference Janssen, Hollman, Reichman, Venema, van Staveren and Katan26.
In conclusion, the present study proved that, following a Mediterranean diet, human subjects not taking aspirin display circulating SA in amounts related to the FV consumption. It is therefore possible that the beneficial effects of regular FV consumption in man could also depend on low chronic SA exposure. To confirm this hypothesis, further studies should define better the SA content in foods and the consequent bioavailability in man. Most importantly, it will be necessary to evaluate whether SA, at the concentrations found in the present study, can exert significant biological effects capable of explaining the protective effects of FV on human health.
Acknowledgements
This work was supported by grant PRIN 2004077043 from MIUR and Università degli Studi di Milano.







