The Research Domain Criteria initiative (RDoC) is part of the National Institutes of Mental Health (NIMH) plan to provide a new framework for conceptualizing mental disorders. As such, projects funded under RDoC are intended to develop ‘new ways of classifying disorders based on dimensions of observable behaviors and brain functions’ (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010). The current study was sponsored through the first RDoC funding request (RFA-MH-12-100) and aims to provide an integrated perspective of genetic and environmental aspects of negative valence systems (NVS) in relation to internalizing disorders (IDs), which include mood and anxiety disorders. As characterized by the RDoC theoretical framework, the NVS domain encompasses biological and psychological systems involved in the response to aversive, threatening, or harmful stimuli, including systems that have a putative relationship to IDs.
Neuroscientists and psychologists have developed dimensional assessment instruments and laboratory tasks that probe cognitive, emotional, biological, and/or behavioral aspects of NVS as phenotypes for study as alternatives to ID clinical symptoms. Such intermediate or ‘endo’ phenotypes might reflect processes more proximal to gene expression than clinical symptoms while also playing an important role in the development of pathological emotional states. Gottesman and Gould (Reference Gottesman and Gould2003) suggested criteria for a putative endophenotype, including (a) association with illness, (b) heritability, and (c) co-segregation with the illness in families.
Early studies suggest that various putative endophenotypic measures might index shared or specific components of ID risk. One hypothesis is that rather than showing associations with specific diagnoses, such measures might be more likely to align with broader constructs proposed in the RDoC matrix; for example, acute threat (fear), potential threat (anxiety), and elements of negative affect (loss, anhedonia, frustrative non-reward). However, many such endophenotypic measures have been examined only in relationship to clinical diagnoses via case-control studies, rather than using more broadly informative, unselected samples. Furthermore, some progress has been made in establishing that such measures satisfy some version of criterion (a), with much less data available regarding criteria (b) and (c) (Savage et al., Reference Savage, Sawyers, Roberson-Nay and Hettema2016). Knowledge about the external predictors and underlying sources of variance of endophenotypes, as gained via epidemiologically and genetically informative studies, is still in progress. In particular, it is not clear how these constructs will map onto the genetic risk structure of IDs in developing children.
Twins are ideal for determining the differential effects of genes and environment and their shared and specific contributions across phenotypes (Kendler, Reference Kendler2001). Under RDoC's specified ‘Units of Analysis’, the Twin Study of Negative Valence Emotional Constructs, also referred to as the Virginia Commonwealth University Juvenile Anxiety Study (VCU-JAS), measures genetic risk factors, self-report, behavior, physiology, and paradigms, as well as specific childhood environmental risk factors in relation to NVS. The measures and experimental tasks were chosen for inclusion in this study in support of its six major aims:
-
1. Via subject level phenotypic analyses, estimate the response structure and underlying latent constructs of a broad suite of possible endophenotypes, including dimensional measures and psychological tasks that probe negative valence emotional states in developing children.
-
2. Examine the relationships between the higher order constructs identified in Aim 1 and internalizing symptom clusters.
-
3. Apply twin modeling approaches to determine the genetic and environmental sources of each putative endophenotypic measure as well as their composite multivariate risk structure.
-
4. Estimate the degree to which common and specific genetic factors identified in Aim 3 overlap the genetic risk structure of IDs.
-
5. Estimate the role of well-established developmental risk factors for IDs in predicting endophenotypic construct measures and how they moderate the relationships with internalizing symptoms.
-
6. Analyze associations and genetic correlations between brain-based phenotypes (structure and neural activity) and the self-report and lab-based measures studied in prior aims.
Materials and Methods
Sample
Recruitment of twin pairs occurred through the Mid-Atlantic Twin Registry (MATR), a Virginia Commonwealth University (VCU) database comprised of twins, other higher order multiples, and their family members who were willing to consider participating in research (Lilley & Silberg, Reference Lilley and Silberg2013). MATR personnel contacted the parents of potentially eligible twins in accordance with their standard operating procedures. They gathered basic demographic information and conducted a brief screen of exclusionary criteria and baseline data that could be used for study assignments. If twins were eligible and a parent agreed to participation of their children, they were enrolled in the study and their contact information was given to the study's project coordinator. In addition to inviting twins already registered in MATR, IRB-approved advertisements and other communications helped ascertain additional twins for this study. Twin pairs were considered ineligible for the study if either child met any of the following exclusion criteria: severe or unstable medical or neurological illness, past seizures without a clear or resolved etiology, intellectual disabilities, substance abuse, recent thoughts of suicide or homicide, episodes of psychosis, or currently taking psychotropic medications besides stimulants for ADHD. These exclusions aimed to (1) decrease the risk of exacerbation of possible medical conditions during the potentially stressful laboratory tasks and (2) minimize the likelihood that physiological responses recorded during the tasks were confounded by the potential effects of these variables. This study was approved by the VCU Institutional Review Board.
Recruitment targeted a sample of 450 twin pairs aged 9–13 years residing in the Mid-Atlantic region around Virginia and Maryland. The transition between childhood and adolescence is a key period for the emergence and progression of IDs, allowing the examination of their developmental aspects to infer underlying mechanisms (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005). For the purposes of biometrical twin modeling, an unselected sample was recruited that should produce an epidemiological distribution of internalizing syndromes. Given the high population prevalence of internalizing symptoms in children, this strategy should produce substantial numbers of children at risk for the target ID symptomatology: generalized anxiety (GAD), social anxiety (SOC), separation anxiety (SAD), panic/somatization (PAN), phobias (PHOB), and depression (DEP). This design provides adequate power to detect significant additive genetic effects explaining around 40% of the variance (heritability) for the proposed quantitative phenotypes (Visscher, Reference Visscher2004).
The sample was limited to Caucasian families, the largest available racial group in the local region. The authors are aware of no twin studies that have examined racial differences in heritability of internalizing phenotypes or their genetic risk structure. Preliminary analyses thus require stratification by race, resulting in the dilution of power to detect genetic effects that might not be shared across races. The inclusion of multiple racial groups would require a substantially expanded timeline and additional resources in order to recruit a sufficiently large, diverse sample. Future molecular analyses would also need to be performed separately by race due to differing allele frequencies across ancestral populations that can induce spurious association results. The investigators believed it prudent to stick with a more homogeneous sample for this study funded early under the RDoC initiative and, if indicated, plan for a replication study in a more racially diverse sample.
Protocols
After obtaining written informed consent from parents and assent from minor children, research protocols were conducted by trained research assistants in laboratory settings at VCU in Richmond, Virginia and at the Intramural Program of the National Institute of Mental Health (NIMH-IRP), part of the National Institutes of Health in Bethesda, Maryland. Involvement of the NIMH-IRP allowed the study to recruit subjects from areas in close proximity to Washington, DC. All data collected during these assessments were de-identified and given a study-generated participant identification number. Twins and willing parents underwent the full assessment of primary study measures during Visit 1. Willing families were brought back to complete one of several possible protocols during Visit 2: (1) participants were administered a reduced set of the same assessments from Visit 1 to examine their test–retest reliability (approximately 2–4 weeks after Visit 1); (2) neuroimaging data were collected from eligible twin participants. Parents and children were financially compensated for their participation.
Measures
A broad suite of NVS-relevant measures were included to examine their reliability, heritability, covariance structure, and how individual differences in these measures contribute to ID symptoms and outcomes. They are described in detail below and listed in Table 1 together with the NVS constructs and phenotypes they assess. The selected measures are multimethod–multitrait and included self-report surveys completed by the twins, parent report measures on the children about ID symptoms and risk factors, parental self-report on their own psychiatric history, and laboratory and neuroimaging paradigms.
TABLE 1 NVS Constructs, Phenotypes, Units of Analysis, and Measures
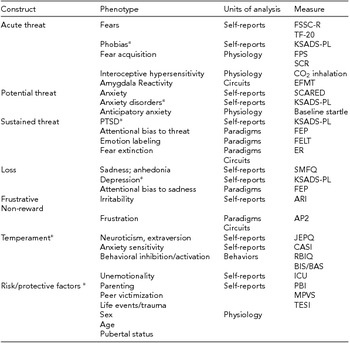
*Not formally included in RDoC matrix. SCARED = screening for childhood anxiety related disorders; SMFQ = short mood and feelings questionnaire; JEPQ = junior eysenck personality questionnaire; CASI = childhood anxiety sensitivity index; ARI = affective reactivity index; BIS/BAS = behavioral inhibition system behavioral activation system; FSSC-R = fear survey schedule for children revised; TF-20 = threat and fearlessness questionnaire - 20-item version; ICU = inventory of callous-unemotional Traits; RBIQ=retrospective behavioral inhibition questionnaire; PBI = parental bonding instrument- Authoritarianism/Coldness/Protectiveness; MPVS = multidimensional peer victimization scale; TESI-PRR = traumatic events screening inventory - parent report revised; KSADS-PL = kiddie schedule for affective disorders and schizophrenia - present & lifetime Version; FEP = face-emotion processing; FELT = facial expression labeling task; FPS = fear-potentiated startle; SCR = skin conductance response; AP2 = affective posner 2; EFMT = emotional face matching task; ER = extinction recall.
In addition to these phenotypic measures available for biometrical twin modeling, salivary DNA samples were collected using Oragene kits (DNA Genotek). DNA was extracted and stored for future genetic association studies that explore the relationship between NVS phenotypes derived from this study and novel genetic loci identified by ongoing genome-wide association studies of IDs (Otowa et al., Reference Otowa, Hek, Lee, Byrne, Mirza, Nivard and Hettema2016; Ripke et al., Reference Ripke, Wray, Lewis, Hamilton, Weissman, Breen and Sullivan2013).
Survey Measures
Several extant survey instruments were selected that cover a broad range of NVS phenotypic domains available by child self-report, parent report, or both. Survey data were collected and managed using REDCap electronic data capture tools hosted at VCU (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009). Among the child report surveys, clinical symptoms were assessed using the Short Mood and Feelings Questionnaire (SMFQ; Angold et al., Reference Angold, Costello, Messer, Pickles, Winder and Silver1995), Screening for Childhood Anxiety Related Disorders (SCARED; Birmaher et al., Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997), Affective Reactivity Index (ARI; Stringaris et al., Reference Stringaris, Goodman, Ferdinando, Razdan, Muhrer, Leibenluft and Brotman2012), and the Fear Survey Schedule for Children Revised (FSSC-R; Ollendick, Reference Ollendick1983). Normal personality and various indices of anxious temperament were assessed using the Junior Eysenck Personality Questionnaire (JEPQ; Eysenck, Reference Eysenck1965), Childhood Anxiety Sensitivity Index (CASI; Silverman et al., Reference Silverman, Fleisig, Rabian and Peterson1991), Behavioral Inhibition System/Behavioral Activation System (BIS-BAS; Carver & White, Reference Carver and White1994), and the Threat and Fearlessness Questionnaire (TF-20, a shorter version of the TF-55 described in Kramer et al., Reference Kramer, Patrick, Krueger and Gasperi2012). In addition, the Multidimensional Peer Victimization Scale (MPVS; Mynard & Joseph, Reference Mynard and Joseph2000) and sex-specific assessments of pubertal stage were administered.
Parents completed the following surveys about each of their twin children: SMFQ-Parent, SCARED-Parent, ARI-Parent, Child Behavior Checklist (CBCL; Achenbach, Reference Achenbach1991), Retrospective Behavioral Inhibition Questionnaire (RBIQ; Reznick et al., Reference Reznick, Hegeman, Kaufman, Woods and Jacobs1992) modified for this age group, Inventory of Callous-Unemotional Traits (ICU; Kimonis et al., Reference Kimonis, Frick, Skeem, Marsee, Cruise, Munoz and Morris2008), Parental Bonding Instrument (PBI; Parker et al., Reference Parker, Tupling and Brown1979), Traumatic Events Screening Inventory – Parent Report Revised (TESI-PRR; http://www.ptsd.va.gov/professional/assessment/child/tesi.asp). A masters- or doctoral-level trained clinician administered the Kiddie Schedule for Affective Disorders and Schizophrenia-Present & Lifetime Version (KSADS-PL; Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997) about each twin during a face-to-face interview with the parent(s); the children were also assessed with the KSADS-PL at the NIMH as part of their standard protocol. In addition, parents answered questions about their twins shown to be informative for estimating zygosity in prior twin studies, such as frequency with which the twins are confused by others, degree of physical similarity, and blood types or DNA tests (if available). A zygosity algorithm was developed for the current study to create a pair (dis)similarity score from those questions, with each item weighted by its relative discriminant predictive ability (Nichols & Bilbro, Jr., Reference Nichols and Bilbro1966; Peeters et al., Reference Peeters, Van, Vlietinck, Derom and Derom1998). Zygosity assignments derived from this algorithm showed high levels of concordance with zygosity determined by DNA testing (κ = 1.0, n = 13) or a medical diagnosis from placental sharing, blood tests, or in-vitro fertilization information (κ = 0.91, n = 112) per maternal report.
Parents completed the following surveys about themselves: short form of the Eysenck Personality Questionnaire (EPQ-SF; Eysenck & Eysenck, Reference Eysenck and Eysenck1975), stressful personal and network life events that occurred in the prior 12 months (adapted from an adult twin study; Kendler et al., Reference Kendler, Karkowski and Prescott1998), and questions about medically relevant events that occurred during the pregnancy and birth of the children. Willing parents also completed an online clinical interview about their own psychiatric history adapted from the CIDI-SF (Kessler et al., Reference Kessler, Andrews, Mroczek, Ustun and Wittchen1998).
Laboratory Paradigms
Twin participants completed five experimental tasks during Visit 1 that probe cognitive, emotional, and psychophysiological components of NVS. Some twins repeated these during Visit 2 to estimate test–retest reliability. Reliability is important for accurately assessing heritability of a measure and has not yet been examined for most of these paradigms. Participants completed tasks in a sequence according to one of four experimental schedules; this was determined randomly before the participants arrived and conserved across twins in a pair. The four conditions attempted to control for order effects while preserving the chronology of certain tasks mandated by the study protocols.
The Face-Emotion Processing (FEP) task utilizes the fact that the processing of facial stimuli is one of the most fundamental human capabilities and an essential part of social communication (Philippot & Feldman, Reference Philippot and Feldman1990). Various FEP tasks have been used to study psychopathology and associated traits, each attempting to capitalize on the finding that facial expressions engage a neural circuit involved in a core, evolutionarily based system independent of cultures (Haxby et al., Reference Haxby, Hoffman and Gobbini2002). The version included in this study engages two cognitive processes, attention, and recognition memory, each of which exhibits somewhat distinct associations with psychopathology (Guyer et al., Reference Guyer, Choate, Grimm, Pine and Keenan2011; Pine et al., Reference Pine, Klein, Mannuzza, Moulton, Lissek, Guardino and Woldehawariat2005). During the FEP task, participants viewed a series of standardized photographs of 32 actors expressing one of four emotions (angry, fearful, happy, sad) displayed for 4 s. During these viewings, participants made one of three ratings: degree of emotion expressed (How sad is the face?), the subject's internal emotional response (How sad does this person make you feel?), and the size of a non-emotional facial feature (How wide is the nose?). Thirty minutes after the FEP task, a surprise recognition memory test was administered. Pairs of two faces displaying neutral expressions, one of which had appeared in the FEP task and one of which was novel, were shown side by side, and participants identified which of the two faces they had previously seen.
The Facial Expression Labeling Task (FELT) assesses the participant's ability to read emotions in others. Youth with psychiatric syndromes often experience social impairment indexed by their ability to read and interpret emotions (Geller et al., Reference Geller, Bolhofner, Craney, Williams, DelBello and Gundersen2000; Rich et al., Reference Rich, Grimley, Schmajuk, Blair, Blair and Leibenluft2008), with information-processing biases that may vary across diagnostic groups (Dalgleish et al., Reference Dalgleish, Taghavi, Neshat-Doost, Moradi, Canterbury and Yule2003). The current study included a FELT paradigm based on a study by Marsh et al. (Reference Marsh, Yu, Pine and Blair2010). Participants were shown faces expressing six basic emotions: anger, disgust, fear, happiness, sadness, or surprise. Participants viewed faces for 500 ms morphed at 10% increments of emotional intensity, ranging from a neutral face (0% intensity) to full emotional (100%) intensity. They were then asked to label the emotion from the six possible choices. Response selections and response latencies were recorded, and percentage accuracy scores were calculated for each emotion at each intensity level.
A fear-potentiated startle (FPS) paradigm was used to assess fear conditioning and extinction. The startle reflex is a psychophysiological measure putatively sensitive to individual differences in emotional reactivity; it is readily measured in humans by recording the eye-blink electromyographic (EMG) response (Landis, Reference Landis1939). Startle is potentiated when elicited in the presence of a stimulus that signals an aversive stimulus like shock (Davis, Reference Davis1986; Grillon & Baas, Reference Grillon and Baas2003). The current study employed a FPS paradigm developed for use with children in which participants viewed photographs of two women: one woman serving as the positive conditioned stimulus (CS+) and the other as the CS− (Britton et al., Reference Britton, Lissek, Grillon, Norcross and Pine2011). The CS+ is paired with a loud, piercing scream as the unconditioned stimulus (UCS). Twins were conditioned to one of the women counterbalanced across twins. Not all CS+ presentations were reinforced with the UCS, thus creating a level of threat unpredictability. Predictable and unpredictable threats putatively distinguish between phasic fear and sustained anxiety (Schmitz et al., Reference Schmitz, Merikangas, Swendsen, Cui, Heaton and Grillon2011) with differential association to anxiety disorders (Grillon et al., Reference Grillon, Lissek, Rabin, McDowell, Dvir and Pine2008). The experimental phases were: habituation, pre-acquisition, acquisition, and extinction. In addition to startle EMG and self-reported distress, skin conductance response (SCR), and electrocardiogram (ECG) were recorded throughout the paradigm.
The carbon dioxide (CO2) inhalation task assesses physiologic and emotional responses when breathing air containing increased concentrations of CO2. The CO2 inhalation procedure has been used extensively by clinical researchers to study individual variation in response to the experience of bodily sensations (interoception), particularly those sensations tied to anxiety sensitivity and panic attacks (Bailey et al., Reference Bailey, Argyropoulos, Kendrick and Nutt2005; Papp et al., Reference Papp, Klein and Gorman1993). CO2 offers several advantages over other ‘panicogens’, including safety, ease of administration, tolerability, and reliability. The two most commonly applied CO2 administration protocols are sustained inhalation of air enriched with lower concentrations of CO2 (5% or 7.5%) versus one or two vital capacity inhalations of air containing high CO2 concentration (35%). Because the latter is substantially more aversive, the milder respiratory stimulating effects of sustained administration of low-concentration CO2 are preferred with children (Pine et al., Reference Pine, Klein, Coplan, Papp, Hoven, Martinez and Gorman2000). This study used a three-phase protocol: a 5-minute baseline breathing room air, 8-minutes breathing 7.5% CO2 enriched air, and a 5-minute recovery period. Participants were unaware of when the CO2 was turned on and off. The Diagnostic Symptom Questionnaire (DSQ) and Subjective Units of Distress (SUDS) ratings were administered at multiple time points to track anxiety and symptomatic reactivity before, during, and after the CO2 challenge. Physiologic indices of respiratory rate, tidal volume, and end-tidal CO2, as well as SCR, heart rate, and heart-rate variability were continuously monitored throughout the paradigm.
The Affective Posner 2 (AP2) task was adapted from a task used in previous studies (Deveney et al., Reference Deveney, Connolly, Haring, Bones, Reynolds, Kim and Leibenluft2013; Rich et al., Reference Rich, Holroyd, Carver, Onelio, Mendoza, Cornwell and Leibenluft2010). It is designed to elicit feelings of frustration in pediatric samples with a particular focus on studying irritability in children. The task provides rigged feedback in the context of a reward task, thereby provoking feelings of frustration. Deception was involved, and the children were debriefed at the end of the task.
Neuroimaging Paradigms
A subset of eligible twin pairs underwent neuroimaging protocols using 3T magnetic resonance imaging (MRI) during Visit 2 at NIMH or VCU. High-resolution structural and resting state functional scans were conducted during each session. Depending on the site and timing of the visit, some participants also performed a mix of the following three tasks while functional MRI (fMRI) data was acquired: the AP2 task, an emotional face-matching task (EFMT; Hariri et al., Reference Hariri, Bookheimer and Mazziotta2000), modified according to Swartz et al. (Reference Swartz, Phan, Angstadt, Fitzgerald and Monk2014), and the extinction recall (ER) portion of the previously described FPS fear-conditioning task (Britton et al., Reference Britton, Grillon, Lissek, Norcross, Szuhany, Chen and Pine2013).
Results
Table 2 lists key demographics for the sample. To date, the study has acquired data from 398 twin pairs (796 individuals) plus one or both parents (514 parents in all). Sixty twin pairs have thus far participated in MRI scans. The zygosity estimation procedure we employed estimated 132 MZ pairs (34.6%), 245 DZ pairs (64.3%), four pairs of unknown zygosity (1.0%), and 35 pairs missing zygosity information. An additional single-item index of parent-rated zygosity was used to assign zygosity to 17 of the 35 same-sex pairs for whom the zygosity questionnaire was not completed; this item showed high concordance (κ = 0.94, n = 252) with the algorithm-assigned zygosity in twin pairs for whom both measures were collected. Table 3 lists basic statistics for the child self-report and parent report survey measures. Within measure and between visit test–retest reliabilities were good to excellent. A wide array of analyses using these and the other assessed measures are underway to address the aims of this study.
TABLE 2 Sample Characteristics (n = 398 Families, 796 Twin Children)

SD = standard deviation; MZ = monozygotic; DZ = dizygotic.
TABLE 3 Statistics for Sum Scores of Survey Measures By Visit
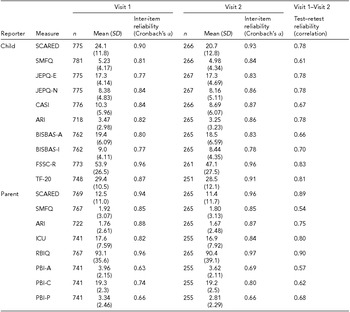
SD = standard deviation; SCARED = screening for childhood anxiety related disorders; SMFQ = short mood and feelings questionnaire; JEPQ-E/N = junior eysenck personality questionnaire - Extraversion/Neuroticism; CASI = childhood anxiety sensitivity index; ARI = affective reactivity index; BISBAS-I/A = behavioral inhibition system behavioral activation system - Inhibition/Activation; FSSC-R = fear survey schedule for children revised; TF-20 = threat and fearlessness questionnaire - 20-item version; ICU = inventory of callous-unemotional traits; RBIQ = retrospective behavioral inhibition questionnaire; PBI-A/C/P = Parental Bonding Instrument - Authoritarianism/Coldness/Protectiveness.
Discussion
The breadth and depth of this study promises an innovative and integrated perspective on the genetic and environmental aspects of NVS constructs. This perspective is not available in studies focused solely on specific psychological paradigms or individual disorder symptomatology. This study is unique in its opportunity to characterize the inter-individual and genetic relationships of a broad suite of dimensional measures and laboratory tasks that probe NVS. It can potentially determine to what extent the genetic factors underlying individual differences in responses to these phenotypes index genetic risk for IDs. Furthermore, paradigms measuring peripheral physiology and brain circuits can provide insights into biological mechanisms of risk. This study also examines the role of specific measures of childhood environment with known, potent effects on IDs (pubertal stage, peer victimization, parenting style, parental psychopathology) on these responses and how they moderate the development of internalizing symptomatology in the context of genetic risk.
As mentioned previously, a primary limitation to this study is the inclusion of Caucasian families only. This precludes the ability to generalize our findings to children of other racial and ethnic groups. A replication study in a more racially diverse sample is needed. The current study was also limited to one assessment in pre-adolescent children. Follow-up with the current cohort in the context of a longitudinal study would estimate the stability versus change of the measures across development, replicability of findings, and predictive value of the endophenotypes for emerging IDs.
Acknowledgments
The authors are very grateful to the twins and parents who have participated in the study over the past several years. JAS was extremely fortunate to have a dedicated team of scientists and research staff who contributed to the success of this study: Andrea Molzhon, Shannon Hahn, Laura Hazlett, Audrey Anderson, Oumaima Kaabi, Elizabeth Long, Diti Sheth, Lisa Ulmer, Lance Rappaport, Jeremy Cornelissen, Aditya Devineni, Lisa Straub, Daniel Deaton, Kevin Kim, Abigail Welch, Alexis Exum, Hiren Kolli, Hannah Mayberry, Tulsi Shah, Casey Guerra, Divya Patel, Ian Muse, Mosa Shahzada, Alana Nichols, Mazin Elmubarak, Pascaline Ezouah, Angela Chung, Lakshmi Ravindra, Hannah Donnelly, Jun Qi, Usama Nasir, Katelyn (Haejung) Shin, Chetna Jhurani, Essam Elrazaq, Jessica Chrisinger, Harper Lorencki, Johnnie Mortensen, Kalina Michalska, Christian Grillon, and Scott Vrana. The authors are also very appreciative of the excellent staff at the Mid-Atlantic Twin Registry, especially Anne Taylor-Morris, Carol Williams, Renolda Gelzinis, Emily Lilley, and Dr Judy Silberg.
Financial Support
This study was supported by the National Institutes of Health (R01MH098055 to JMH and NIMH-IRP-ziamh002781 to DSP) and the Brain and Behavior Research Foundation (BBRF Grant 21984 to JMH). REDCap and the Mid-Atlantic Twin Registry were supported through the NIH Center for Advancing Translational Research Grant Number UL1TR000058.





