The relationship between dietary fat intake and chronic disease risk, including type 2 diabetes (T2DM)(Reference Neuenschwander, Barbaresko and Pischke1), Alzheimer’s disease(Reference Ruan, Cheng and Zhu2) and certain types of cancer(Reference Gopinath, Cheema and Chaludiya3,Reference Di Sebastiano and Mourtzakis4) , has been the subject of considerable investigation with the greatest focus on CVD(Reference Lichtenstein5,Reference Hooper, Martin and Jimoh6) . The latter relationship has been investigated since the 1950s(Reference Page, Stare and Corcoran7). However, over the past several decades, a marked shift away from a focus on total fat intake(Reference Ornish, Brown and Scherwitz8) to type of fat(9) has occurred. Contemporary focus is on limiting SFA intake(Reference Arnett, Blumenthal and Albert10–Reference Kirkpatrick, Sikand and Petersen12) and replacing SFA with unsaturated fatty acids (UFA)(Reference Arnett, Blumenthal and Albert10–Reference Kirkpatrick, Sikand and Petersen12), particularly PUFA. This recommendation is based on robust evidence from both observational studies and clinical trials that shows beneficial health outcomes, particularly lower risk of CVD, when SFA are replaced by UFA(13,Reference Retterstol and Rosqvist14) . CVD, which includes coronary artery disease (CAD) and cerebrovascular disease (i.e. stroke), continues to be the leading cause of morbidity and mortality in the USA(Reference Tsao, Aday and Almarzooq15) and globally(16).
In the USA, SFA intake generally exceeds recommendations(Reference Shan, Rehm and Rogers17). Globally, estimates are that non-optimal intakes of n-6 PUFA (<12 % total daily energy [TDE]) and SFA (>10 % TDE) account for 10·3 % and 3·6 % of CAD mortality, respectively(Reference Wang, Afshin and Yakoob18). However, despite multiple lines of complementary and concordant evidence indicating that n-6 PUFA intake is cardioprotective, some authors claim that n-6 PUFA intake leads to pro-inflammatory and pro-oxidative states(Reference Mariamenatu and Abdu19–Reference Yamashima, Ota and Mizukoshi21), which is contributing to a growing social media movement against the use of vegetable oils (also called seed oils)(Reference Dennett22,Reference Dickson23) . The goal of this narrative review is to provide health professionals, especially dietitians and other clinicians, with the information they need to address any concerns their patients/clients may have about oils containing predominantly UFA, including MUFA and PUFA.
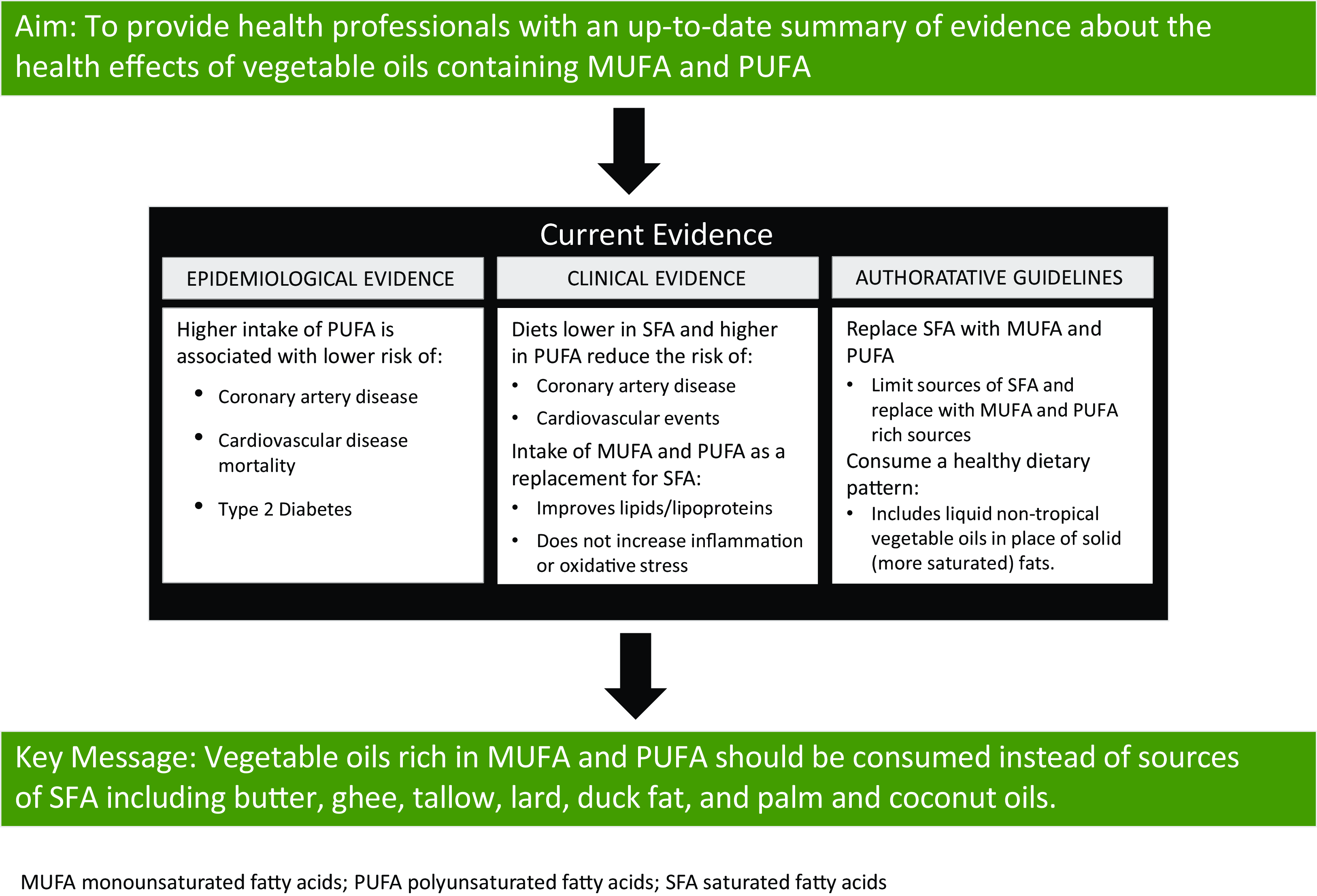
As such, the following topics are covered in this review: (1) fatty acid composition of commonly consumed vegetable oils; (2) epidemiological evidence on the association between intake of vegetable oils high in UFA and chronic disease risk with an emphasis on CVD; (3) data from randomised controlled trials (RCT) examining the effect of intake of vegetable oils high in UFA on chronic disease risk with an emphasis on CVD; (4) biological relevance of the dietary n-6:n-3 fatty acid ratio and (5) guidance for health professionals on vegetable oil use. Non-systematic literature searches were used to identify research on each of these topics. Key conclusions are summarised in Table 1.
Table 1. Key conclusions from the evidence reviewed

Oil composition
Edible plant oils are commonly referred to as vegetable oils and include oils derived from vegetables, nuts, seeds, fruits and cereal grains. In recent online discussions about oils made from seeds, the term ‘seed oils’ is used(Reference Dennett22,Reference Dickson23) rather than the more commonly used term ‘vegetable oils’, although ‘seed oils’ has been occasionally used in the scientific literature(Reference Scrimgeour40–Reference Tarjuelo, Rabadan and Alvarez-Orti43). Seed oils include oils derived from sunflower, cottonseed, safflower, canola, sesame, grapeseed, rice bran, soyabean and corn. While the fatty acid composition of these oils varies substantially, all are high in UFA, including MUFA and PUFA, while being relatively low in SFA (Table 2).
Table 2. Fatty acid content of selected oils (g/100 g) listed in order of total PUFA content *

* USDA FoodData Central, Standard Release Database. https://fdc.nal.usda.gov/.
† Mid-oleic (most commonly used sunflower oil).
‡ High oleic (primary safflower oil of commerce).
Fatty acids in oils are predominately in the form of triglycerides, which consist of three fatty acids esterified to a glycerol molecule. Fatty acids are comprised of a hydrocarbon chain that varies from four to twenty-four carbons with a methyl group (also known as an omega carbon) at one end and a carboxylic acid group at the other end (Fig. 1). SFA contain no double bonds within the hydrocarbon chain, whereas MUFA contain a single carbon–carbon double bond and PUFA contain at least two carbon–carbon double bonds. The omega system is commonly used to describe the chemical structure of fatty acids with one or more double bonds. In this system, the position of the first carbon–carbon double bond counting from the methyl end of the hydrocarbon chain is used. In the case of n-6 and n-3 PUFA, the first double bond is at carbon 6 and 3, respectively (Fig. 1).

Fig. 1. Structure of the two essential fatty acids, linoleic acid and alpha-linolenic acid.
Fatty acid intake recommendations
Humans can endogenously synthesise SFA and MUFA in sufficient quantities to meet biological needs. In contrast, humans cannot synthesise the n-6 PUFA linoleic acid (LA; 18:2n-6) or the n-3 PUFA α-linolenic acid (ALA; 18:3n-3). Thus, LA and ALA are classified as essential fatty acids and must be consumed in the diet or taken as supplements(Reference Vannice and Rasmussen44,Reference Djuricic and Calder45) . LA deficiency is characterised by rough and scaly skin and dermatitis(Reference Kris-Etherton, Taylor and Yu-Poth46). For LA, the adequate intake established by the USA Institute of Medicine (now the National Academy of Medicine) is 17 g/d and 14 g/d for men 19–50 years and ≥51 years, respectively, and is 12 g/d and 11 g/d for women 19–50 years and ≥51 years, respectively(47). These adequate intakes are based on median intakes in the USA where deficiency is non-existent among healthy individuals. Similarly, adequate intakes for ALA are based on USA median intakes where n-3 fatty acid deficiency is non-existent in healthy individuals. The adequate intake for ALA is 1·6 and 1·1 g/d for men and women, respectively(47). The European Food Safety Authority recommendations for LA and ALA are 4 % TDE and 0·5 % TDE, respectively(48). The FAO/WHO recommends 2·5–9 % of TDE be from LA and ALA intake to be >0·5 % TDE for adults(49).
Oils high in UFA and CVD risk
Epidemiologic evidence
Evidence from multiple meta-analyses of prospective cohort studies consistently shows that higher PUFA intake (primarily LA) and higher circulating LA levels are associated with lower risk of CVD(Reference Maki, Eren and Cassens31–Reference Sacks, Lichtenstein and Wu33,Reference Djuricic and Calder45) . Evidence for the relationship between MUFA intake and CVD is less consistent(Reference Lotfi, Salari-Moghaddam and Yousefinia29), but generally shows higher intake of MUFA from plant sources is associated with lower risk of CVD(Reference Schwingshackl and Hoffmann30). Findings from meta-analyses demonstrate that higher intake of PUFA, compared with SFA, MUFA, or carbohydrates, is associated with reduced risk of CAD(Reference Farvid, Ding and Pan24,Reference Li, Guasch-Ferre and Li25,Reference Maki, Eren and Cassens31,Reference Wang32) .
A meta-analysis of thirteen cohort studies showed that the highest LA intake category was associated with a 15 % lower risk of a CAD event (pooled relative risk [RR] 0·85; 95 % confidence interval [CI] 0·78, 0·92) and a 21 % lower risk of CAD death (RR 0·79; 95 % CI 0·71, 0·89)(Reference Farvid, Ding and Pan24). Similar results were reported by Li et al. (Reference Li, Guasch-Ferre and Li25) in a more recent systematic review and meta-analysis of eighteen prospective cohort studies that examined LA intake. A higher v. lower intake of PUFA was associated with a significantly lower risk of CVD mortality (RR 0·87; 95 % CI 0·82, 0·92). It was also found that each 5 % increase in energy intake from LA was associated with a 7 % lower risk of CVD mortality (RR 0·93; 95 % CI 0·91, 0·95)(Reference Li, Guasch-Ferre and Li25).
Circulating LA concentrations reflect dietary intake because LA cannot be endogenously synthesised(Reference Hodson, Eyles and McLachlan50,Reference Skeaff, Hodson and McKenzie51) . Results from meta-analyses examining the relationship between circulating LA concentrations and CVD are consistent with the results from dietary intake studies(Reference Maki, Eren and Cassens31,Reference Wang32,Reference Djuricic and Calder45) . In a meta-analysis of thirty cohort studies (median follow-up 2·5–31·9 years, 15 198 incident cardiovascular events among 68 659 participants), Marklund et al. (Reference Marklund, Wu and Imamura26) found that higher circulating LA levels were associated with a significantly lower risk of total CVD (hazard ratio [HR] 0·93; 95 % CI 0·88, 0·99), CVD mortality (HR 0·78; 95 % CI 0·70, 0·85) and ischaemic stroke (HR 0·88; 95 % CI 0·79, 0·98), as well as a nominally lower risk of total CAD (HR 0·94; 95 % CI 0·88, 1·00). Similarly, in a meta-analysis of twenty-two prospective cohort studies that examined LA biomarkers, Li et al. (Reference Li, Guasch-Ferre and Li25) observed that a higher concentration of plasma and tissue LA was associated with an 8 % lower risk of CVD mortality.
Results from pooled analyses and meta-analyses of prospective cohort studies modelling the replacement of SFA with UFA show predicted reductions in risks for CAD events and mortality(Reference Maki, Eren and Cassens31,Reference Wang32) . For example, in an analysis of data from three USA cohorts, the Nurses’ Health Study I and II and the Health Professionals Follow-up Study, substitution of 5 % of TDE from SFA with PUFA was associated with a significant reduction in CAD risk (HR 0·75; 95 % CI 0·67, 0·84)(Reference Li, Hruby and Bernstein52). Replacement of SFA with MUFA (HR 0·85; 95 % CI 0·74, 0·97) and carbohydrate from whole grains (HR 0·91; 95 % CI 0·85, 0·98) was also associated with significantly lower CAD risk, whereas substitution with carbohydrates from refined starches and added sugars was not(Reference Li, Hruby and Bernstein52). Farvid et al. (Reference Farvid, Ding and Pan24) modelled the effect of substituting 5 % of TDE from SFA with LA and found a 9 % lower risk of CAD events (RR 0·91; 95 % CI 0·87, 0·96) and a 13 % lower risk of CAD mortality (RR 0·87; 95 % CI 0·82, 0·94).
It is notable that, after reviewing the available evidence, the 2020 USA Dietary Guidelines Advisory Committee concluded that ‘strong evidence demonstrates that replacing saturated fatty acids with PUFA in adults reduces the risk of CHD events and cardiovascular disease mortality’(13). This conclusion is consistent with evidence-based analyses by other public health and scientific organisations(Reference Arnett, Blumenthal and Albert10–Reference Kirkpatrick, Sikand and Petersen12,Reference Retterstol and Rosqvist14,Reference Sacks, Lichtenstein and Wu33,Reference Mach, Baigent and Catapano53–Reference Visseren, Mach and Smulders55) .
Clinical evidence
Significant clinical evidence supports the beneficial effect of replacing SFA with UFA, particularly PUFA, on cardiovascular events and key risk factors, including lipids/lipoproteins, blood pressure, inflammation and oxidative stress.
In RCT examining diets lower in SFA that included high-PUFA oils, clinically relevant reductions in CVD events have been observed(Reference Hooper, Martin and Jimoh6,Reference Sacks, Lichtenstein and Wu33,Reference Mozaffarian, Micha and Wallace34) . In the most recent (2020) Cochrane Review, which included thirteen RCT (sixteen comparisons, 53 758 participants, mean duration 4·7 years), reducing SFA intake lowered the risk of combined cardiovascular events by 17 % (RR 0·83; 95 % CI 0·70, 0·98), and the quality of evidence was assessed as moderate(Reference Hooper, Martin and Jimoh6). Further analyses examining the replacement macronutrient showed that replacing SFA with PUFA was associated with a nominal 21 % (RR 0·79; 95 % CI 0·62, 1·00; eight trials) reduction in the risk of combined cardiovascular events, whereas replacement of SFA with carbohydrates (RR 0·84; 95 % CI 0·67, 1·06; five trials), MUFA (RR 1·00; 95 % CI 0·53, 1·89; one trial) or proteins (RR 0·97; 95 % CI 0·91, 1·03; four trials) was not significantly associated with cardiovascular event risk.
An earlier meta-analysis of RCT (median duration 4·25 years) that included 13 614 participants showed that replacing 5 % of TDE from SFA with equivalent energy from PUFA was associated with a 10 % reduction in CAD risk (RR 0·90; 95 % CI 0·83, 0·97)(Reference Mozaffarian, Micha and Wallace34). This effect estimate was similar to the predicted CAD risk reduction based on the total cholesterol to HDL-cholesterol ratio (RR 0·91; 95 % CI 0·87, 0·95), which suggests that improvements in lipids/lipoproteins account for most of the protection against CAD. These findings are consistent with those from a meta-analysis conducted for an American Heart Association Presidential Advisory that included four trials, which provide the core RCT evidence for replacement of SFA with PUFA(Reference Sacks, Lichtenstein and Wu33).
These four trials(Reference Dayton, Pearce and Hashimoto56–Reference Miettinen, Turpeinen and Karvonen61) provide the core evidence because of the quality of study design, execution and intervention adherence (Table 3). Six trials(Reference Watts, Lewis and Lewis62–Reference Frantz, Dawson and Ashman67) were identified that did not meet the inclusion criteria in Table 3 and, therefore, provide low-quality evidence. A meta-analysis of the four high-quality studies showed that replacement of SFA with PUFA was associated with a 29 % reduction in risk of CAD (RR 0·71; 95 % CI 0·62, 0·81). While there are RCT showing that intake of high-PUFA-containing oils is not associated with reduced risk of CVD(Reference Ramsden, Zamora and Leelarthaepin66,Reference Ramsden, Hibbeln and Majchrzak68) , these have fundamental flaws, which preclude meaningful conclusions(Reference Virtanen69). Limitations include (1) mixed dietary interventions in which PUFA and carbohydrate replaced SFA; (2) insufficient duration; (3) low adherence and (4) few events. Collectively, the available high-quality RCT evidence shows that replacement of SFA with PUFA-containing oils lowers CVD risk to a magnitude similar to statin therapy(Reference Cholesterol Treatment Trialists, Mihaylova and Emberson70).
Table 3. Criteria for inclusion of RCT in the 2017 American Heart Association Presidential Advisory on dietary fats and CVD

Oils high in UFA and CVD risk factors
Although it is well established that LDL-cholesterol has a causal role in the development of CVD(Reference Ference, Ginsberg and Graham71,Reference Boren, Chapman and Krauss72) , a point of debate among scientists and clinicians is whether diet-related reductions in LDL-cholesterol translate to CVD risk reduction(Reference Krauss and Kris-Etherton73–Reference Kris-Etherton and Krauss75). A systematic review and meta-analysis of forty-nine RCT of pharmacological and dietary interventions (312 175 participants, 24 % women; mean baseline LDL-cholesterol level of 3·16 mmol/l (122·2 mg/dl), 39 645 major vascular events) showed the RR reduction for major vascular events was proportional to LDL-cholesterol-lowering achieved. Specifically, per 1 mmol/l (39 mg/dl) decrease in LDL-cholesterol, the RR of a major vascular event was reduced by 23 % (RR 0·77; 95 % CI 0·75, 0·79)(Reference Silverman, Ference and Im76).
It has also been suggested that LDL-cholesterol lowering in response to dietary reductions in SFA does not confer protection against CVD(Reference Boren, Chapman and Krauss72) because some evidence shows SFA replacement lowers larger LDL particle subspecies(Reference Bergeron, Chiu and Williams77), which are not strongly associated with CVD risk(Reference Boren, Chapman and Krauss72). Generally, smaller, denser LDL particles are considered more atherogenic(Reference Boren, Chapman and Krauss72). However, it is important to note that statins preferentially lower larger LDL particles and significantly lower CVD risk(Reference Mora, Caulfield and Wohlgemuth78). In addition, epidemiological analyses show that the association between LDL particle size or subclass distribution and CVD risk is attenuated to nonsignificance after adjustment for LDL particle concentration(Reference Ip, Lichtenstein and Chung79). Clinically, LDL-cholesterol concentration is used to estimate CVD risk(Reference Grundy, Stone and Bailey80) because, under most conditions, LDL-cholesterol concentration is highly correlated with LDL particle number(Reference Ference, Ginsberg and Graham71).
In most individuals, LDL particles constitute ∼90 % of circulating apolipoprotein B (apoB)-containing lipoproteins in the fasting state(Reference Ference, Ginsberg and Graham71). Each LDL particle contains one apoB molecule; thus, measurement of the apoB concentration reflects the total circulating concentration of lipoproteins with atherogenic potential. Critically, it is the trapping of apoB-containing lipoproteins in the artery wall that initiates and drives the atherosclerotic process(Reference Sniderman, Thanassoulis and Glavinovic81). Therefore, examining apoB concentration is informative to CVD risk reduction estimates.
The results of RCT consistently show improvements in atherogenic lipids and lipoproteins when UFA, particularly PUFA, are consumed instead of SFA. A systematic review and meta-regression analysis that included eighty-four RCT (duration ranging from 13 to 91 d) involving 2353 participants showed that when 1 % of TDE from SFA is isocalorically replaced with PUFA (predominately LA), lowering of total cholesterol (mean change –2·47 mg/dl; 95 % CI –2·71, –2·24), LDL-cholesterol (–2·13 mg/dl; 95 % CI –2·36, –1·93), total cholesterol to HDL-cholesterol ratio (–0·034; 95 % CI –0·04, –0·028) and apoB (–10·2 mg/dl; 95 % CI –12·4, –8·1) is observed(Reference Mensink35). Similarly, when 1 % of TDE from SFA is isocalorically replaced with MUFA, total cholesterol (–1·78 mg/dl; 95 % CI –1·97, –1·55), LDL-cholesterol (–1·62 mg/dl; 95 % CI –1·82, –1·43), total cholesterol to HDL-cholesterol ratio (–0·027; 95 % CI –0·033, –0·022) and apoB (–7·8 mg/dl; 95 % CI –9·5, –6·0) are reduced. Thus, high-quality evidence supports intake of PUFA and MUFA in place of SFA to improve lipids/lipoproteins.
The effects of fatty acids on CVD risk and lipoprotein levels align with the results of analyses focusing on the intake of specific vegetable oils. For example, systematic reviews and meta-analyses of RCT show intake of canola oil(Reference Ghobadi, Hassanzadeh-Rostami and Mohammadian82,Reference Amiri, Raeisi-Dehkordi and Sarrafzadegan83) and rice bran oil(Reference Jolfaie, Rouhani and Surkan84) lowers total cholesterol and LDL-cholesterol levels. Similarly, RCT examining soyabean oil(Reference Baer, Henderson and Gebauer85–Reference Zhang, Ping and Chunrong91), corn oil(Reference Maki, Hasse and Dicklin92–Reference Maki, Lawless and Kelley94), cottonseed oil(Reference Polley, Oswell and Pegg95,Reference Prater, Scheurell and Paton96) and sunflower oil(Reference Perez-Jimenez, Espino and Lopez-Segura97,Reference Wardlaw and Snook98) consistently show lipid/lipoprotein improvements. Finally, in a network meta-analysis of RCT (duration ranging from 3 to 17 weeks) where direct and indirect evidence on the effects of thirteen oils and solid fats (safflower, sunflower, canola, hempseed, flaxseed, corn, olive, soyabean, palm and coconut oils as well as beef fat, lard and butter) was synthesised, it was shown that replacement of 10 % of TDE from butter with an equivalent amount of safflower, sunflower, canola, olive, flaxseed, corn or soyabean oil lowered LDL-cholesterol by 10–16 mg/dl(Reference Schwingshackl, Bogensberger and Bencic99). In summary, replacement of dietary sources of SFA with oils rich in PUFA and MUFA consistently improves lipids and lipoprotein levels.
Researchers have also investigated the effects of dietary fatty acids on blood pressure. However, in contrast to the effects on lipids and lipoproteins, RCT generally show replacement of SFA with MUFA or PUFA has little to no impact on this metric(Reference Mutanen, Kleemola and Valsta100–Reference Vafeiadou, Weech and Altowaijri103). A 2018 Cochrane Review of RCT comparing higher v. lower intake of n-6 PUFA for a minimum duration of 12 months found no clinically relevant effects on systolic or diastolic blood pressure, although this finding was based on only two studies(Reference Hooper, Al-Khudairy and Abdelhamid104). In agreement, in the Dietary Intervention and VAScular function study, replacing ∼9·5 % of TDE from SFA with MUFA or n-6 PUFA for 16 weeks did not affect 24-hour systolic or diastolic blood pressure in individuals at moderate CVD risk(Reference Vafeiadou, Weech and Altowaijri103). However, replacing SFA with MUFA did reduce nighttime systolic blood pressure (mean difference –4·9 mmHg; P = 0·019). Finally, in the OmniHeart RCT, a higher fat, MUFA-rich diet (48 % kcal carbohydrate, 37 % kcal total fat, 21 % kcal MUFA, 10 % kcal PUFA, 6 % kcal SFA) modestly lowered systolic (mean difference –1·3 mmHg, P = 0·005) and diastolic (–0·8 mmHg, P = 0·02) blood pressure after 6 weeks compared to a carbohydrate-rich diet (58 % kcal carbohydrate, 27 % kcal total fat, 13 % kcal MUFA, 8 % kcal PUFA, 6 % kcal SFA)(Reference Appel, Sacks and Carey105). In summary, evidence from RCT suggests replacing SFA with MUFA may modestly improve blood pressure, whereas PUFA have no effect.
As noted previously, a commonly expressed concern about LA is that it increases chronic inflammation. However, the available evidence does not support this concern. A recent systematic review and meta-analysis that included thirty RCT with a duration of 4 to 24 weeks demonstrated that higher intake of LA did not increase tumor necrosis factor-α (standardised mean difference [SMD] –0·01; 95 % CI –0·19, 0·17), interleukin-6 (SMD 0·11; 95 % CI –0·07, 0·29), adiponectin (SMD 0·17; 95 % CI –0·17, 0·50), monocyte chemoattractant protein 1 (SMD 0·14; 95 % CI –0·33, 0·60), or C-reactive protein (SMD 0·09; 95 % CI –0·05, 0·24)(Reference Su, Liu and Chang36). In agreement, after systematically reviewing fifteen RCT (duration 2 to 9 weeks) involving healthy participants, Johnson and Fritsche(Reference Johnson and Fritsche37) concluded that there is virtually no evidence to show that addition of LA to the diet increases the concentration of inflammatory markers.
Another concern is that n-6 PUFA, including LA, promote oxidative stress(Reference DiNicolantonio and O’Keefe20,Reference Mercola and D’Adamo106,Reference Ramsden, Hennebelle and Schuster107) . Oxidative stress is defined by a disturbance in the balance between production of reactive oxygen species (free radicals or chemical species that contain unpaired electrons) and antioxidant defenses, which may lead to tissue damage(Reference Betteridge108). Oxidative stress has been implicated in the development of many chronic diseases, including CVD, cancer, T2DM and neurological diseases(Reference Pizzino, Irrera and Cucinotta109). PUFA are susceptible to oxidation because they contain multiple double bonds(Reference Richaud, Audouin and Fayolle110). This susceptibility has given rise to the concern that greater intake of PUFA results in PUFA-enrichment of LDL particles, thereby increasing their susceptibility to oxidative modification. This susceptibility has been demonstrated in in vitro and ex vivo experiments(Reference Reaven, Parthasarathy and Grasse111,Reference Tsimikas, Philis-Tsimikas and Alexopoulos112) . However, the results of most RCT show no effect of n-6 PUFA on markers of oxidative stress, including oxidised LDL(Reference Sanders38) and F-2 isoprostanes, a marker of lipid peroxidation(Reference Da Silva, Bilodeau and Julien39). As reviewed by Birben et al. (Reference Birben, Sahiner and Sackesen113), aerobic organisms have integrated antioxidant systems, which include enzymatic and non-enzymatic antioxidants that are effective in blocking harmful effects of reactive oxygen species.
The lack of effect of n-6 PUFA on markers of oxidative status is illustrated by research involving soybean oil. Of the four RCT, with a duration of 4–12 weeks, examining the effects of soybean oil (∼51 % LA) with oils lower in LA, only one showed an increase in a marker of oxidative status (decrease in small, dense LDL oxidation lag time)(Reference Utarwuthipong, Komindr and Pakpeankitvatana89). However, given the in vitro test used to measure lag time in this study and a substantial reduction in LDL-cholesterol in response to soybean oil intake, the overall health effect is likely beneficial. Furthermore, in two trials, no effects on markers (thiobarbituric acid reactive substances, malondialdehyde, oxidised LDL-cholesterol) of oxidative status were observed(Reference Karupaiah, Chuah and Chinna114,Reference Wu, Du and Wen115) , and, in one trial, trolox equivalent antioxidant capacity actually increased in response to soybean oil(Reference Costa, Pereira de Melo and Faro Reis116).
Finally, it is established that chronic hyperlipidaemia promotes oxidative stress, with more pronounced effects in individuals with obesity(Reference Galassetti and Pontello117). Therefore, total cholesterol and LDL-cholesterol lowering induced by replacing SFA with UFA, including PUFA, would be expected to reduce oxidative stress.
UFA and T2DM
Epidemiologic evidence
The association between PUFA or LA intake and risk of developing T2DM has been examined in numerous cohort studies, especially in Western countries. For example, in the Nurses’ Health Study I, a 5 % increase in energy from PUFA (mainly LA) was associated with a marked lowering of T2DM risk (RR 0·63; 95 % CI 0·53, 0·76)(Reference Salmeron, Hu and Manson118). In agreement are the results of another USA study involving 35 988 older women. During the 11-year follow-up period, 1890 women developed T2DM. After adjusting for potential confounders, RRs across increasing quintiles of vegetable fat intake were 1·00, 0·90, 0·87, 0·84 and 0·82 (P = 0·02). Lower risk of incident T2DM was also associated with modelled substitution of PUFA with SFA(Reference Meyer, Kushi and Jacobs119).
Consistent with the results of the two studies discussed above are those from the Health Professionals Follow-Up Study, which involved 42 504 men aged 40–75 years at study entry. LA intake was associated with a lower risk of developing T2DM in men < 65 years of age (RR 0·74; 95 % CI 0·60, 0·92) and in those with a body mass index (BMI) < 25 kg/m2 (RR 0·53; 95 % CI 0·33, 0·85)(Reference van Dam, Willett and Rimm120). However, no relationship was found between LA intake in older men or men with obesity (BMI > 30 kg/m2). More recently, a pooled analysis of the Nurses’ Health Study I and II and the Health Professionals Follow-Up Study, which involved over 200 000 USA men and women, showed that dietary LA intake was significantly inversely related to risk of incident T2DM over the nearly 3-decade follow-up period (HR 0·92; 95 % CI 0·87, 0·98)(Reference Zong, Liu and Willett121).
PUFA intake has also been found to be protective against T2DM in cohorts outside of the USA. For example, in the European Prospective Investigation of Cancer-Norfolk study, the energy-adjusted dietary PUFA:SFA ratio was inversely associated with the risk of T2DM (per standard deviation change, odds ratio 0·84; 95 % CI 0·75, 0·94)(Reference Harding, Day and Khaw122). However, a later publication from this cohort reported no significant association between dietary LA and incident T2DM(Reference Patel, Sharp and Jansen123).
More important than the results of individual studies are the findings of systematic reviews and meta-analyses of epidemiological evidence. These reviews are generally supportive of the benefits of PUFA. For example, the authors of a 2014 meta-analysis of four cohort studies concluded that an increase in PUFA intake, mainly n-6 PUFA, from 3 % to ∼6 % of energy in exchange for carbohydrate or SFA may be associated with a 20 % reduction in T2DM risk(Reference Schwab, Lauritzen and Tholstrup27). They also noted that tissue LA is inversely associated with the development of T2DM. These results align with a more recent systematic review and meta-analysis of 31 cohort studies involving 297 685 participants (22 639 incident T2DM cases) with dietary intake assessment and 84 171 participants (18 458 incident T2DM cases) with biomarker measurements(Reference Mousavi, Jalilpiran and Karimi28). Higher dietary LA intake was associated with a 6 % lower risk of T2DM (RR 0·94; 95 % CI: 0·90, 0·99). In a dose-response analysis, each 5 % increment in energy from LA was associated with a 10 % lower risk of T2DM. The summary RR for incident T2DM per standard deviation increase in LA concentrations in blood compartments or adipose tissue was 0·85 (95 % CI 0·80, 0·90).
Clinical evidence
The results of RCT are generally supportive of the protective effects of LA intake on metabolic changes relevant to developing and/or managing T2DM. For example, the results of a meta-analysis of 102 RCT that included 239 dietary intervention arms involving 4220 adults found that replacing 5 % of TDE from SFA with PUFA significantly lowered blood glucose, hemoglobin A1c, C-peptide and homeostatic model assessment-insulin resistance(Reference Imamura, Micha and Wu124). Furthermore, PUFA significantly improved insulin secretion capacity whether replacing carbohydrate, SFA or MUFA. Imamura et al. (Reference Imamura, Micha and Wu124) concluded that, in comparison to carbohydrates, SFA, or MUFA, consistent favorable effects were seen with PUFA.
Three years later, Wanders et al. (Reference Wanders, Blom and Zock125) published the results of a meta-analysis that included thirteen RCT and nineteen comparisons of plant-derived PUFA with controls that examined glucose metabolism and insulin resistance. In contrast to the analysis by Imamura et al. (Reference Imamura, Micha and Wu124), PUFA did not significantly affect fasting glucose; however, PUFA lowered fasting insulin and homeostatic model assessment-insulin resistance. Finally, in contrast to the meta-analyses conducted by Imamura et al. (Reference Imamura, Micha and Wu124) and Wanders et al.,(Reference Wanders, Blom and Zock125) a meta-analysis of ten parallel and twenty crossover RCT involving 1586 participants failed to find that replacing SFA with MUFA or PUFA had significant effects on insulin sensitivity. The authors noted that many of the trials were relatively short-term and that longer term studies evaluating glucose homeostasis are needed(Reference Lytrivi, Gomes Da Silveira Cauduro and Kibanda126).
In conclusion, epidemiologic and clinical trial evidence suggests that replacing SFA with PUFA may reduce the risk of developing T2DM and favorably affects metabolic changes related to diabetes.
Biological relevance of the n-6:n-3 fatty acid ratio
The n-6:n-3 fatty acid ratio is calculated by summing all n-6 PUFA in circulation or the diet divided by the sum of all n-3 PUFA. The n-6:n-3 ratio was first popularised by Simopoulos(Reference Simopoulos and Robinson127) and Lands(Reference Lands128) in the 1990s. The concept is based on the established competition between the two essential fatty acids – LA and ALA – for metabolism by desaturase enzymes in the synthesis of long-chain n-3 PUFA, especially arachidonic acid (AA; 20:4n-6), eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3). Since, ALA conversion to EPA and DHA can be accelerated by reducing intake of LA, the ‘balance’ idea appeared reasonable. As appreciation of the health importance of n-3 PUFA grew throughout the 1990s, ways to increase tissue levels of EPA and DHA took on new importance, which led to the vilification of n-6 PUFA and a high dietary n-6:n-3 PUFA ratio.
However, there are several flawed assumptions about the use of the dietary n-6:n-3 ratio as a metric, which severely limit its utility. For example, the components of the n-6:n-3 ratio are rarely defined. Generally, three n-6 PUFA are in the diet (LA, AA and trace amounts of gamma-linolenic acid) and another four n-6 metabolites are present in the blood (dihomo-gamma linolenic acid, adrenic acid, eicosadienoic acid and n-6 docosapentaenoic acid). In comparison, there are four dietary n-3 PUFA, mostly ALA but also EPA, n-3 docosapentaenoic acid and DHA. In the blood, DHA is the most prevalent. Depending on the analytical methods used, sometimes all 11 PUFA are used in the calculation of the n-6:n-3 ratio, whereas, in some cases, the calculation will include far fewer. Since it is rarely reported which PUFA are included, the ratio is non-specific.
In addition, identical ratios can be calculated from different absolute amounts of individual PUFA. A diet containing 15 g of n-6 PUFA and 1 g of n-3 PUFA has the same ratio (15:1) as a diet containing 5 g of n-6 PUFA and 0·33 g of n-3 PUFA. A specific dietary n-6:n-3 ratio can be achieved by an almost infinite set of dietary patterns, some of which could be deficient in both fatty acid classes. Thus, dietary recommendations should not be made based on the n-6:n-3 ratio, but on the absolute intake of n-3 and n-6 PUFA.
Furthermore, the notion that n-6 PUFA are pro-inflammatory and that n-3 PUFA are anti-inflammatory(Reference Lands129,Reference Bibus and Lands130) contrasts with the new understanding that such broad categorisation of n-6 and n-3 PUFA is far too simplistic(Reference Innes and Calder131) and has little to no direct support from studies in humans(Reference Johnson and Fritsche37,Reference Fritsche132,Reference Ferrucci, Cherubini and Bandinelli133) . In fact, evidence shows that higher LA levels are associated with reduced inflammatory status(Reference Asp, Collene and Norris134–Reference Belury, Cole and Bailey136). Higher inflammatory status is observed when EPA and DHA levels are low(Reference Fontes, Rahman and Lacey137) (i.e. the ratio is high), but the problem is not the presence of n-6 PUFA, rather the relative absence of n-3 PUFA. Even if evidence indicated that n-6 PUFA and AA are pro-inflammatory, this would not suggest the same of LA since the assumption that lowering LA intake will lower tissue levels of AA is not supported by available evidence(Reference Rett and Whelan138). Tracer studies have shown that <0·2 % of dietary LA is converted to AA(Reference Hussein, Ah-Sing and Wilkinson139).
Finally, oxylipins, lipid mediators produced from metabolism of both n-6 and n-3 PUFA, may explain the diverse and complex effects of this class of fatty acids(Reference Shearer and Walker140). In some cases, n-6 oxylipins have similar beneficial roles as n-3 oxylipins, although they are not as potent(Reference Shearer and Walker140). The bulk of the evidence now supports the cardiovascular benefits of both n-6 and n-3 PUFA. There are many conceptual limitations to the dietary n-6:n-3 ratio that render it clinically and biologically irrelevant. As observed by Lucas, ‘What is the usefulness of the ratio of n-6 to n-3, which is good divided by good?’(Reference Lucas141).
Guidance for health professionals on oils high in UFA
Dietary guidance for general health and CVD prevention focuses on dietary patterns rather than single foods or nutrients(9–Reference Kirkpatrick, Sikand and Petersen12,Reference Mach, Baigent and Catapano53–Reference Visseren, Mach and Smulders55) . Recommendations are based on evidence from RCT and observational studies conducted in various populations that has demonstrated healthful dietary patterns are associated with lower risk of CVD(Reference Hu, Wu and Dearborn142–Reference Morze, Danielewicz and Hoffmann144). Several healthful dietary patterns have been described, such as the Mediterranean, Dietary Approaches to Stop Hypertension, Healthy U.S.-style and plant-based patterns. Common elements to healthful dietary patterns include an emphasis on minimally processed foods, including fruits, vegetables, whole grains, healthful sources of proteins (e.g. fish, seafood, beans, lentils, tofu and other soy foods, nuts and seeds) and non-tropical, liquid plant oils in place of solid (more saturated) fats. Healthful dietary patterns are also low in processed meats, refined grains, added sugars, salt, SFA and trans fatty acid(9–Reference Kirkpatrick, Sikand and Petersen12,Reference Mach, Baigent and Catapano53–Reference Visseren, Mach and Smulders55,Reference Gardner, Vadiveloo and Petersen145) .
Vegetable oils containing PUFA can be routinely used in cooking with some considerations for proper use. Concerns surrounding the use of PUFA-containing oils include UFA oxidation and the production of other potentially harmful by-products(Reference Yamashima, Ota and Mizukoshi21). The smoke (burning) point is important for determining the type of oil to use in cooking (i.e. high v. low heat). Several research groups and/or organisations have provided smoke point measurements for a variety of edible oils(Reference De Alzaa, Guillaume and Ravetti146–149). Several oils with moderate to high amounts of PUFA have a high smoke point, such as avocado, peanut, canola and sunflower oils, and can be used with higher heat cooking without adverse consequences (Table 4).
Table 4. Smoke point of vegetable oils*

* Values represent typical smoke points for commercially available edible oils based on tests conducted on various oil batches at various laboratory facilities. The values do not represent a statistically valid mean or indicate the range of values from a single source for each of the oils.
In contrast, oils like extra-virgin olive oil have a lower smoke point and should be used for low-heat cooking only or in recipes that do not require cooking (e.g. salad dressing) (Table 4). Deep frying with low smoke point oils should be avoided because the temperature of oils during deep frying exceeds the recommended temperature (180 °C or 356° F) to avoid the production of harmful by-products. The repeated use of frying oil (i.e. 8–10 frying cycles), especially at higher cooking temperatures and with intermittent heating and cooling of the oil, results in the increased production of free fatty acids, SFA and trans fatty acid(Reference Andrikopoulos, Dedoussis and Falirea150–Reference Bhat, Maganja and Huang155). Although the repeated use of frying oils is a potential concern with commercial establishments, reusing frying oil may be a practice in some populations (e.g. Asian Indians)(Reference Bhardwaj, Passi and Misra152). Thus, consumers should receive education to avoid reusing oils when cooking.
Proper storage of vegetable oils is important to prevent them from going rancid and developing an unpleasant smell or flavour. Heat, light and exposure to oxygen increase the risk of oils turning rancid(Reference Okparanta, Daminabo and Solomon156). Proper storage of vegetable oils includes keeping them in a dark place (e.g. pantry or cupboard), reducing air exposure by placing the cap on tightly between uses and keeping oils at room temperature. Oils should not be stored on a countertop near a stove or oven because this will increase both light and heat exposure. For freshness and quality, it is recommended that vegetable oils be used within 6–12 months of purchase, if stored properly, and within 3–5 months after opening, if stored properly(157).
Summary and conclusions
For many decades, the relationship between dietary fat intake and health has been rigorously investigated with an emphasis on CVD. In many countries, current dietary guidelines do not include recommendations to limit total fat intake, rather, the focus is on the type of fat to consume. Strong and consistent evidence demonstrates that higher intake of PUFA is associated with lower risk of incident CVD. In addition, replacement of SFA with PUFA reduces the risk of CAD events and CVD mortality. Less data are available for the relationship between MUFA intake and CVD, although the existing evidence suggests higher intake of MUFA from plant sources is associated with lower risk of CVD. In alignment with observational evidence, results from clinical trials show higher intake of PUFA and MUFA from plant-sources improves major CVD risk factors, including levels of atherogenic lipids and lipoproteins. Observational and clinical evidence also suggests that diets higher in UFA reduce the risk of developing T2DM and increase insulin sensitivity.
One topic of debate within the fatty acid field is the clinical relevance of the dietary ratio of n-6 to n-3 fatty acids. At one point, the consensus was that a high ratio was considered to be harmful because LA and ALA compete for desaturation enzymes, and because metabolites of n-6 AA were considered to be pro-inflammatory. However, the utility of this ratio has been rejected by health agencies throughout the world. One reason for this rejection is the recognition that the in vivo conversion of LA to AA is negligible, and another reason is that some metabolites of AA exert anti-inflammatory effects. There is also concern that, because of its multiple double bonds, LA intake could promote oxidative stress. However, clinical trial evidence shows the intake of n-6 PUFA does not increase markers of inflammation or oxidative stress. Nevertheless, because carbon–carbon double bonds are susceptible to oxidation, high n-6 PUFA oils can become rancid if improperly stored. Therefore, consideration of how these oils are stored and how they are used in cooking, especially frying, is important.
In conclusion, authoritative health and scientific organisations recommend intake of n-6 PUFA-containing vegetable oils, including seed oils, as part of healthful dietary patterns. Specifically, vegetable oils rich in UFA should be consumed instead of rich sources of SFA, such as butter, tallow, lard, palm and coconut oils, duck fat and ghee.
Acknowledgements
None.
This work was supported by the Soy Nutrition Institute Global, United Soybean Board, Corn Refiners of America, National Corn Growers Association, Canola Council of Canada and USA Canola Association. These funders had no role in the design, analysis or writing of this article.
K. S. P., K. C. M., P. C. C., M. A. B., M. M., C. F. K. and W. S. H. conducted literature searches and wrote sections of the manuscript, K. S. P. & M. M. drafted the manuscript, all authors critically reviewed the manuscript.
Financial support for K. S. P., K. C. M., M. A. B., C. F. K., and W. S. H. was provided by the Soy Nutrition Institute Global, United Soybean Board, Corn Refiners of America, National Corn Growers Association, Canola Council of Canada and USA Canola Association. In the last 3 years, K. S. P. has received research grants from Cotton Incorporated, National Cattlemen’s Beef Association, Hass Avocado Board, American Pecan Council, American Egg Board, American Pistachio Growers and McCormick Science Institute. Over the last 3 years, K. C. M. has received research funding and/or consulting fees from 89Bio, Acasti Pharmaceuticals, Beren Therapeutics, Cargill, Eli Lilly & Company, Hass Avocado Board, Indiana University Foundation, Matinas Biopharma Holdings, Inc., Medifast, National Dairy Council, NewAmsterdam Pharma, North Sea Therapeutics, Pharmavite. P. C. C. acts as an consultant/advisor to BASF, Baxter Healthcare, Bunge, Cargill, Danone Nutricia Research, dsm-fermenich, Fresenius-Kabi, Smartfish. M. A. B. serves on the American Society for Nutrition board of directors and has received research funding from the United Soybean Board. M. M. is employed by Soy Nutrition Institute Global, which is funded by the United Soybean Board and membership dues from organisations involved in the manufacture and/or sale of soy foods or soy ingredients. C. F. K. is an employee of Midwest Biomedical Research and has no additional conflicts of interest to report beyond those disclosed for K. C. M., except that she serves on the National Lipid Association Board of Directors. W. S. H. holds stock in OmegaQuant Analytics, LLC, a laboratory that offers fatty acid testing.