INTRODUCTION
The HIV-infected population constitutes a heterogeneous group of individuals of different age, gender, ethnic background, geographic setting, access to medical care and other demographic aspects. Age [Reference Perez and Moore1, Reference Grabar2] and gender [Reference Kuyper3–Reference Fardet6] as differential clinical, analytical and evolutive factors have already been analysed in different cohorts of HIV-infected patients. A large amount of information has also been reported regarding different clinical, immunological, virological and evolutive aspects of patients with either the sexual or parenteral transmission categories of the infection. However, a few studies have compared these aspects simultaneously in both transmission groups, sometimes with discordant results. Thus, whereas some authors reported an influence of HIV transmission categories on CD4 counts [Reference Fardet6, Reference Caro-Murillo7], others failed to find such differences [Reference Smith8]. Therefore the issue remains largely unsolved.
Several factors may explain these apparently discordant results, such as different sample size, design, epidemiological settings, ethnic background, access to medical care, periods of inclusion and antiretroviral regimens used, among others. Therefore, a large study involving a homogeneous population from these important points of view is desirable to evaluate the possible influence of different transmission categories on clinical, immunological and virological parameters.
The Grupo Español para el Estudio Multifactorial de la Adherencia (GEEMA) study prospectively recorded much data from a large number of HIV-infected patients recruited from 69 hospitals across Spain (see Appendix), who initiated a new antiretroviral regimen that included nelfinavir as the backbone. These facts, among other factors, allowed us to study a large and relatively homogeneous population in search of possible differences in the diverse HIV transmission categories.
Therefore the aim of this study was to evaluate the comparative demographic, clinical, virological and immunological parameters of HIV-infected individuals according to the HIV transmission categories, both at baseline and during highly active antiretroviral therapy (HAART), in a well-characterized, nationwide, large cohort of patients who were followed up for 1 year.
METHODS
Many sociodemographic, clinical, viroimmunological and therapeutic data were recorded for the patients of the GEEMA cohort, including route of acquisition of the HIV infection. All patients had initiated a HAART regimen composed of nelfinavir plus at least two other antiretroviral drugs, mostly nucleoside reverse transcriptase inhibitors (NRTI), between January 1998 and December 1999. Patients were evaluated at baseline and at 3, 6 and 12 months from the onset of nelfinavir treatment. Data were recorded in a standardized form at each participating institution and were introduced into a computerized database at one single centre. Regarding the HIV transmission categories, patients were divided into four groups: injecting drug users (IDUs), men who have sex with men (MSM), heterosexual transmission, and other/miscellaneous transmission routes, which included haemophiliacs and patients that had acquired the infection through transfusion or other parenteral transmission routes. Medical care, analytical studies and antiretroviral drugs are provided free of charge to all HIV-infected patients in Spain. Therefore, no major bias was expected in relation to different access to medical care or treatment in the different transmission categories.
CD4 counts were measured by flow cytometry and viral load by branched DNA and RNA–PCR assays. The limit of detection of viral load measurements was established at 200 copies/ml. Adherence was assessed by a simplified medication adherence questionnaire [Reference Knobel9]. Patients were classified as fully adherent if they showed good adherence at all evaluations. If adherence was suboptimal at any evaluation, patients were considered non-adherent, regardless of the adherence observed at other time points. Individual HIV transmission categories were available for a total of 3039 patients who comprised the study group. Data were analysed by an intention-to-continue-treatment approach, ignoring eventual changes in treatment during follow-up.
Statistical analyses
The parameters under study did not follow a Gaussian distribution according to the Kolmogorov–Smirnov test. Consequently non-parametric tests were used for statistical analyses. The Mann–Whitney U test was used for the comparison of continuous variables in two groups and the Kruskal–Wallis test for the comparison in more than two groups. Categorical variables were analysed with the χ2 test. The independent predictive values of different variables on CD4 counts and viral load were assessed by stepwise multiple linear regression analyses. The independent associations between baseline parameters and clinical progression or death, adherence, suppression of viral load and losses to follow-up were evaluated by stepwise logistic regression analyses. A multivariate general linear model repeated-measures procedure was performed to evaluate the comparative effects over time of HAART on the CD4 counts and viral load in the four transmission categories. SPSS version 14.0 (SPSS Inc., USA) was used for statistical analyses. A P value <0·05 for a two-sided test was considered to be statistically significant.
RESULTS
A total of 3039 patients were included in the study. Most were men (73·1%), and had received prior antiretroviral therapy at the time of inclusion (79·5% experienced, 20·5% naive). Table 1 depicts the baseline sociodemographic, clinical and laboratory characteristics of the patients classified according to the four transmission categories, as well as the statistical comparisons among them.
Table 1. Baseline characteristics of the patients
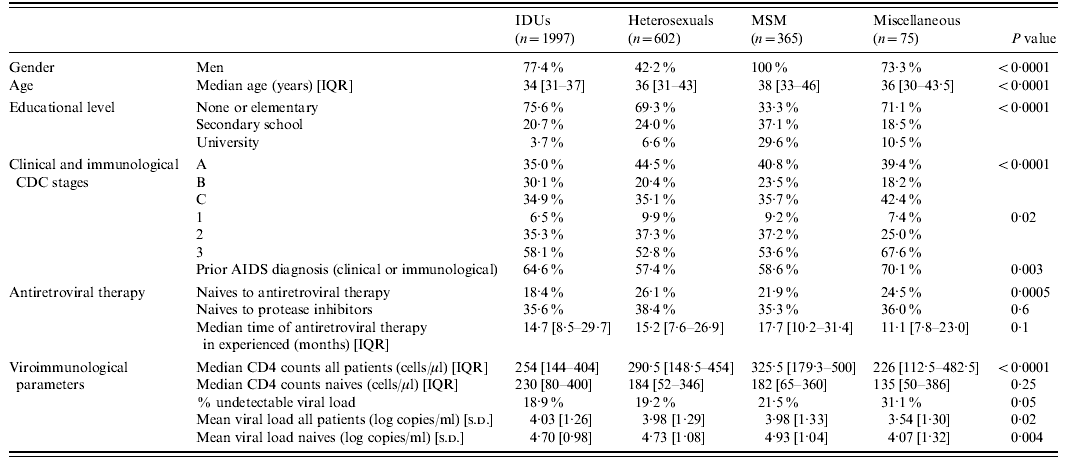
IQR, Inter-quartile range; IDUs, injecting drug users; MSM, men who have sex with men.
Regarding the antiretroviral regimens used at the time of inclusion in the study, most patients received only NRTI in addition to nelfinavir, without significant differences in the diverse transmission categories (IDUs 75·9%, heterosexuals 79·4%, MSM 74·2%, miscellaneous 72·0%; P=0·2). Adverse events severe enough to stop nelfinavir treatment occurred in 13·1% of IDUs, 12·4% of heterosexuals, 12·8% of MSM, and 11·5% of the miscellaneous group (P=0·9).
Clinical progression or death during follow-up was observed in 9·4% of IDUs, 10·2% of heterosexuals, 6·8% of MSM, and 6·3% of the miscellaneous group (P=0·4). Missed cases at the 12-month appointment were very similar in heterosexual (21·9%), MSM (21·9%) and miscellaneous (21·3%) categories, but significantly more frequent in the IDU group (33·1%, P<0·0001). Adherence rate was also significantly different in the four categories (IDUs 38·3%, heterosexuals 54·2%, MSM 60·7%, miscellaneous 68·2%, P<0·0001). Patients actively engaged in injecting drug use or who were receiving substitutive methadone therapy missed the 12-month evaluation more frequently and had poorer adherence rates than the other patients of the IDU group (44·8% vs. 32·1%, P=0·0002, and 26·5% vs. 39·0%, P=0·001, respectively).
Figure 1 shows the course over time of CD4 counts and Figure 2 the course of viral load in each of the four transmission categories. Patients who had acquired the infection through sexual contact (MSM or heterosexual) had significantly higher CD4 counts than the other patients, a pattern also observed in both the antiretroviral-naive and experienced patients (data not shown). The multivariate general linear model repeated- measures procedure revealed that the course over time of CD4 counts was significantly different in the four transmission categories (P=0·01), particularly during the initial 3 months of therapy (P=0·0001). Regarding post-hoc inter-group comparisons, the most marked differences were observed between the IDU and MSM groups (P<0·0001) and between the IDU and heterosexual groups (P=0·06). On the contrary, the general linear model did not find differences in the course of viral load in the four transmission categories (P=0·4).
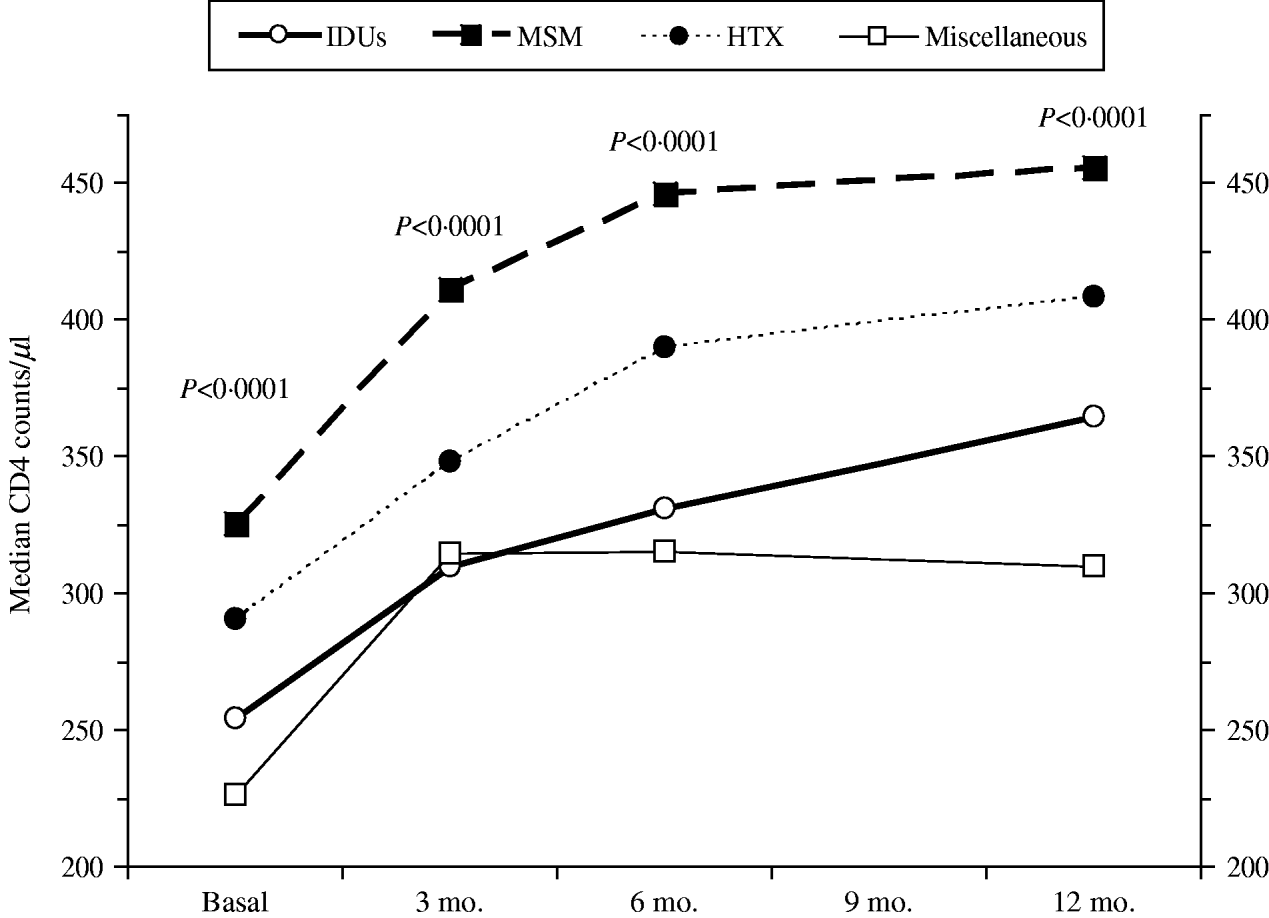
Fig. 1. CD4 counts over time according to the HIV transmission categories: men who had sex with men (MSM), heterosexuals (HTX), injecting drug users (IDUs), and the miscellaneous group.
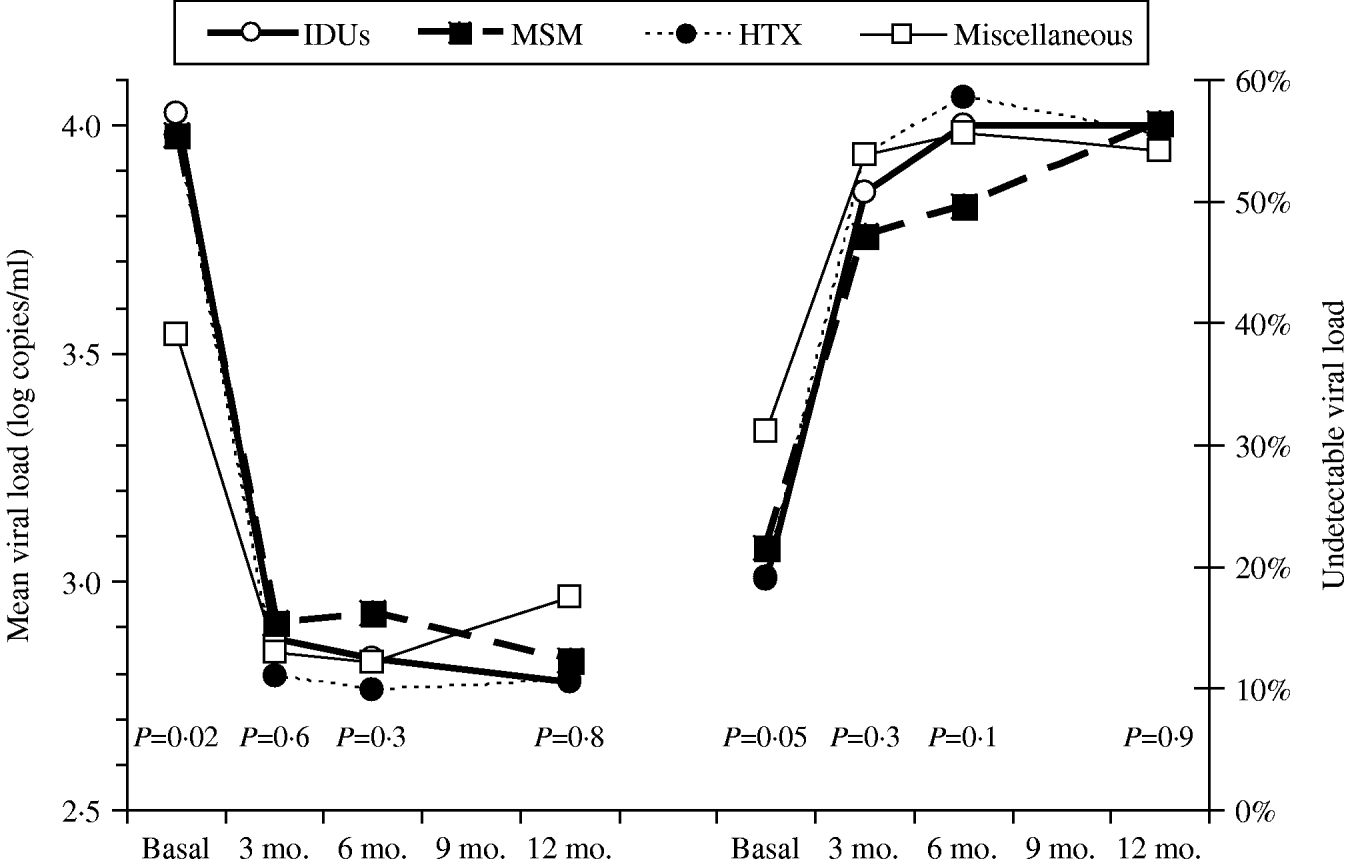
Fig. 2. Viral load (left panel) and rates of suppressed viral load (right panel) over time according to the HIV transmission categories: men who had sex with men (MSM), heterosexuals (HTX), injecting drug users (IDUs), and the miscellaneous group.
Multiple regression analyses were performed to evaluate the independent association of transmission categories, as well as many other parameters, with viral load and CD4 counts both at baseline and at 12 months. Of the diverse parameters analysed, baseline CD4 counts were significantly associated with HIV transmission categories (P=0·01), immunological CDC stage (P<0·0001), baseline viral load (P<0·0001), HAART experience (P<0·0001) and clinical CDC stage (P=0·03). Viral load at 12 months was also associated with transmission categories (P=0·0006), naive status (P<0·0001), baseline CD4 counts (P<0·0001), baseline viral load (P<0·0001), CD4 counts at 12 months (P<0·0001), CDC clinical stage (P<0·0001), adherence (P<0·0001), age (P=0·02) and CDC immunological stage (P=0·04). On the contrary, there was no significant relationship between transmission categories and baseline viral load or final CD4 counts.
Table 2 shows the results of the logistic regression analyses performed to evaluate the independent association of diverse baseline parameters (CD4 counts, viral load, gender, age, transmission categories, prior HAART experience and clinical and immunological CDC stages) with clinical progression or death, adherence, missing of the 12-month evaluation and suppressed viral load at 12 months. As can be seen, transmission categories had a significant impact on adherence to antiretroviral therapy, failure to fulfil the 12-month appointment and viral suppression at the end of the follow-up period. On the contrary, no such association was found with clinical outcomes.
Table 2. Logistic regression analyses
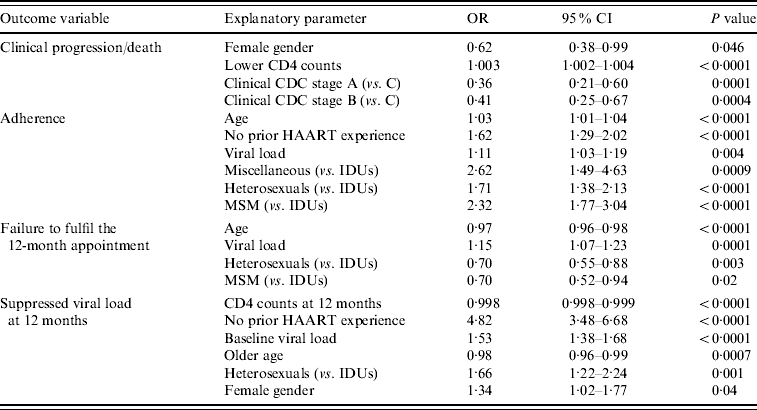
OR, Odds ratio; CI, confidence interval; HAART, highly active antiretroviral therapy; IDUs, Injecting drug users; MSM, men who have sex with men.
DISCUSSION
We found differences in multiple baseline and follow-up parameters according to the different transmission categories. Thus there were significant differences in gender, age, educational level, clinical and immunological CDC stages, prior antiretroviral therapy, CD4 counts and viral load at baseline. Regarding the viroimmunological markers, multivariate analyses revealed that baseline CD4 counts showed an independent association with transmission categories, but such an association was not found with baseline viral load.
Similarly to our findings in naive and experienced patients, other studies have also found higher CD4 counts at baseline in MSM than in other transmission categories in patients not receiving HAART [Reference Fardet6, Reference Caro-Murillo7], although other authors have reported the opposite [Reference Von Overbeck10], and a large study conducted prior to the HAART era did not find appreciable differences in CD4 counts at the time of AIDS diagnosis according to different transmission routes [Reference Phillips11]. We also found that the slope of the time-course curves, i.e. the response over time to HAART, was also different in the groups, especially during the first 3 months after the onset of the nelfinavir-based regimen. Regarding virological parameters, some authors did not find differences in viral load at baseline according to transmission categories in antiretroviral-naive patients [Reference Rezza12], whereas others reported that, adjusting for other covariates, IDUs had poorer viral load suppression than other transmission groups [Reference Rodríguez-Arenas13, Reference Greub14]. In our series, the differences observed at baseline in viral load and viral suppression disappeared shortly after the onset of the new therapeutic regimen when analysing crude data, but transmission categories had an independent association with both viral load and viral suppression after adjustment for covariates.
We observed that IDU patients had significantly lower CD4 counts at baseline and during follow-up than MSM and heterosexual groups, but not lower than the miscellaneous group, although the reduced sample size of the latter was probably responsible for the lack of statistical significance. These findings raise the possibility of a role of hepatitis C virus (HCV) co-infection in conditioning blunted immunological responses, as has been suggested by some [Reference Greub14–Reference Nunez17], but not all [Reference Anderson, Guest and Rimland18, Reference Sullivan19] studies, because HCV co-infection is much more common in IDUs than in other transmission categories across different ethnic groups [Reference Mohsen, Murad and Easterbrook20, Reference Buffington21]. Unfortunately the patients' HCV status was not recorded in the GEEMA database and, consequently, this issue cannot be addressed in our study. However, most of the differences between the heterosexual and IDU groups were derived from their different baseline set points, and IDU patients also had significantly increased CD4 counts following HAART. Therefore, the effect of HAART clearly overcomes any potentially negative effect of HCV on CD4 recovery, although it cannot be excluded that HCV co-infection could have conditioned somewhat lower CD4 responses to HAART in our patients.
Unsurprisingly, adherence to antiretroviral therapy was poorer in IDUs than in the other groups in univariate and multivariate analyses, particularly in individuals engaged in active drug use. However, the poorer CD4 course of the IDU group did not seem to be due to differences in adherence, because the latter was not selected by the multivariate model to explain the CD4 counts one year after the onset of therapy (P=0·2). On the contrary, we found that adherence had a significant impact on the course of viral load, a finding that supports previous reports that also described poorer virological outcomes in non-adherent patients [Reference O'Connell22–Reference Mannheimer24]. However, our results do not agree with the findings of one of these studies that found similar rates of HIV suppression in IDUs than in non-IDUs once lower levels of adherence were taken into account [Reference Wood23]. In fact we found that transmission categories had a significant influence on viral load and viral suppression at 12 months after adjustment for adherence, and that such an influence was also observed when both adherent and non-adherent patients were analysed separately (data not shown).
However, other protease inhibitors boosted with ritonavir, which have better pharmacological profiles, fewer number of pills, higher genetic barriers, better tolerance and more potency against HIV, could help to overcome the poorer adherence of IDUs. On the other hand, concerns about the development of drug resistance in IDUs owing to their poorer adherence do not seem to be justified according to the results of a large study on naive patients, which found that resistance to all major classes of antiretrovirals were similar in IDUs and non-IDUs after a long follow-up [Reference Wood25].
Similarly to other studies that reported more frequent losses to follow-up in IDU patients [Reference Fardet6, Reference Lanoy26], we found that the IDU group failed to fulfill the 12-month evaluation more commonly than the other groups in univariate and multivariate analyses. On the contrary we did not find differences in the transmission categories in the rate of adverse events. Other studies also found no differences between IDUs and non-IDUs regarding the stopping of indinavir or nelfinavir therapy, although such differences were observed in the case of efavirenz treatment [Reference Hirschel27].
We also failed to find significant differences in the clinical outcomes (progression or death) during follow-up, although MSM had slightly better outcomes than IDUs. Perhaps the relatively short follow-up period and the low number of patients who died or progressed may have precluded the emergence of statistical differences. However, the impact of transmission categories on clinical outcome does not seem very important as other, stronger parameters such as gender, CD4 counts and clinical CDC stages were included in the model. Other large studies also failed to find significant differences in clinical outcomes following HAART in diverse HIV transmission groups [Reference Fardet6, Reference Von Overbeck10], although some authors found higher mortality rates from AIDS in IDU patients [Reference Rodríguez-Arenas13, Reference Greub14].
Our study has several advantages over other reports published on this topic. A major point is the fact that all of our patients received an antiretroviral regimen based on the same drug, nelfinavir, in most cases combined with two NRTIs, whereas patients included in other large series received diverse, mostly unknown antiretroviral regimens. Therefore our comparison of the different transmission categories is quite reliable, minimizing bias such as, for example, the use of selected regimens for expectedly less adherent patients such as IDUs. In addition, our study was multicentre, nationwide and accurately reflected the situation across Spain, reducing bias such as particularly prevalent transmission categories or management habits in more restricted settings. Moreover, the ethnic and racial variability common to large studies, and its consequences in demographic, clinical and viroimmunological factors [Reference Anastos28, Reference Hessol and Palacio29], was minimal in our series as about 90% of patients were Caucasian of Spanish origin. In addition, the prospective collection of data in our study provided additional advantages over other series based on historic databases, and the fact that all HIV patients in Spain have free access to medical care and antiretroviral therapy minimizes bias related to poorer access in the less socially advantaged transmission categories. Finally, all our patients were included at a time when the standard of care was to provide antiretroviral therapy to all patients regardless of their clinical, virological or immunological situation. This fact allows the evaluation of the full spectrum of the HIV infection, an analysis that could not be performed today because the current treatment guidelines recommend treatment only for patients with moderate to advanced HIV disease.
Limitations to our study include the relatively short follow-up period, the fact that current antiretroviral regimens may be different from those used in our study, potential difficulties in the extrapolation of our results to other countries with different epidemiological settings, possible mistakes in the recording and transcription of such a large amount of data and the different methods used for the determination of viral load. However, considering our large sample size it is expected that such potential shortcomings were evenly distributed in all transmission groups and, therefore, their impact on the results would have been minimal. In addition, the use of different viral load assays is acceptable for multicentre studies [Reference Schuurman30] and, moreover, this limitation would not affect to sociodemographic, clinical and immunological parameters and outcomes.
We conclude that there are demographic, clinical, virological and immunological differences in the different HIV transmission categories at baseline, and that some of them persisted or increased during antiretroviral therapy. In particular, MSM had more favourable CD4 profiles than the heterosexual and IDU groups. Compliance with both antiretroviral therapy and medical appointments were also influenced by transmission categories, particularly in the case of IDUs, but adherence issues did not explain all differences observed. Our findings underscore the importance of these epidemiological factors and the convenience of adapting medical interventions and therapeutic strategies to them, as well as the need for considering IDU-related issues as a relevant part of the HIV management strategies.
ACKNOWLEDGEMENTS
The GEEMA study was partially funded by Roche Farma, Spain, in the organizational aspects. The sponsor did not have any role in the study design, in the analysis or interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
APPENDIX: Investigators of GEEMA
Teresa Vázquez Castañón, H. Alvarez Buylla, Asturias. Ana Mariño Callejo, H. Arquitecto Marcide-Novoa Santos, La Coruña. Daniel Podzamczer Palter, H. Bellvitge, Barcelona. Ramón Canet, H. Can Misses, Ibiza. José Manuel Antunez Galvez, H. Carlos Haya, Málaga. Ma Victoria Gordillo, H. Carlos III, Madrid. Víctor Roca Arbones, H. Clínico San Carlos, Madrid. Luis Force, H. Consorcio Sanitario de Mataró, Barcelona. Jordi de Otero, H. Creu Roja, Barcelona. Isabel Gracia Marce, H. Creu Roja, Hospitalet, Barcelona. Ma José López Alvarez, H. de Calde, Lugo. José Luis Mostaza Fernández, H. del Bierzo, León. Hernando Knobel, H. del Mar, Barcelona. Luis Morano Amado, H. do Meixoeiro, Pontevedra. Josep Cucurull, H. Figueres, Gerona. Carlos Tornero Estébanez, H. Francesc de Borja, Gandia, Valencia. Julio Collazos, H. Galdakano, Vizcaya. Elisa Martínez Alfaro, H. General de Albacete, Albacete. Luis Caminal Montero, H. General de Asturias, Asturias. Jordi Uso Blasco, H. General de Castellón, Castellón. Elena Losada, H. General de Galicia, Santiago, Coruña. Enric Pedrol, H. General de Granollers, Barcelona. Manuel Rodríguez Zapata, H. General de Guadalajara, Guadalajara. Ester Dorca, H. General de Manresa, Barcelona. Alfredo Cano Sánchez, H. General de Murcia, Murcia. Josep Vilaró, H. General de Vic, Barcelona. Juan F. Lorenzo, H. General Yagüe, Burgos. Ma Luisa García-Alcalde, H. de Cabueñes, Asturias. Ignacio Suárez Lozano, H. Infanta Elena, Huelva. Javier Juega Puig, H. Juan Canalejo, La Coruña. José Joaquín Peraire Forner, H. Juan XXIII (Universitari Rovira I Virgili – Institut de Estudis Avançat), Tarragona. José López Aldeguer, H. La Fe, Valencia. Juan González García, H. La Paz, Madrid. Francisco Pasquau Liaño, H. Marina Baixa, Villajoyosa, Alicante. Carmen Fariñas Alvarez, H. Marques de Valdecilla, Santander. Rafael Ojea de Castro, H. Montecelo, Pontevedra. Carlos Barros Aguado, H. de Móstoles, Madrid. David Dalmau, H. Mutua de Tarrasa, Barcelona. José A. Iribarren, H. Ntra. Sra. de Aránzazu, Guipúzcoa. Fernando Marcos Sánchez, H. Ntra. Sra. del Prado, Toledo. Eva León Jiménez, H. Ntra. Sra. de Valme, Sevilla. Manuel Cervantes, H. Parc Tauli, Barcelona. Manuel Camba Esteve, H. Policlinico Vigo, Pontevedra. Alberto Arranz Caso, H. Príncipe de Asturias, Madrid. Ricardo Rodríguez Real, H. Provincial Rebullón, Pontevedra. Joan Colomer, H. Provincial Sta. Caterina, Gerona. Teodoro Martín Jiménez, H. Puerta de Hierro, Madrid. Eugenio Pérez Guzmán, H. Puerta del Mar, Cádiz. Antonio Vergara de Campos, H. Puerto Real, Cádiz. José Luis Casado Osorio, H. Ramón y Cajal, Madrid. José Ma Kindelan Jaquotot, H. Reina Sofía, Córdoba. Ma José Tuya Moran, H. San Agustín, Asturias. José Ma Cuadrado Pastor, H. San Juan, Alicante. Antonio Delegido Sánchez, H. Sant Pau I Santa Tecla, Tarragona. Pere Domingo Pedrol, H. Sant Pau, Barcelona. Raúl Rodríguez Pérez, H. Santa María Nai- Cabaleiro Goas, Orense. Alberto Terrón Pernia, H. Sas de Jerez, Cádiz. Joseba Portu. H. Txagorritxu, Vitoria. Víctor Carcaba Fernández, H. Valle del Nalón, Asturias. Isabel Ruiz Camps, H. Valle Hebrón, Barcelona. Ismael Piñas Forcadell, H. Verge de la Cinta, Tarragona. Eduardo Rodríguez de Castro, H. Verge del Toro, Menorca. Paloma Geijo, H. Virgen de la Luz, Cuenca. Fernando Cuadra García-Tenorio, H. Virgen de la Salud, Toledo. Manuel Marquez Solero, H. Virgen de la Victoria, Málaga. Carlos Galera Peñaranda, H. Virgen de la Arrixaca, Murcia. Miguel Angel Colmenero Camacho, H. Virgen de la Macarena, Sevilla. José Adolfo García Henarejos, H. Virgen del Rosell, Murcia. Antonio Ocampo, H. Xeral Xies, Vigo.
DECLARATION OF INTEREST
None.






