Introduction
A small number of older adults, over 50 years old, in nursing home and in geriatric settings have behavioral or psychological symptoms or syndromes that can exhaust caregivers and professionals (Monfort, Reference Monfort1995). Most often, the symptoms and syndromes are the result of interactions between biological, psychological and social factors, risk factors, and protection factors. Dementia and psychiatric disorders are the most frequent risk factors. Since 1994, Neuro Psychiatric Inventory for Nursing Homes (NPI-NH) is used for patients with dementia (Cummings et al., Reference Cummings1994) or without dementia (Korner et al., Reference Korner2008; Squelard et al., Reference Squelard2012), and sometimes with psychiatric diagnosis (Baranzini et al., Reference Baranzini2013; Iverson et al., Reference Iverson, Hopp, DeWolfe and Solomons2002). For these older adults, professionals would need advice and support (Droes et al., Reference Droes2005) regarding appropriate medical or psychosocial interventions (Van Mierlo et al., Reference Van Mierlo, Van der Roest, Meiland and Droes2010).
The initial NPI scale, composed of 10 items, was followed in 1997 by a 12-item version including assessments of sleep and appetite (Cummings, Reference Cummings1997). A nursing home version (NPI-NH) with minor modifications was developed to interview professionals involved in the daily care of patients (Wood et al., Reference Wood2000; Lange et al., Reference Lange, Hopp and Kang2004). Translated into 92 languages, including French (Robert et al., Reference Robert1998; Sisco et al., Reference Sisco2000), the NPI and, later, the NPI-NH became the most commonly used rating scale for assessing challenging behavior in older patients.
Despite its many strengths, the NPI-NH has some limitations. NPI-NH interrater reliability has been observed to be poor, whereas the CMAI interrater reliability is good (Zuidema et al., Reference Zuidema2011). The need to train interviewers (Boada et al., Reference Boada, Tarraga, Modinos, Lopez and Cummings2005) and the overly long administration time have led to the development of the NPI-Q, a brief version with a self-administered questionnaire replacing the interview. The NPI-Q assesses only severity because severity and frequency ratings are highly correlated on the NPI (Kaufer et al., Reference Kaufer2000). Missing items and the need for clinical judgment led to the NPI-Clinician (NPI-C), which includes 78 new items (de Medeiros et al., Reference de Medeiros2010). When an error is made on a frequency or a severity score, this error is multiplied.
Because of these limitations, we developed the Psychogeriatric Inventory of Disconcerting Symptoms and Syndromes (PGI-DSS). The questions are replaced by unambiguously worded items formed by blocks of words that are immediately understandable (Zuidema et al., Reference Zuidema2011).
Methods
Scale construction
The construction of this measure lasted from 2003 to 2012. A Balint group of geriatricians and psychiatrists met four times a year. This group listened to emotionally exhausted professional caregivers talking about violence and refusal behaviors, followed by the selection of their verbatim. An unexpected discovery was that some patients, without any violent or refusal behaviors, had the ability to exhaust professional caregivers because particular repetitive behaviors required greater presence and vigilance. Over the study period, another finding was that exhaustion could stem from either disconcerting words (DW) or disconcerting acts (DA). This explains why the tool first comprised two, then three, and in the end, four syndromes. The number of items rose from eight items in 2003–2006 (Monfort et al., Reference Monfort2006), to nine items in 2009, to 12 items in 2010 (Monfort et al., Reference Monfort, Lezy-Mathieu and Hugonot-Diener2010, and finally to 16 items in 2011–2012. A factor analysis of this 16-item scale produced the final mature scale, which was produced in 2012 (16 items distributed in four syndromes, shown in Table 2).
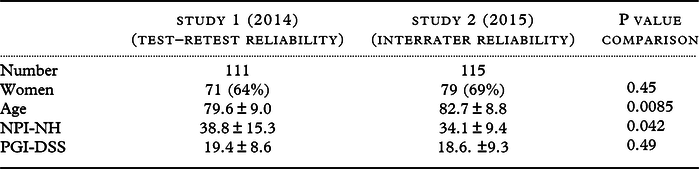
Table 1. Comparison of the characteristics of the patients included in Study 1 and Study 2
Disconcerting Older Person Inventory (PGI-DSS)
Neuro Psychiatric Inventory – Nursing Home Version (NPI-NH)
Table 2. Construct validity of the 16-item PGI-DSS assessed by principal component factor analysis and Cronbach’s alpha (226 patients)

Scale description
The PGI-DSS is a single A4-format worksheet that is foldable into four parts, easy-to-read in the palm of the hand and pocket sized (PGI-DSS is available in Appendix 1 and is also available for download in open access at authors’ website: www.psychoge.fr). The reverse side serves as a guide. A central drawing illustrates the caregiver’s “emotional keyboard.” This keyboard is surrounded by four drawings that illustrate the four disconcerting syndromes (violence, refusal, words, acts) linked to four emotional risks and in reaction, four inappropriate psychological attitudes, and four targeted psychosocial interventions. The front of the page gives the 16-item scale with the four syndromes, four items per syndrome, and four blocks per item, with each block containing words corresponding to four degrees of severity. Thus, the score ranges from zero to 64: the qualitative clinical refinement provided by each block of words is associated with a quantitative evaluation provided by its numerical value. Scoring indications are printed directly on the front page. The rater reads aloud the four blocks of words describing the severity of each item. To rate each item in a minimum amount of time, the items are read from left to right, from the most severe block to the least severe block. When a participant identifies the symptom as present, he/she simply has to raise a hand and say yes. The rater’s task consists of circling the block of words that describe the most severe situation reported in this way. Thus, the score circled is the highest score observed for the chosen period of reference. This avoids endless discussion about severity.
Participants and settings
Data were obtained from two studies that began in 2012 and included 111 patients and 115 patients. The raters were a geriatrician or a psychologist belonging to the unit where the professional caregivers were working. They asked the questions on the NPI-NH and read the PGI-DSS items aloud to the nursing staff. The interviews were conducted in the absence of the patients. The participants were nurses and nursing assistants involved in the daily care of the patient. The patients were over 50 years old and had stable symptoms over the previous week. The raters had no contact with the patients. The settings were 30 geriatric care units and nursing homes with units dedicated to patients with behavioral and psychological disorders. Two independent digital captures were performed, and input errors were corrected.
Outcomes
Age, sex, and NPI-NH and PGI-DSS scores were collected for all patients in the units in a given week. No further investigations were made. No attempt to collect medical or psychological diagnoses was made. Professionals’ distress was not measured. The French version of the Neuro-Psychiatric-Inventory, Nursing Home version (NPI-NH), i.e. the NPI-ES (Sisco et al., Reference Sisco2000), used in this study was the official French version provided by the French Health Ministry. Its use is mandatory in most French nursing homes and geriatric care units, and the clinician raters were all familiar with and trained over the years on the NPI-ES. The poor reliability of the appetite and sleep items (Leung et al., Reference Leung, Lam, Chiu, Cummings and Chen2001; Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008) led to the use of the 10-item version (Canevelli et al., Reference Canevelli2013) with scores ranging from zero to 120. The French version of the PGI-DSS is known as Echelle d’évaluation chez les Personnes Agées des symptômes et syndromes DEconcertants (EPADE).
Design
Study 1 was designed to assess test–retest (same rater) reliability. On day one, a rater administered the NPI-NH and the PGI-DSS to a sample of nursing staff. In the following week, the same rater administered the NPI-NH and the PGI-DSS to the same sample of nursing staff. Study 2 was designed to accurately assess interrater (between raters) reliability. On day one, a first rater administered the NPI-NH and the PGI-DSS to a first sample of nursing staff. In the following week, a second rater administered the NPI-NH and the PGI-DSS to a second sample of nursing staff.
Approval
Patients were not interviewed. Information on the PGI-DSS and the NPI-NH was obtained from nurses and nursing assistants. The data were strictly anonymous with no possibility of identifying the patients. In accordance with the present provisions of French law, the Comité de Protection des Personnes Ile de France II (Ethics Committee) confirmed that this study did not require its approval.
Statistical analysis
External validity
The correlation between the NPI-NH and PGI-DSS scores was calculated with the total sample, i.e. 226 patients. To calculate the cutoff score, receiver operating characteristic (ROC) curve analysis was used to find a cutoff score corresponding to the NPI-NH cutoff required for admission to special units dedicated to patients with behavioral and psychological symptoms of dementia. This cutoff value is a score of 7 or greater on one item out of seven “productive” items: delusions, hallucinations, agitation-aggression, elation-euphoria, disinhibition, irritability-lability, and aberrant motor behavior (http://www.cmrr-nice.fr).
Internal validity
The analysis was conducted on the total sample, first by evaluating the factor structure using a principal component analysis (PCA) with an orthogonal rotation procedure (varimax rotation) and second by assessing its reliability using Cronbach’s alpha coefficient.
Reliability
Test–retest reliability (n = 111) and interrater reliability (n = 115) were calculated with the intraclass correlation coefficient (ICC) and the 95% confidence interval (CI). Data are expressed as the means ± standard deviation (SD) or frequencies (percentages). All tests were two-sided. P-values < 0.05 were considered significant. Statistical analyses were performed using the SAS 9.4 statistical package (SAS Institute Inc) and R (version 3.4.3) for the computation of the ICC.
Results
Sample description
The two study populations were similar in terms of sex distributions (Table 1). The patients scored in Study 1 were younger than those in Study 2 (80 ± 9 versus 83 ± 9 years old, p = 0.0085). Ages ranged from 61 to 102 years.
Convergent validity
The correlation coefficient between the NPI-NH and the PGI-DSS was good (r = 0.70; p < 0.0001) (Figure 1).
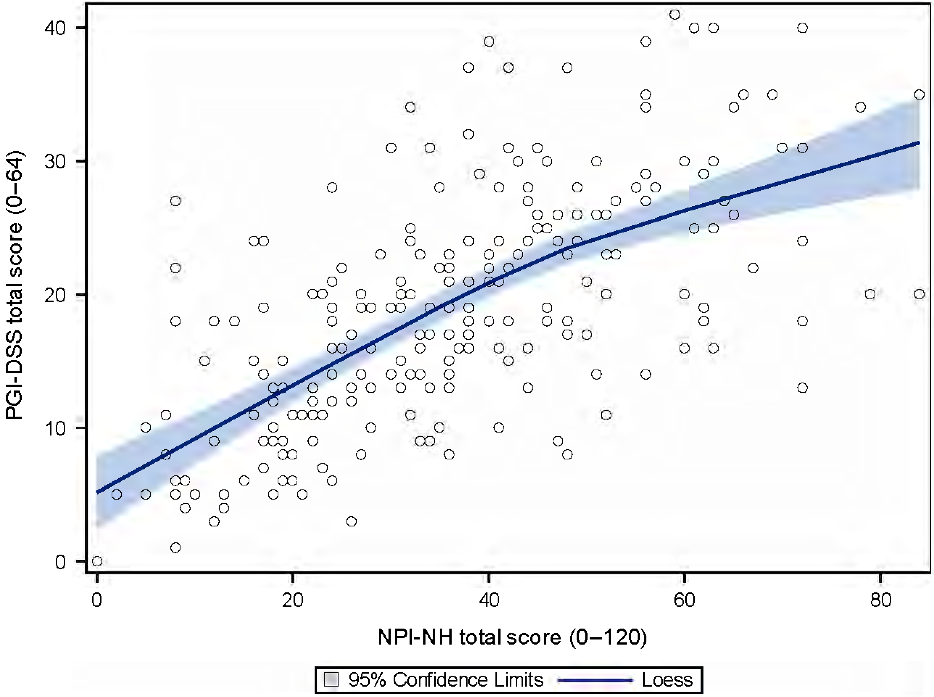
Figure 1. Convergent validity between the PGI-DSS score and the NPI-NH score (226 patients). Blue line: local regression line based on locally estimated scatterplot smoothing with the 95% confidence interval.
Threshold
Given a specificity of 87% and a sensitivity of 63%, the PGI-DSS threshold score corresponding to the NPI threshold score was 17 (Figure 2).

Figure 2. Receiver operating characteristic curve (ROC curve) to define the threshold score for the PGI-DSS. The dashed lines are the Sensitivity and 1-Specificity for a score of 7 or higher for one item among seven productive items (delusions, hallucinations, agitation-aggression, elation-euphoria, disinhibition, irritability-lability, aberrant motor behavior) of the NPI-NH.
Factor analysis
Four factors with factor loadings > 0.4 explained 53.4% of the total variance. Items 1 to 4 showed high factor loadings for the first factor, Disconcerting Violence (DV). Items 5 to 8 showed high factor loadings for the second factor, Disconcerting Refusal (DR) (Table 2). Items 9 to 12 showed high factor loadings for the third factor, DW. Items 13 to 16 showed high factor loadings for the fourth factor, DA. In comparison, four factors explained 59.7% of the total variance on the NPI-NH.
Internal consistency
For the PGI-DSS (226 patients), the Cronbach’s alpha value was good (Cronbach’s alpha = 0.695) and increased slightly for four items (DR1, DW3, DA1, and DA4) with a maximum of 0.704. In comparison, the value for the NPI-NH was lower (Cronbach’s alpha = 0.474) with the deletion of one item resulting in a large increase (Cronbach’s alpha = 0.521 after deletion, data not shown).
Reliability
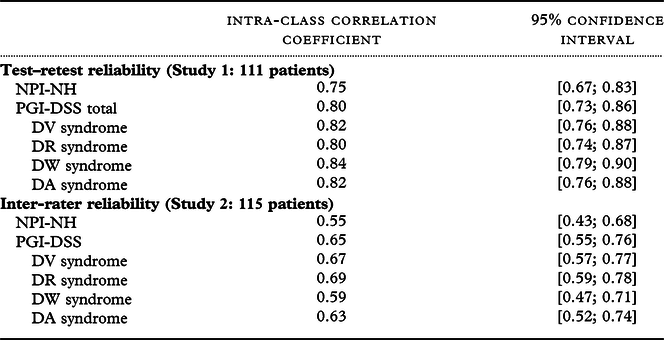
The PGI-DSS had a higher test–retest ICC (0.80 [0.73; 0.86]) than the NPI-NH (0.75 [0.67; 0.83]). The PGI-DSS had a higher interrater ICC (0.65 [0.55; 0.76]) than the NPI-NH (0.55 [0.43; 0.68]) (Table 3).
Table 3. Reliability (study 1 test–retest reliability and study 2 interrater reliability)
DV: Disconcerting Violence,
DR: Disconcerting Refusal,
DW: Disconcerting Words,
DA: Disconcerting Acts
Discussion
These results suggest that the PGI-DSS could be a valid and reliable scale. The convergent validity between the PGI-DSS and the NPI-NH was high. The four statistical factors in the PGI-DSS coincided almost perfectly with its four clinical syndromes. The internal consistency of the PGI-DSS was much higher than that of the NPI-NH. The test–retest and interrater reliability ICCs for the PGI-DSS were higher than the ICCs for the NPI-NH. These results could be explained by the construction of the PGI-DSS, with two differences from the NPI-NH. First, the wording in the PGI-DSS was selected from the professional caregiver verbatim so that it was immediately understandable by nurses. Second, raters were asked to read words rather than to ask questions.
Face/Content validity
During the nine years of the construction, the Balint group selected the symptoms (construct) which were considered at risk to exhaust the caregivers (measurement).
Construct validity
Construct validity of PGI-DSS is open to debate because it relies only on experience and judgment of the Balint group and especially JCM, AML, and AP, experts in the field of psychiatry and geriatrics, with daily practice in units dedicated to older people with behavioral and psychological symptoms.
Concurrent validity
External validity. The high convergent validity (r = 0.70; p < 0.0001) was similar to or higher than the convergent validity between the NPI-NH and the BEHAVE-AD (r = 0.38 to 0.72) (Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008). The PGI-DSS threshold score corresponding to the NPI threshold score was 17 (specificity: 87%, sensitivity: 63%).
Internal validity (statistical homogeneity of the items explored by factorial validity and internal consistency).
Four clear-cut statistical factors, with factor loadings > 0.4, explained more than 50% of the variance on the PGI-DSS. The excellent correspondence with the four clinical syndromes (Table 2) was the result of the nine-year construction period, involving nurses and including a factor analysis that contributed to guiding the development of the tool. In contrast, the number of statistical factors in the NPI-NH varies across translations, from five (Lange et al., Reference Lange, Hopp and Kang2004; Wang et al., Reference Wang2012; Chen et al., Reference Chen2018), to four (Baranzini et al., Reference Baranzini2013), to four or three (Selbaek and Engedal, Reference Selbaek and Engedal2012) or three factors (Reuther et al., Reference Reuther, Dichter, Bartholomeyczik, Nordheim and Halek2016). Despite these discrepancies, the relative consistency of the neuropsychiatric syndromes points to the importance of replacing the total score by a consideration of different neuropsychiatric syndromes (Aalten et al., Reference Aalten2008).
The internal consistency of the PGI-DSS (Cronbach’s alpha = 0.695) was much higher than that of the NPI-NH (0.474). With one exception, this PGI-DSS Cronbach’s alpha value was higher than most of the NPI-NH internal consistency values reported in six previous NPI-NH studies, with alpha values ranging from 0.55 to 0.83 (Wood et al., Reference Wood2000; Lange et al., Reference Lange, Hopp and Kang2004; Baranzini et al., Reference Baranzini2013; Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008; Chen et al., Reference Chen2018; Reuther et al., Reference Reuther, Dichter, Bartholomeyczik, Nordheim and Halek2016). The internal consistency of the PGI-DSS was also higher than that of the recent NPH-Diary with an alpha value of 0.581 (Morganti et al., Reference Morganti, Soli, Savoldelli and Belotti2018), higher than that of the Agitated Behavior Scale with an alpha value of 0.661 (Hellweg and Schuster-Amft, Reference Hellweg and Schuster-Amft2016), and higher than that of the recent Cohen Mansfield Agitation Inventory – Observation (CMAI-O) with an alpha value of 0.61 (Griffiths et al., Reference Griffiths2019).
Reliability (intra- and inter-rater)
The test–retest intraclass coefficient (ICC), i.e. the same rater or intrarater reliability, was higher for the PGI-DSS than for the NPI-NH (0.80 and 0.75, respectively). Five previous studies have assessed NPI-NH test–retest reliability and found lower (Zuidema et al., Reference Zuidema2011), similar (Wood et al., Reference Wood2000; Iverson et al., Reference Iverson, Hopp, DeWolfe and Solomons2002), or higher values (Chen et al., Reference Chen2018) up to 0.961 (Baranzini et al., Reference Baranzini2013).
The interrater ICC, i.e. between-rater reliability was higher for the PGI-DSS than for the NPI-NH, 0.65 and 0.55, respectively. Only three previous studies have assessed NPI-NH interrater reliability, and they found lower (0.42) (Zuidema et al., Reference Zuidema2011) or higher values (0.991) (Baranzini et al., Reference Baranzini2013), even reaching 1.00 with 100% concordance between the raters for delusions, hallucinations, euphoria, and irritability (Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008). These low and high interrater values were derived from two different methods. One study (Zuidema et al., Reference Zuidema2011) compared two interviews and obtained low values, while the other two studies (Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008; Baranzini et al., Reference Baranzini2013) used a single interview and compared the ratings of two raters present together. This single interview was conducted either by one of the raters or alternately by the two raters. This method naturally led to high values for interrater reliability, which explains why the interrater reliability was unexpectedly higher than the intrarater reliability (Baranzini et al., Reference Baranzini2013). As a result, NPI-NH interrater reliability was estimated paradoxically as either excellent (Selbaek et al., Reference Selbaek, Kirkevold, Sommer and Engedal2008) or poor (Zuidema et al., Reference Zuidema2011).
Clinical validity
The homogeneity of items and statistical significance of coefficient correlations can accompany a lack of clinical significance. As the innovative clinimetric approach was not used, neither our study nor previous NPI-NH studies provided an answer to questions pertaining to the microanalytical level of clinical validity, i.e. scalability and sensitivity. However, a discussion on the scalability of the two scales can be initiated: does their total score cover the same spectrum of neuro-psycho-geriatric symptoms? The convergent validity between the PGI-DSS and the NPI-NH suggests that these tools capture a similar broad spectrum of psychopathology. Nevertheless, this information is captured by two very different investigations. Comparisons are interesting for discussion.
The comparison of the assessment of symptoms by the PGI-DSS and the NPI-NH is not an easy task because there are, on the one hand, four groups (syndromes) and, on the other hand, 10 groups (domains).
The first comparison concerns the symptoms included in “DV” and the symptoms of aggression included in “agitation-aggression.” Whereas “DV” includes symptoms related exclusively to nonverbal, verbal, and physical violence, the “agitation-aggression” domain is explored by questions relating to physical violence and agitation. The “DV syndrome” excludes agitation, which is explored by the “disconcerting acts syndrome.”
The second comparison concerns the symptoms included in “Disconcerting Refusal” (refusal, opposition, passivity, apathy) and the symptoms included in “apathy-indifference” and “agitation-aggression.” Apathy, as explored by the NPI-NH, is often considered a scale in itself (Jones et al., Reference Jones2019). In comparison, each subsyndrome in the PGI-DSS refusal syndrome is regarded as a continuum ranging from the most severe (refusal) to the least severe (loss of motivation). The concept of this continuum was already present in the Pittsburg Agitation Scale (PAS) (Rosen et al., Reference Rosen1994), with a syndrome entitled “Resisting care.” This grouping underscores the risk of refusal when caregivers stimulate people who exhibit apathy. This continuum of behaviors from procrastination to striking out could also result from refusal with a mask of apathy. Another explanation could be the need that some patients may have to remain quiet. For these patients, stimulation, solicitation, help, and care, instead of being perceived as appropriate, can trigger violence toward caregivers.
The third comparison concerns the symptoms included in “Disconcerting Words” and its four subsyndromes and symptoms included in four NPI-NH domains: anxiety, depression-dysphoria, delusions, and hallucinations. The PGI-DSS does not explore melancholic symptoms such as guilt, incurability, or indignity. Exploration of moral pain is limited to suicidal ideation. The PGI-DSS includes delusions and hallucinations in the same subsyndrome. This grouping can help caregivers who can have difficulties in disentangling delusions from hallucinations.
The fourth comparison concerns the symptoms included in “Disconcerting Acts” and symptoms included in three NPI-NH domains: “agitation-aggression,” “disinhibition,” and “aberrant motor behavior.” These domains address the issue of restless behaviors and the importance of criteria enabling a distinction as to what is restlessness and what is not (Regier and Gitlin, Reference Regier and Gitlin2016). Assessment of these criteria can be easy or can lead to endless discussions when a patient presents numerous symptoms.
A remark is needed about the symptoms explored by the NPI-NH that the PGI-DSS does not cover. No single block of words in the PGI-DSS explores symptoms related to “feeling too good or too happy,” “laughing inappropriately,” “having a childish sense of humor,” and “telling jokes or saying things that are not funny to others.”
These comparisons of symptoms explored by the PGI-DSS and the NPI-NH raise the question of the correlation between the length of an exploration and its validity. Surprisingly, length cannot guarantee clinical validity (Duppen et al., Reference Duppen2019). For example, to screen for the severity of depression, only six items were shown to have better validity than long, traditionally used questionnaires (Fava et al., Reference Fava, Rafanelli and Tomba2012). Clinical reality can be adequately described using only a handful of items (Bech, Reference Bech2012).
Clinical utility
The first advance concerns the ease of administration and brevity. Acceptance of the PGI-DSS by caregivers is linked to its construction, making use of the narratives of caregivers, who can thus recognize their modes of speech when talking about symptoms. The administration time for the four syndromes in the PGI-DSS is less than 4 minutes. This should be compared with the four groups in the PAS, which take less than one minute to complete (Rosen et al., Reference Rosen1994). Conversely, the NPI-NH interview, intended to last 15 minutes (Lange et al., Reference Lange, Hopp and Kang2004) or longer (Kaufer et al., Reference Kaufer2000), can take up to 30 minutes (Chen et al., Reference Chen2018; Noblet-Dick et al., Reference Noblet-Dick2013) when patients have numerous symptoms (Cummings et al., Reference Cummings1994; Kang et al., Reference Kang2004). Short and easy-to-administer, our study also showed that the PGI-DSS can be used in various settings, such as geriatric care units and nursing homes. These three characteristics are shared by other new psychogeriatric tools, such as the Short Well-being Instrument for Older people (SWIO) (Duppen et al., Reference Duppen2019).
The second advance concerns caregiver roles. Caregivers’ perceptions of behavioral disturbances are an underused resource that can be exploited by the PGI-DSS. Much more than an inventory, the PGI-DSS gives the floor to caregivers by using their own words. They thus realize how useful it is to put the right words on the symptoms. There is some interest, and even pleasure for some caregivers, in attributing the right level of severity to a symptom that they have observed. Providing information in this manner, a key area of support (Queluz et al., Reference Queluz2019), could contribute to improving their sense of competence and self-efficacy (van der Lee et al., Reference van der Lee, Bakker and Droes2019). The process of obtaining the scores for the four syndromes, far from being the end, serves as a written base and a kind of agreement (Droes et al., Reference Droes2005) for the clinical staff, enabling the instatement of care. Helped by the central drawing of the “emotional keyboard” depicting legitimate emotions, caregivers feel free to say that they have been frightened by violence, embarrassed by refusals (Politis et al., Reference Politis, Mayer, Passa, Maillis and Lyketsos2004), distressed by repetitive, or upset by repetitive acts (Regier and Gitlin, Reference Regier and Gitlin2016). Clinicians are able to explain that these emotions are human, thereby alleviating the caregivers’ guilt. Dialogue is improved between geriatricians, psychologists, and nurses. Thinking collectively about psychotherapy and narrative skills (Piver, Reference Piver2019), “in a context of positive mental health” (Fulcheri and Carrozzino, Reference Fulcheri and Carrozzino2017), helps prevent or defuse inappropriate interventions. It contributes to psychosocial diagnoses and helps attune appropriate psychosocial interventions to the syndromes that have been observed (Isik et al., Reference Isik, Soysal, Solmi and Veronese2019). This collective work helps staff overcome emotions. Once teams are reassured, care can go further. Caregivers can give themselves the chance to discover the causes of the syndromes observed. Hypotheses can be made, for example, using an analgesic test. This collective assessment of symptoms and syndromes, at once enabling interventions to be attuned and identifying reversible causes, could be one of the first steps in the direction of a biopsychosocial psychogeriatric integrative care approach, embracing (Sands, Reference Sands2012) the exposome (Wild, Reference Wild2005). According to this concept, which refers to the exposure of the genome to environmental factors, symptoms should be considered as being caused by interactions among numerous variables. Symptoms should no longer be linked to a specific pathology. Apart from dementia, which can be present or absent, the challenge of the exposome and of psychogeriatrics is to find a reversible cause for symptoms.
Does the word “disconcerting” represent an advance? In the past, symptoms were often regarded as problematic or disruptive, patients as difficult (Monfort, Reference Monfort1995) and caregivers as distressed. These qualifiers contributed subconsciously (Sargent-Cox, Reference Sargent-Cox2017) to negative images of the aging process. Symptoms should not lead to the use of negative qualifiers. Patients should not be referred to by the name of their symptoms. Caregivers can be disconcerted by symptoms, but distress and exhaustion are not mandatory.
The word “distressing” underscores the negative dimension of emotional exhaustion (EE). The word “disconcerting” (déconcertant, in French) implies this emotional risk, but not necessarily EE stricto sensu. Meaning “to confuse,” the French etymology of déconcertant is borrowed from Middle French desconcerter, divisible into des-, a prefix denoting reversal or cancellation, and concertare from ecclesiastical Latin meaning “acting for a common purpose” or “playing together harmoniously.” This word for our scale does in fact pinpoint the challenge for professional caregivers: to go along and interact harmoniously with older adults, without being distressed by their “disconcerting” symptoms.
Limitations
Because of the context of a significant burden in French hospitals and in French nursing homes, and in the absence of any grants, the study was designed to minimize the additional workload that might have resulted from the implementation of the study. Thus, information on patient characteristics has been reduced to a minimum. As a result, our study has obvious limitations due to the absence of any inclusion and exclusion criteria. Other limitations are due to the absence of a cognitive assessment and any medical or psychological diagnosis. Therefore, the study population, distributed across French territory, was highly heterogeneous.
This heterogeneity is demonstrated by a wide age range (from 61 to 102 years). Although our study was open to people over the age of 50 years, those included were over the age of 60. This heterogeneity is also demonstrated by a wide distribution of NPI-NH scores (scores from 0 to 84 out of a total of 120) and probably a wide range of cognitive impairments and a wide spectrum of pathologies. The design of our study, with minimal additional workload, should facilitate the implementation of replication studies. This strength is hampered by patient heterogeneity, which can limit the generalizability of results.
Future work, including patients over 60 years, with a PGI-DSS score greater than 17, should assess the mean administration time and evaluate its psychometric properties in various languages, cultures, populations, and settings. Studies of correlations between the four PGI-DSS syndromes and the factor structure of the Zarit Burden Interview could confirm the existence of a distinct factor, i.e. a conceptual continuum labeled “worry about caregiving performance” (WaP), ranging from “inadequacy” to “guilt” (Lau et al., Reference Lau, Ali, Lim and Lim2019).
A clinimetric approach (Fava et al., Reference Fava, Rafanelli and Tomba2012), with macro- and microanalyses, should be used to assess the clinical validity of the PGI-DSS. This innovative evaluation method, defined as the science of clinical measurements (Fava et al., Reference Fava, Rafanelli and Tomba2012), could be used to confirm the threshold of 17 points, which discriminates patients with disconcerting symptoms from patients without them.
The ability to discriminate patients with dementia from patients with psychiatric disorders should also be tested. To be used as an outcome measurement for interventions (Moniz-Cook et al., Reference Moniz-Cook2008), studies comparing the sensitivity to change of the PGI-DSS and the NPI-NH should estimate the number of points necessary to avoid misinterpretation due to measurement error (Iverson et al., Reference Iverson, Hopp, DeWolfe and Solomons2002). Its usefulness in telemedicine and its impact on psychoeducation should also be assessed.
Other studies could explore links between syndromes and causes. For example, DR could be linked with hypoactive delirium and hidden depression, DW and DA with hidden obsessive–compulsive disorders, DV, DW, and DA with manic episodes.
Conclusion
The PGI-DSS is a brief and easy-to-administer tool usable in geriatric care units and nursing homes. Developed to overcome the imitations of the NPI-NH, this new tool could have similar or superior statistical properties compared with the NPI-NH. While the 10 domains of the NPI-NH have clinical utility for clinicians, the four understandable syndromes of the PGI-DSS could enable inappropriate attitudes among caregivers to be avoided and guide psychosocial interventions. Beyond this, the PGI-DSS could improve dialogue between caregivers and clinicians and help clinical staff to find reversible causes.
Conflicts of interest declaration
None.
Description of authors’ roles
Sophie Tezenas du Montcel (STdM), Jean-Claude Monfort (JCM), and Anne Marie Lezy (AML) conceived of and designed study 1 and study 2. JCM, AML, and AP contributed to the acquisition of data. JCM collected the data. JCM and AML independently performed digital captures and then corrected input errors together. STdM carried out the statistical analysis and wrote the statistical part of the methods section and the statistical part of the results section. JCM, AML, and AP designed and drafted the article, with the exception of the statistical parts. JCM, AML, AP, and STdM gave final approval for the version to be published. JCM and STdm had full access to all the data in the study. JCM had final responsibility for the decision to submit the article for publication.
Acknowledgments
The authors would like to acknowledge the professionals who contributed to data acquisition in each location: Claire Thollenaz-Picard (EHPAD du CASVP [Julie Siegfried], Paris), Françoise Guillemette (EHPAD du CASVP [Herold], Paris), Catherine Bayle (Hôpital Broca, AP-HP and EHPAD Péan, ACPPA, Paris), Benoit Houbin (GH Paul Guiraud, Villejuif), Camille Lejeune (CH de Rouffach, Rouffach), Céline Morvan (Fondation Bonsauveur, Bégard), Cyril Hazif-Thomas (CHU de Brest), Catherine Rémy (CH de Bonneval), Anne Le Néchet (CH d’Antibes), Gaëlle Marie-Bailleul (CH de Dax), Pierre Le Mauff (CH de Tréguier), Fatima Moulan (CH de Tréguier), Philippe Babadjian (CH d’Argenteuil), Patricia Perrier (SSR d’Amélie les Bains), Laurence Gangnant (EHPAD de Malnoue, Emerainville), Gaelle Lejeune (EHPAD Résidence Obert, Wambrechies), Anne Desmaret (EHPAD Résidence, Mons en Bareuf), Isabelle Bathelier (CHR Unité de Psychogériatrie, Orléans), Sabine Vaccaro (CH, Chartres), Véronique Meunier (CHI des Portes de l’Oise, Beaumont sur Oise), Françoise Illes (EHPAD, Antrain), Fabienne Faivre-Desrousseaux (Hôpital de la Porte Verte, Versailles), Alexandra Choquet (EHPAD Lasserre, Issy Les Moulineaux), Magali Frapart (EHPAD du Château, Ay en Champagne), Juliette Levilion (EHPAD Alésia, Paris), Damien Simon Amedulo (CH de Carignan), and Karim Hamadachi (CH de Grasse). They would also like to thank their own institutions: CH Sainte-Anne, Paris; Hôpital Corentin – Celton, AP-HP, Issy les Moulineaux; and CH du Mans, Le Mans.
Supplementary material
To view supplementary material for this paper, please visit https://doi.org/10.1017/S1041610220000496







