Increased intake of brassica vegetables and brassica-containing foods (e.g. mustard, horse radish) may be of benefit for cancer preventionReference Steinmetz and Potter1, Reference Verhoeven, Goldbohm, van Poppel, Verhagen and van den Brandt2. This is thought to be partly due to the presence of glucosinolates (thioglucoside phytochemicals) in brassica vegetables. They are accompanied in the plant by the thioglucosidase enzyme, myrosinase (thioglucoside glucohydrolase, EC 3.2.3.1). Following tissue disruption (during cutting or chewing), glucosinolates come in contact with myrosinase and the resulting hydrolysis yields a range of active aglycone products including isothiocyanates, nitriles and cyano-epithioalkanesReference Fenwick, Heaney and Mullin3. Isothiocyanates influence xenobiotic metabolising enzyme expression and apoptosisReference Talalay4–Reference Smith, Mithen and Johnson6 and may explain the cancer-protective effect of brassica vegetable consumptionReference Rose, Faulkner, Williamson and Mithen7. However, not all epidemiological studies demonstrate a protective role for brassica vegetablesReference Michels, Giovannucci, Kaumudi, Rosner, Stampfer, Fuchs, Colditz, Speizer and Willett8, Reference Flood, Velie, Chaterjee, Subar, Thompson, Lacey, Schairer, Troisi and Schatzkin9 and this may be due to differences in release of breakdown products in different situations.
In general, man consumes brassica vegetables following processing. Myrosinase is denatured by the application of heat during cookingReference Krul, Humblot, Philippe, Vermeulen, van Nuenen, Havenaar and Rabot10 and this has implications for the amount and composition of breakdown products available to man. Consumption of raw vegetables containing plant-derived myrosinase activity produces rapid hydrolysis of glucosinolates and the release of isothiocyanates may primarily occur in the upper digestive tractReference Rouzaud, Rabot, Ratcliffe and Duncan11. The isothiocyanates are absorbed and rapidly excreted as conjugates in the urine. Consumption of cooked brassica with denatured myrosinase permits intact glucosinolates to pass through the upper digestive tract and enter the colonReference Michaelsen, Otte, Simonsen and Sorensen12, Reference Rouzaud, Young and Duncan13 where they may be hydrolysed by the gut microbiota. The different sites of release and absorption of hydrolysis products of glucosinolates may have implications for the cancer-protective properties of brassica vegetables.
Apoptosis was increased in the colon crypts of dimethylhydrazine-treated rats following absorption of allyl isothiocyanate from fresh Brussels sprout juice or raw sprouts in the upper intestine. Therefore a systemic delivery of glucosinolate metabolites to the colonic epithelium may be effective for chemoprotectionReference Smith, Mithen and Johnson6. However, the release of allyl isothiocyanate from purified sinigrin (with no plant myrosinase) into the colon has also been shown to enhance apoptosis and reduce the formation of aberrant crypt foci in dimethylhydrazine-treated rats, indicating that a localised production of allyl isothiocyanate may also be of benefitReference Smith, Lund and Johnson14. The most effective route of delivery of glucosinolate metabolites is not yet clear. Indeed it is probable that both systematic and luminal delivery are important for maximising the chemoprotective effect of brassica vegetables.
Some genera of the human intestinal microflora (e.g. Bifidobacterium, Lactobacillus and Bacteroides) possess myrosinase-like activityReference Nugon-Baudon, Rabot, Wal and Szylit15, Reference Elfoul, Rabot, Khelifa, Quinsac, Duguay and Rimbault16. Three species of bifidobacteria of human origin have been shown to be capable of degrading the glucosinolates sinigrin and glucotropaeolin in vitro Reference Cheng, Hashimoto and Uda17. Lactobacillus agilis and Bacteroides thetaiotaomicron, isolated from human faeces, have also been reported to convert sinigrin to allyl isothiocyanate in vitro Reference Elfoul, Rabot, Khelifa, Quinsac, Duguay and Rimbault16, Reference Palop, Smiths and ten Brink18. Incubation of human faeces with cooked watercress (in which plant myrosinase had been denatured) in vitro also yielded isothiocyanatesReference Getahun and Chung19. Bacteria may hydrolyse glucosinolates consumed in a cooked vegetable meal and release hydrolysis products directly into the lumen of the colon. Enhancement of selected microbial populations in the colon of human faecal flora-associated rats alters the biological effects of glucosinolates consumed in rapeseed meal and may also alter vegetable glucosinolate effectsReference Roland, Rabot and Nugon-Baudon20. Bifidobacteria residing in the colon may be enhanced following the consumption of prebioticsReference Kruse, Kleessen and Blaut21. Prebiotics are fermentable, indigestible food ingredients that selectively stimulate growth and/or activity of a limited number of bacterial species resident in the colonReference Gibson, Beatty, Wang and Cummings22. Given the availability of pre- and probiotic supplements and the potential for myrosinase-like activity in lactobacilli and bifidobacteria, an investigation into the effect of enhanced microflora populations on glucosinolate metabolism in man is needed.
In the current study we sought to enhance colonic bifidobacterial populations in human subjects by the use of a commercially available prebiotic to investigate the influence of changes to the colonic bacterial population on the hydrolysis of glucosinolates. We hypothesised that any changes to glucosinolate hydrolysis brought about through prebiotic supplementation would be manifest following consumption of cooked cabbage but not after consumption of cabbage retaining myrosinase activity.
Experimental methods
Subjects
Twelve healthy, Caucasian, non-smoking adult volunteers (three male, nine female; mean age 38·1 (sem 2·43), range 25–51 years; mean BMI 25·0 (sem 1·09), range 19·8–31·5 kg/m2) were recruited from academic institutions in Aberdeen. After discussion with the study investigator the volunteers provided a record of informed consent. The volunteers did not consume medical drugs or supplements during the study period. The protocol and all procedures were approved by the NHS Trust Grampian Research Ethics Committee.
Study design
The subjects were randomly split into two groups prior to participating in a crossover study design. Group 1 consumed the recommended amount of a commercially available prebiotic formulation (92 % inulin–oligofructose +8 % glucose–fructose-sucrose; Beneo™, DKSH/Orafti Great Britain Ltd, Beckenham, Kent, UK) for a 21 d treatment period which included a test meal period during the last 5 d. A 21 d control period, during which no supplement was consumed, followed immediately and this control period also concluded with a 5 d test meal period. The second group completed the control and treatment periods in reverse order (Fig. 1). During the prebiotic treatment period, volunteers were provided with sachets of pre-weighed quantities (5 g) of the prebiotic supplement. The contents of each sachet were dissolved in 100 ml water immediately prior to consumption. One prebiotic sachet was consumed twice daily (at breakfast and evening meal) for 21 d. This level of consumption represented 50 % of the maximum daily dose rate recommended by the manufacturers. The 10 g daily prebiotic dose contained approximately 0·04 MJ (10·7 kcal) which could lead to an increase in energy intake of 0·5 % assuming a normal energy intake of 8·3 MJ/d (2000 kcal/d). This increase in energy intake was much lower than the natural day-to-day variation in energy intake of 20–30 %Reference Bingham23 and was not considered sufficient to challenge the interpretation of the results. In general, compliance to the study protocol was good. Out of the forty-two inulin doses one volunteer missed four and two volunteers each missed two doses.

Fig. 1 Study design indicating the timing of cabbage meals and faecal and urine collections. Black rectangles indicate the consumption of a cabbage - containing meal.
Vegetable treatments
Within each 5 d test meal period each volunteer was offered a test meal on day 1 and again on day 4 at 12.30 hours. The second test meal was consumed 72 h after the first to permit clearance of urinary metabolites between the cabbage meals. Clearance has been shown to occur within 24 h in previous workReference Rouzaud, Young and Duncan13. For 2 d prior to consumption of the first test meal and until the end of each test meal period, subjects were asked to avoid consumption of potential sources of glucosinolates. A list of glucosinolate-containing vegetables and foodstuffs was provided at the outset of the study and verbal reminders were given at each test meal. To check volunteer compliance, a simple food diary was completed for 2 d prior to, and during, each test meal period.
Each test meal consisted of a 150 g portion of white cabbage with a standard meal of chicken and rice (containing no glucosinolates), followed by a glucosinolate-free dessert course (summer fruit and ice cream). Alongside each cabbage meal each volunteer was provided with a 70 ml drink consisting of an extract made from 50 ml water and 9 g ground broccoli seeds (GEO organic broccoli seeds; UK Juicers Ltd, York, UK), in which myrosinase had been denatured by heating (80°C for 10 minReference Rouzaud, Young and Duncan13). Solid matter was removed by sieving the seed and water mixture before 20 ml fresh orange juice and 10 g Canderel (aspartame) artificial sweetener were added to improve palatability. The drink contained high concentrations of the nitrile hydrolysis product of glucoraphanin, the metabolism of which is being investigated in a further study.
A homogeneous source of large white cabbage (var. Colmar; Kettle Produce, Cupar, UK) was used. Each cabbage was divided into a series of nine longitudinal wedges. Each wedge weighed 150 g and contained consistent proportions of inner and outer leaves. Residual cabbage was discarded. Each cabbage wedge was roughly chopped. Four portions were microwave cooked for 2 min (750 W; Whirlpool UK Ltd, Croydon, Surrey, UK) in a dish containing 16 ml water and covered with pierced PVC cooking film while a further four were microwave cooked for 5·5 min under the same conditions. Cooking times were chosen to provide meals with similar sinigrin content but different myrosinase activities (2 min – lightly cooked, retaining active, naturally available plant myrosinase; 5·5 min – fully cooked, containing denatured natural myrosinase) and were chosen based on the results of a previous studyReference Rungapamestry, Duncan, Fuller and Ratcliffe24. Cabbage treatments were offered in a randomised order to each individual. One portion from each cooking treatment per cabbage along with the remaining raw portion was reserved for subsequent analysis. These portions were placed on a plate and left at room temperature for the duration of the volunteers' meal. At the end of each meal, these cabbage portions were snap-frozen in liquid nitrogen and maintained at − 20°C for analysis of glucosinolate concentrations and myrosinase activity. As the type and quantity of active ingredients may vary between individual cabbage heads, the identity of the cabbage head consumed by each volunteer was recorded to permit accurate determination of the intake of glucosinolates and myrosinase by each volunteer.
Cabbage samples were freeze-dried, ground and maintained at − 20°C prior to glucosinolate and myrosinase analyses. The glucosinolate content of the cabbage samples was determined using HPLC by the methodsReference Minchinton, Sang, Burke and Truscott25, 26 described in Rungapamestry et al. Reference Rungapamestry, Duncan, Fuller and Ratcliffe24. The myrosinase activity of the cooked cabbage was determined using a UV visible spectrophotometer (Cary 50; Varian Ltd, Yarnton, Oxford, UK) to measure the rate of disappearance of sinigrin from a test mixtureReference Shapiro, Fahey, Wade, Stephenson and Talalay27. Standard curves were run each day using commercially prepared myrosinase isolated from Sinapis alba (white mustard seed; Sigma Aldrich, Poole, UK). One unit of myrosinase activity is defined as that which will produce 1·0 μmol glucose/min from sinigrin at pH 6·0 and 25°C.
Urine collection and analysis
Prior to consumption of the test meal each volunteer provided a spot sample of urine to enable the determination of background concentrations of glucosinolate metabolites. Total urine collections were obtained for 24 h after each cabbage test meal. The 24 h collection was separated into four time periods (0–4, 5–9, 10–19 and 20–24 h). Since volunteers were free-living, urine samples were kept in insulated bags containing cool blocks to reduce possible degradation of the compounds of interest during the collection period. The pH of the urine was not modified. For each collection period, urine volume was measured by a study investigator and recorded. Four 20 ml aliquots from each collection receptacle were placed in sealed tubes (Sterilin, Stone, Staffordshire, UK) and maintained at − 20°C until analysis.
Urinary metabolites of isothiocyanates derived from glucosinolates contained in the cabbage meals were quantified in the urine samples. The major aliphatic glucosinolate in cabbage is sinigrin which, on hydrolysis by myrosinase, forms allyl isothiocyanate. Allyl isothiocyanate is metabolised by the mercapturic acid pathway prior to urinary excretion as N-acetyl (allylthiocarbamoyl)-l-cysteine (hereafter referred to as allyl mercapturic acid; AMA). AMA excreted in the urine was measured using the methodReference Rouzaud, Young and Duncan13 as adapted from Mennicke et al. Reference Mennicke, Kral, Krumbiegel and Rittmann28 AMA and N-acetyl (phenylthiocarbamoyl)-l-cysteine (phenyl mercapturic acid) were synthesised according to Mennicke et al. Reference Mennicke, Gorler and Krumbiegel29 for use as standards in the analysis. Phenyl mercapturic acid (dissolved in deionised water–ethanol, 50 : 50, v/v) was used as an internal standard and calibration curves for AMA (R 2 0·9993 (sem 0·0002)) were analysed within each batch of samples for quantification of the amount of AMA excreted. Each sample was analysed in duplicate. Discrepancy between duplicate samples was always less than 5 %.
Faecal collection and analysis
At three points during the study volunteers provided a faecal sample to enable changes in bifidobacterial numbers relative to the total bacteria population to be monitored. Faecal samples were obtained at the outset of the study for determination of baseline bifidobacterial population ratios and also at day 16 of both the prebiotic treatment and control periods (Fig. 1). At each time-point, the subjects placed a 10 g sub-sample of faeces into a collection tube. Samples were further aliquoted before being maintained at − 20°C for later analysis by real-time PCR.
Quantitative real-time PCR
Standard template DNA was prepared from the 16S rRNA gene of Bifidobacterium pseudocatenulatum DSM 20 438 by amplification with primers 27F and RP2 and purification as described previouslyReference Louis, Duncan, McCrae, Millar, Jackson and Flint30. Standard curves were prepared as described previouslyReference Belenguer, Duncan, Calder, Holtrop, Louis, Lobley and Flint31 with either universal primers UniF (GTGSTGCAYGGYYGTCGTCAReference Maeda, Fujimoto, Haruki, Maeda, Kokeguchi, Petelin, Arai, Tanimoto, Nishimura and Takashiba32, modified) and UniR (ACGTCRTCCMCNCCTTCCTCReference Maeda, Fujimoto, Haruki, Maeda, Kokeguchi, Petelin, Arai, Tanimoto, Nishimura and Takashiba32, modified), or Bifidobacterium-specific primers BifF (TCGCGTCYGGTGTGAAAGReference Rinttilä, Kassinen, Malinen, Krogius and Palva33) and g-Bifid-R (GGTGTTCTTCCCGATATCTACAReference Matsuki, Watanabe, Fujimoto, Miyamoto, Takada, Matsumoto, Oyaizu and Tanaka34). Both primer sets resulted in very similar standard curves (PCR efficiency 95·9 (sem 0·92) % for universal primers and 96·7 (sem 1·34) % for Bifidobacterium-specific primers).
DNA from faecal samples was extracted using the DNA Spin Kit for Soil (MP Biomedicals Europe, Illkirch, France). DNA concentrations were determined with a NanoDrop ND 1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). DNA was diluted to 0·5 ng/μl in 5 μg/ml herring sperm DNA for amplification with universal primers UniF and UniR and 5 ng/μl in 5 μg/ml herring sperm DNA for amplification with Bifidobacterium-specific primers BifF and g-Bifid-R. Herring sperm DNA (5 μg/ml) was used for all DNA dilutions as carrier DNA, as it significantly increased the accuracy of the data, especially at low DNA concentrations. The absence of cross-reactivity with either primer pair was confirmed by real-time PCR under the same conditions as described later.
PCR reactions were performed as described previouslyReference Belenguer, Duncan, Calder, Holtrop, Louis, Lobley and Flint31. Starting quantities of all bacterial and bifidobacterial 16S rRNA genes were determined using the iCycler IQ software version 3.1 (Bio-Rad Laboratories Inc., Hercules, CA, USA) to calculate the percentage of bifidobacterial genes. The detection limit was 0·01 % of total bacterial 16S rRNA genes.
Statistical analyses
Data are expressed as means and their standard errors. One volunteer withdrew from the study after completion of the control period and prior to data collection for the prebiotic period. Total glucosinolate concentrations, sinigrin concentrations and myrosinase activity in lightly and fully cooked cabbage were subjected to general ANOVA with cooking treatment as the main effect. Samples from the same cabbage head were treated as a block in the ANOVA to correct for background variation between heads of cabbage.
Changes in faecal bifidobacterial populations were assessed by ANOVA using individual as a block to account for inter-individual variation. Orthogonal contrasts were used to compare bifidobacterial populations in faecal samples collected at the three time-points, day 0 (baseline), day 16 and day 37 (Fig. 1). The potential effect of baseline bifidobacterial population on the stimulatory effect of inulin was investigated using analysis of covariance on bifidobacterial populations after 16 d of inulin supplementation using the background population (day 1) of bifidobacteria as a covariate. As the two groups of volunteers experienced the inulin and control treatments in different orders, the effect of order of treatment was investigated using ANOVA to detect the interaction between group of volunteers and prebiotic treatment.
AMA output over 24 h was used as a variate to assess the effect of cooking treatment and prebiotic treatment on allyl isothiocyanate production. Each individual's total AMA excretion was expressed as a proportion of the individual's intake of sinigrin in the cabbage meal to derive a recovery for AMA. The main effects of cooking treatment and prebiotic treatment together with their interaction, on AMA recovery, were assessed within individuals. Individuals were used as blocks and AMA excretion in different time periods was compared using orthogonal contrasts. The interaction between cooking time and AMA excretion in each time period was also investigated.
To determine if the extent of change in the bifidobacterial population recorded after the inulin treatment was related to the extent of change in AMA excretion after the differently cooked cabbage meals, a correlation analysis was performed.
All statistical analyses were performed using GenStat version 8.1 (Lawes Agricultural Trust, Harpenden, Hertfordshire, 2005).
Results
Glucosinolate concentrations in cabbage
Raw cabbage contained the following glucosinolates (mean μmol/g DM): sinigrin, 9·54 (sd 4·01); glucoiberin, 4·97 (sd 3·14); glucobrassicin, 2·56 (sd 0·91); progoitrin, 1·39 (sd 0·47); gluconapin, 0·89 (sd 0·28); 4-methoxyglucobrassicin, 0·69 (sd 0·22); 4-OH glucobrassicin, 0·24 (sd 0·06); gluconapoleiferin, 0·07 (sd 0·02); neoglucobrassicin, 0·05 (sd 0·05); glucoraphanin, 0·02 (sd 0·02). Glucosinolate concentrations in cooked cabbage did not differ from raw values (Fig. 2). The proportion of sinigrin to total glucosinolates did not differ between the 2 and 5·5 min cooking treatments (2 min = 43·7 %; 5·5 min = 43·1 %).

Fig. 2 Total glucosinolate (![]() ) and sinigrin (□) concentrations in raw (0 min), lightly cooked (2 min) and fully cooked (5·5 min) cabbage. Values are means with their standard errors depicted by vertical bars (n 9 for lightly cooked and fully cooked cabbage; n 5 for raw cabbage).
) and sinigrin (□) concentrations in raw (0 min), lightly cooked (2 min) and fully cooked (5·5 min) cabbage. Values are means with their standard errors depicted by vertical bars (n 9 for lightly cooked and fully cooked cabbage; n 5 for raw cabbage).
Myrosinase activity in cabbage treatments
Myrosinase activity was reduced from 21·5 (sem 13·29) units/g DM (291·8 units/150 g fresh weight portion consumed) in raw cabbage to 2·44 (sem 0·25) (35·5 units/150 g fresh weight portion consumed) and 0·87 (sem 0·05) units/g DM (17·67 units/150 g fresh weight portion consumed) in cabbage cooked for 2 and 5·5 min, respectively. Myrosinase activity was higher in cabbage following a 2 min period of microwave cooking than following the 5·5 min cooking period (P = 0·043).
Faecal bifidobacterial population
Inulin supplementation increased the relative proportion of bifidobacteria in faecal samples by approximately two-fold (P < 0·001, Fig. 3). In two volunteers the percentage of bifidobacterial 16S rRNA genes was below the detection limit in baseline samples. The presence of a small population of bifidobacteria was detected in one volunteer after inulin supplementation but no change was noted for the other. Although retained in the analysis these results were not sufficient to eliminate the increase shown overall. Baseline bifidobacterial populations varied markedly between individuals as indicated by a large range in values (the minimum population was below detection and maximum was 4·6 % of the total bacterial genes) and standard error (mean 1·80 (sem 0·45) bifidobacterial genes as a percentage of total genes) but the stimulatory effect of inulin supplementation was not dependent on the baseline population (P = 0·236) or the order in which the prebiotic and control periods were experienced (P = 0·162).
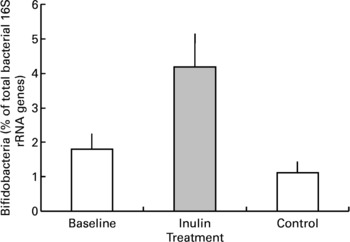
Fig. 3 Faecal bifidobacterial population prior to (baseline, n 12), after 16 d of inulin supplementation (inulin, n 11) and following a 16 d control period (control, n 12). Values are means with their standard errors depicted by vertical bars.
Urinary excretion of glucosinolate metabolites
All mercapturic acid excretion data were corrected for individual glucosinolate intakes. Following the 48 h period of abstinence from brassica vegetables and glucosinolate-containing foods immediately prior to each cabbage meal, mercapturic acid concentrations in baseline urine samples were essentially zero (Fig. 4). AMA excretion in the 24 h following the meal was greater following consumption of lightly cooked cabbage (mean recovery 23·3 (sem 1·9) %) than fully cooked cabbage (mean recovery 7·7 (sem 0·76) %, P < 0·001). Prebiotic supplementation did not significantly influence AMA excretion after either cabbage cooking treatment (prebiotic treatment mean recovery 14·6 (sem 2·01) %, control mean recovery 15·8 (sem 2·40) %, P = 0·880). The pattern of AMA excretion over time was different between the cooking treatments (P < 0·001). Consumption of lightly cooked cabbage resulted in a peak of mercapturic acid excretion between 4 and 9 h following the cabbage meal in contrast to the excretion after consumption of cooked cabbage which peaked between 9 and 19 h (Fig. 4). Excretion of AMA in the urine was negligible by the end of the 24 h period irrespective of prebiotic treatment or cabbage cooking time.
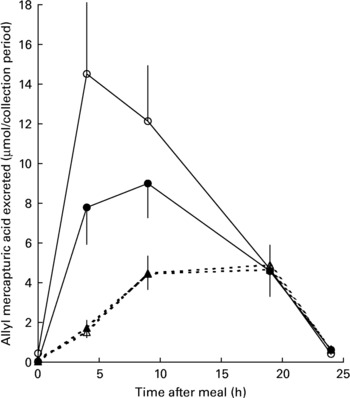
Fig. 4 Excretion of allyl mercapturic acid (μmol/collection period) during the 24 h following meals containing lightly cooked (2 min) cabbage during prebiotic treatment (●, n 11) and control (○, n 12) periods or fully cooked (5·5 min) cabbage during prebiotic treatment (▲, n 11) and control (△, n 12) periods. Values are means with their standard errors depicted by vertical bars.
Despite the significant increase in the proportion of faecal bifidobacteria during the prebiotic treatment period there was no association between the change in bifidobacteria and the change in AMA excretion after consumption of the fully cooked cabbage meal (R − 0·416; P = 0·179). Although ten of the eleven volunteers that completed both the prebiotic and control periods demonstrated an increase in faecal bifidobacteria in response to inulin supplementation, the extent of change had no relation to either the extent or even direction of change of AMA excretion between periods (Fig. 5).
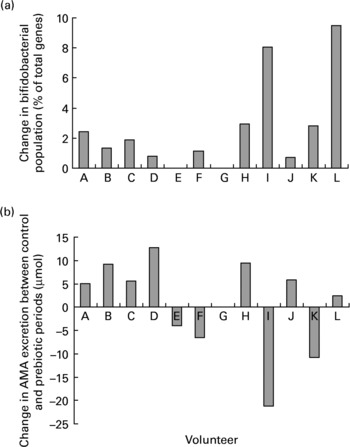
Fig. 5 Individual changes in (a) faecal bifidobacterial populations (% of total bacterial 16S rRNA genes) and (b) excretion of allyl mercapturic acid (AMA) after consumption of a fully cooked (5·5 min) cabbage meal, between control and prebiotic treatment periods. Volunteer G withdrew from the study before the start of the inulin consumption period.
Discussion
Hydrolysis of glucosinolates in the colon or caecum has been known for some timeReference Michaelsen, Otte, Simonsen and Sorensen12. The gastrointestinal microflora of rats and poultry have the ability to hydrolyse glucosinolatesReference Nugon-Baudon, Rabot, Wal and Szylit15, Reference Slominski, Campbell and Stanger35, Reference Nugon-Baudon, Szylit and Raibaud36 as demonstrated by the development of negative side-effects of isothiocyanate exposure such as goitre following excessive progoitrin consumption even in the absence of dietary myrosinase. The myrosinase activity of the intestinal microflora is physiologically relevant as the biological effects of consumption of cruciferous vegetables do not occur when germ-free animals are provided with a glucosinolate-rich but myrosinase-free dietReference Nugon-Baudon, Szylit and Raibaud36. This activity is not confined to laboratory animals; human microflora also show myrosinase-like activity as demonstrated when mechanical and antibiotic bowel cleansing in man decreased the isothiocyanate excreted following consumption of a cooked brassica homogenate from 11 to 1 % of the doseReference Shapiro, Fahey, Wade, Stephenson and Talalay37. As the intake of cooked brassica (i.e. glucosinolate consumption) leads to a low delivery of isothiocyanates to the gastrointestinal tract relative to consumption of the raw vegetable (i.e. isothiocyanate consumptionReference Shapiro, Fahey, Wade, Stephenson and Talalay27) but is a common form of ingestion, there could be advantages in maximising isothiocyanate production through manipulation of the microflora.
Although not fully investigated, several genera of the human colonic microflora such as Bifidobacterium, Lactobacillus and Bacteroides have been reported to possess myrosinase-like activityReference Nugon-Baudon, Rabot, Wal and Szylit15–Reference Cheng, Hashimoto and Uda17. Enhancement of some or all of these species may reasonably be expected to increase excretion of mercapturic acids following the consumption of a cooked brassica meal. Ingestion of prebiotics (indigestible, fermentable oligosaccharides) such as inulin has been widely reported to increase bifidobacterial populations in the human colonReference Kruse, Kleessen and Blaut21Reference Gibson,38, Reference Tuohy, Finlay, Wynne and Gibson39. A 16 d period of inulin consumption was sufficient to increase faecal bifidobacterial populations significantly relative to the total bacterial microflora in the current study. Baseline faecal samples from two volunteers did not harbour a detectable population of bifidobacteria and little or no enhancement of the population was shown in these individuals. Large inter-individual variation in faecal bifidobacterial populations has been reportedReference Lay, Sutren, Rochet, Saunier, Dore and Rigottier-Gois40 and interestingly some rats do not display myrosinase-like activity in their colonic microfloraReference Michaelsen, Otte, Simonsen and Sorensen12. However, despite having undetectable populations of bifidobacteria, the two volunteers demonstrated evidence of colonic myrosinase-like activity due to their excretion of mercapturic acids after consumption of the fully cooked cabbage. This indicated that microbial glucosinolate breakdown does occur in the absence of high numbers of bifidobacteria, presumably through the action of other bacterial species, possibly lactobacilli or bacteroides, which have been shown to display myrosinase-like activityReference Nugon-Baudon, Rabot, Wal and Szylit15, Reference Elfoul, Rabot, Khelifa, Quinsac, Duguay and Rimbault16.
Despite the strong, positive influence of inulin consumption on faecal bifidobacteria there was no effect of this increase on urinary output of mercapturic acid following consumption of the fully cooked cabbage meal. This may have been because the additional fermentable carbohydrate (inulin) could have led to a decrease in colonic pH due to the increased production of fermentation acids by the gut microbiota. A reduction in pH has been demonstrated when human flora-associated rats were fed a diet containing 10 % inulin in one studyReference Humblot, Lhoste, Knasmuller, Gloux, Bruneau, Bensaada, Durao, Rabot, Andrieux and Kassie41, although not shown in a second study by the same groupReference Humblot, Bruneau, Sutren, Lhoste, Dore, Andrieux and Rabot42. A decrease in the pH of the hydrolysis environment may be of importance since nitrile rather than isothiocyanate formation is favoured in an acidic environmentReference VanEtten and Daxenbichler43–Reference Uda, Kurata and Arakawa45. It is possible that rather than forming isothiocyanate or nitrile breakdown products, glucosinolates could be hydrolysed to amines, or that isothiocyanates formed in the colon could be degraded to amines prior to absorptionReference Combourieu, Elfoul, Delort and Rabot46.
There is some evidence that inulin supplementation can influence liver detoxification enzyme activityReference Roland, Nugon-Baudon, Andrieux and Szylit47. This appears to be associated with changes to the SCFA profile of the colon. Our use of urinary mercapturic acids as markers of isothiocyanate production in the digestive tract assumed consistent post-absorptive metabolism of isothiocyanates via the mercapturate pathway across treatments; treatment-related alterations in isothiocyanate metabolism could have undermined this assumption. Since we found no evidence that inulin altered urinary concentrations of mercapturic acids, this was not a major concern for this experiment but in further work this possible interference could be excluded by simultaneously monitoring post-absorptive metabolism of homologous isothiocyanates as has been done previouslyReference Rouzaud, Young and Duncan13.
Isothiocyanates are highly reactive compounds and could react rapidly with compounds present in the colon to form other derivativesReference Cheng, Hashimoto and Uda17 or could have been metabolised to different final products by the microflora prior to absorption by the hostReference Rouzaud, Rabot, Ratcliffe and Duncan11. It is also likely that inulin has positive effects on other, perhaps currently unidentified, bacterial species within the complex colonic environment. However, as inulin supplementation did not lead to a statistically significant effect on AMA excretion, microbial changes in the microflora were not investigated further. Differences in glucosinolate-mediated toxic effects observed in different animal species seem to be due to differences in their microfloral compositionReference Nugon-Baudon and Rabot48. Altering human microfloral composition may, therefore, have varied effects on glucosinolate metabolism. However, as the proportion of the total human microflora accounted for by the bifidobacteria (0·6–15·6 %Reference Lay, Sutren, Rochet, Saunier, Dore and Rigottier-Gois40) is relatively small it is possible that even large increases in the population will have only negligible effects on glucosinolate metabolism.
In the current study, we have demonstrated that consumption of lightly cooked cabbage is associated with a large and rapid excretion of AMA (the digestive metabolite of allyl isothiocyanate, released upon breakdown of the glucosinolate sinigrin) similar to that which follows consumption of raw cabbageReference Rouzaud, Young and Duncan13. AMA output was significantly greater following consumption of lightly cooked than after consumption of fully cooked cabbage. It was also apparent that the production of allyl isothiocyanate was more rapid as excretion of AMA peaked between 4 and 9 h following ingestion of the lightly cooked cabbage meal compared to a peak at 9–19 h after consumption of fully cooked cabbage. This agrees with other studies which have compared mercapturic acid output following intake of cooked or raw brassicaReference Rouzaud, Young and Duncan13, Reference Getahun and Chung19, Reference Shapiro, Fahey, Wade, Stephenson and Talalay27, Reference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung49. Cooking treatment may also alter the proportions of breakdown products arising from plant myrosinase-catalysed breakdown of glucosinolates. A protein, called epithiospecifier protein, has been found in some brassicas and acts to direct glucosinolate breakdown towards epithionitrile rather than isothiocyanate formation in raw vegetablesReference Matusheski, Juvik and Jeffery50. However, short periods of heat treatment (2 min) denature epithiospecifier protein and result in maximal production of isothiocyanatesReference Rungapamestry, Duncan, Fuller and Ratcliffe24. The greater output of AMA after consumption of lightly cooked rather than fully cooked cabbage indicates that brassica vegetables may be subjected to a short period of heat treatment, prior to consumption, which may enhance their palatability while retaining their maximum potential to deliver isothiocyanates to the digestive tract.
It is notable that after both cabbage treatments urinary mercapturic acid output was less than could be expected if all of the sinigrin ingested was transformed into allyl isothiocyanate and metabolised to AMA. Other authors have noted this phenomenon, reporting glucosinolate recoveries as mercapturic acids of 0·30–0·67 after intake of raw watercressReference Chung, Morse, Eklind and Lewis51, 0·10 and 0·30 after consumption of steamed and raw broccoli, respectivelyReference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung49, and 0·15 and 0·37 after consumption of microwaved and raw cabbage respectivelyReference Rouzaud, Young and Duncan13. There are several reasons why this may be the case. On hydrolysis, glucosinolates break down into a variety of products. The formation of nitrile breakdown products may be favoured in acidic conditions, such as in the stomach, but we have yet to identify a measurable urinary metabolite for glucosinolate-derived nitriles, so have no information on the extent and kinetics of nitrile metabolism. Intact glucosinolates may pass through the digestive tract and be excreted in the faeces but, to date, this has been demonstrated only in germ-free ratsReference Rouzaud, Rabot, Ratcliffe and Duncan11, Reference Kassie, Rabot, Kundi, Chabicovsky, Qin and Knasmuller52.This is unlikely to occur in a normal human digestive tract as glucosinolates are not recovered in rat faeces when a colonic microflora is presentReference Rouzaud, Rabot, Ratcliffe and Duncan11.
In conclusion, an increase in the relative faecal bifidobacterial population had no influence on the excretion of allyl isothiocyanate which we interpret as being reflective of their having no influence on production. It appears that the most effective way of increasing isothiocyanate production is to limit the length of time of cooking for brassica vegetables prior to consumption.
Acknowledgements
Financial support for this project was provided by the Food Standards Agency (project code T01027, ‘Influence of cooking and processing of brassica vegetables on the release of beneficial and harmful metabolites of glucosinolates in the digestive tract’). P. Louis is supported by the Scottish Executive Environment and Rural Affairs Department. The authors especially thank the volunteers for their participation in, and compliance with, the study protocol; Mrs Heather Scott for her help in serving the cabbage meals; M. Brewer and G. Holtrop from Biomathematics & Statistics Scotland for their statistical advice; and DKSH/Orafti Great Britain Ltd for supplying Beneo™.







