Globally, more than 20 million infants are born with low birth weight (LBW), 95 % of which are born in developing countries(Reference Wardlaw, Blanc and Zupan1). Incidence rate of LBW varies significantly across countries ranging from 6 % to 18 %, and majority of LBW infants occur in Asia(Reference Wardlaw, Blanc and Zupan1). In China, the rates of LBW range from 4·2 % to 12·4 % in different provinces between rural and urban areas(Reference Liang-Ming, Yu-Lin and Xin-Li2). Although there is a relatively low incidence rate of LBW in China, it contributes significantly to the worldwide incidence of LBW due to its large population size(Reference Wardlaw, Blanc and Zupan1). LBW infants face an increased risk of morbidity and mortality over the life course(Reference McCormick3). They suffer from various chronic diseases in lifetime, such as type 2 diabetes(Reference Hales, Barker and Clark4), hypertension(Reference Law, de Swiet and Osmond5), CVD(Reference Osmond, Barker and Winter6) and chronic obstructive pulmonary disease(Reference Barker, Godfrey and Fall7). Therefore, LBW remains an important public health problem globally.
Potential biological mechanisms behind LBW include prematurity (gestational age <37 weeks) and intrauterine growth restriction(Reference Christian, Lee and Donahue Angel8). It is believed that several risk factors are associated with LBW, the most important of which are socio-economic factors and medical risks before or during gestation and maternal lifestyles(Reference Valero de Bernabe9). However, these reported risk factors cannot not fully explain the high incidence of LBW.
It has been reported that the risk of LBW is also associated with intra-uterine malnutrition, which is mainly due to alterations of placental circulation. Moreover, LBW is also associated with the lack of micronutrient intake such as Ca, which is a critical component of human bone and contributes to 1 %–2 % of body mass(10). During pregnancy and lactation, Ca requirements are increased by 50–100 % in order to maintain Ca balance(Reference Diogenes, Bezerra and Rezende11) and to satisfy fetal growth requirements. Sufficient Ca is particularly important during maternal–fetal transfer. It has been reported that Ca deficiency is associated with pre-eclampsia and intra-uterine growth restriction(Reference Michael and Weisman12). Some studies show that Ca supplementation can reduce the risk of LBW by 17 %(Reference Nils and Haram13). However, currently, there is no consensus on the role of routine Ca supplementation in lowering the risk of LBW(Reference Buppasiri, Lumbiganon and Thinkhamrop14). Numerous studies have investigated the associations between Ca supplementation and hypertension or pre-eclampsia as primary outcomes of LBW(Reference Villar and Repke15–Reference Wanchu, Malhotra and Khullar25), while only few studies have focused on the associations between dietary Ca intake and the risk of LBW or small for gestational age (SGA). Such evidence is especially scarce in the Chinese population. To our knowledge, there are few studies that investigate the association between Ca supplementation, dietary Ca intake and the risk of LBW and SGA infants by various clinical subtypes in the Chinese population.
Therefore, we conducted a large-scale birth cohort study in Lanzhou City, Gansu Province, China, with the aim to comprehensively examine the association between Ca intake (from supplementation and from food) and the risk of LBW and SGA.
Materials and methods
Study population
A study of a birth cohort was conducted at Gansu Provincial Maternity and Child Care Hospital, the largest maternity and child care hospital in Lanzhou City (Gansu Province, China), from January 2010 to December 2012. Pregnant women who came to the hospital for delivery at 20 weeks of gestation or more, and those with no mental illness, over 18 years old were eligible to participate in the study. There were a total of 14 535 pregnant women who came to the hospital for delivery. Among them, 176 were judged to be ineligible for the study (13 had mental illness, 39 were younger than 18 years of age and 124 gave birth at less than 20 gestational weeks). Moreover, nulliparous in this study excluded current pregnancy. Thus, a total of 14 359 eligible women were approached for participation. However, of the 14 350 women approached for participation, there were 3712 who declined to participate and 105 did not complete in-person interviews, so yielded 10 542 (73·4 %) women. After excluding women who gave birth to infants with birth defects, missing birth weight data, birth weights under 500 g or exceeding 4000 g and those who had chronic hypertension and diabetes, the final sample size was 9595. Among them, 708 women gave birth to LBW infants and 768 women gave birth to SGA infants.
All study procedures were approved by the human investigation committee of the hospital. Eligible women were informed of study procedure when they arrived at the hospital for delivery. After obtaining their written consent, trained study interviewers conducted in-person interviews at the hospital using a standardised and structured questionnaire. The majority of women (84 %) were interviewed within 1 to 3 d after delivery. The questionnaire collected information regarding demographic factors, reproductive and medical history, smoking behaviour, alcohol and tea consumption, occupational and residential history, physical activity level, and diet before pregnancy and during pregnancy. Information on birth outcomes and pregnancy complications were abstracted from the medical records.
Definition and classification
LBW was defined as a birth weight less than 2500 g (up to and including 2499 g) irrespective of gestational age(Reference Wardlaw, Blanc and Zupan1). Normal birth weight (NBW) was defined as a birth weight between 2500 g and less than 4000 g. SGA was defined as birth weight below the 10th percentile of a sex- and gestational-age-specific reference birth weight distribution(Reference Christian, Lee and Donahue Angel26). Average for gestational age (AGA) was defined as birth weight more than 10th and below 90th percentile of a sex- and gestational-age-specific reference birth weight distribution(Reference Christian, Lee and Donahue Angel26). Large for gestational age (LGA) was defined as a weight, length or head circumference which laid above the 90th percentile for gestational age. Preterm-SGA included born before 37 complete weeks of gestation and weight <10th population centile. Term-SGA included born between 37 and 42 complete weeks of gestation and weight <10th population centile(Reference Zhao and Shaofang27).
Birth weight was measured in grams by trained professional nurses within the baby’s first hour of life. Gestational age was calculated in completed weeks based on the date of self-reported last menstrual period. Since there was a lack of references in the Chinese population, we applied United States national references to construct SGA and AGA in the current study population(Reference Oken, Kleinman and Rich-Edwards28,Reference Min, Jie and Min29) .
Ca supplementation and dietary Ca intake
The influence factors included Ca supplementation and dietary Ca intake from food. Information on Ca supplement use was collected in the following four periods: before conception (12 months before pregnancy), first trimester (1–13 weeks), second trimester (14–27 weeks) and third trimester (more than 27 weeks). In each period, duration and frequency of Ca supplementation use alone and Ca-containing multivitamins were ascertained. Ca supplement users were defined as those who took Ca supplements alone or Ca-containing multivitamins before conception and/or during pregnancy. Non-users were defined same as above. Dietary information was collected via a semi-quantitative FFQ. Dietary Ca intake was estimated based on the frequency of consumption and portion size of food items using the Chinese Standard Tables of Food Consumption(30).
Statistical analysis
Chi-square or Fisher’s exact tests were used to compare selected characteristics between LBW and NBW, and between SGA and AGA. Student’s t test was utilised to compare the differences in infants’ mean birth weights between Ca users and non-users. Unconditional logistic regression models were used to estimate OR and 95 % CI for the association between Ca supplementation, dietary Ca intake, LBW and SGA. Potential confounding variables were adjusted in the final models, including maternal age (less than 25, 25–29 and more than 30 years old), pre-pregnancy BMI (weight (kg)/height (m2)), weight gain during pregnancy, gestational weeks, parity (nulliparous or multiparous), education levels (<college or ≥college), family monthly income per capita (less than 3000 or more than 3000 RMB in income), maternal employment (yes or no), Ca supplementation (yes or no) and dietary Ca intake (mg/d). Additional adjustments for vaginal bleeding, smoking (active and passive), alcohol consumption during pregnancy, gestational diabetes and infants’ gender did not result in material changes in the observed associations and thus were not included in the final models. All analyses were performed using SAS software, version 9.3 (SAS Institute, Inc.).
Results
Baseline characteristics
Among the 9595 women with a singleton live birth, 7·39 % (n 708) gave birth to SGA infants with a birth weight less than 2500 g, and 8·00 % (n 768) were born with SGA (Table 1). Among the 768 cases of SGA, 18·62 % (n 143) were preterm-SGA (less than 37 weeks) and 81·38 % (n 625) were term-SGA infants (more than 36 weeks). Compared with women who delivered infants that were NBW or AGA, respectively, women who delivered LBW or SGA infants were more likely to be less than gestational week (less than 37 weeks), have lower education, less family income and weight gain during pregnancy, and higher percentages of exposure to passive/active smoking. Women who were diagnosed with vaginal bleeding were also more likely to have LBW and SGA infants. Women who delivered infants with LBW or SGA were less likely to be nulliparous and employed when compared with women who delivered infants with NBW or AGA. There were no significant differences in gestational diabetes, alcohol consumption during pregnancy and infants’ gender between women who delivered infants with LBW and NBW as well as women who delivered infants with SGA and AGA. Women who delivered infants with LBW were more likely to be either younger (< 25 years old) or older (≥ 30 years old) than women who delivered infants with NBW, while women who delivered infants with SGA were more likely younger than women who delivered infants with AGA. There was no obvious difference in pre-pregnancy BMI between women who delivered infants with LBW and NBW. However, women who delivered infants with SGA had a higher percentage of women with low BMI (<18·5 kg/m2) pre-pregnancy compared with women who delivered infants with AGA.
Table 1 Baseline characteristics of the study population in a large-scale Chinese birth cohort (n 9595)
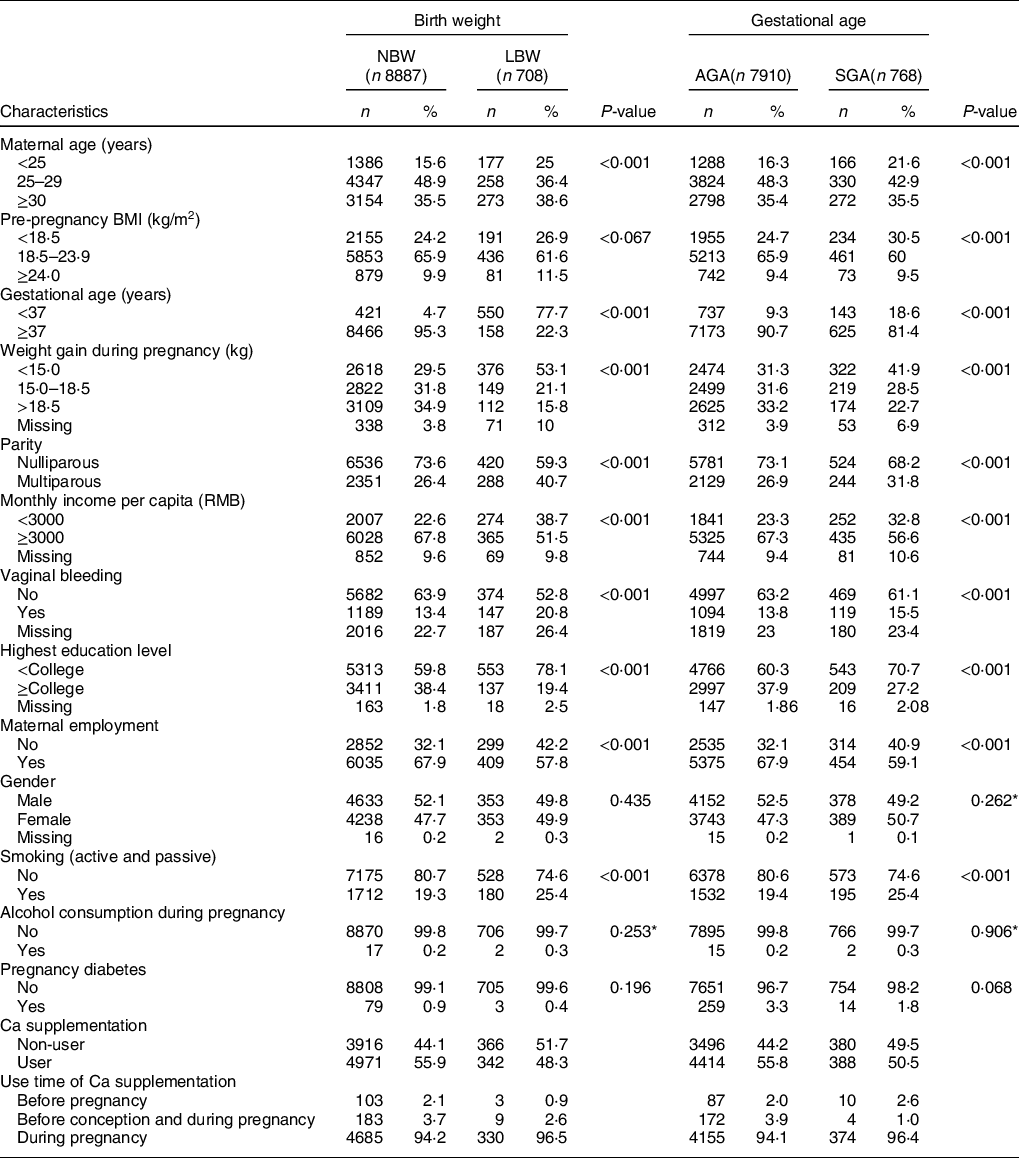
NBW, normal birth weight; LBW, low birth weight; AGA, average for gestational age; SGA, small for gestational age.
Association between Ca intake and the risk of low birth weight
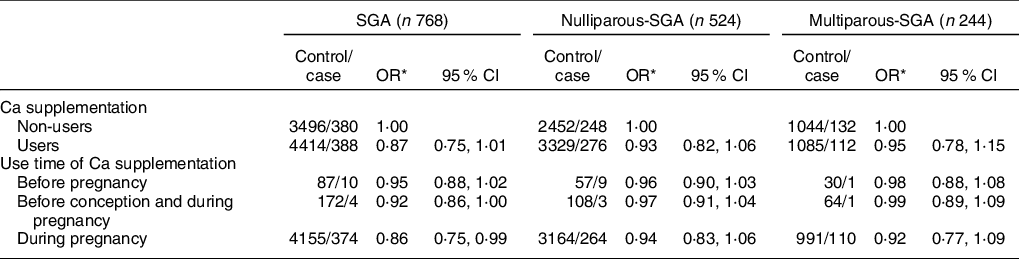
As shown in Table 2, compared with non-users, users with Ca supplementation were associated with a reduced risk of LBW overall (OR: 0·77, 95 % CI: 0·63, 0·95) (P < 0·05). More specifically, the significantly reduced risk was observed for women who had used Ca supplementation before conception and during pregnancy (OR: 0·44, 95 % CI: 0·19, 0·99) and during pregnancy only (OR: 0·80, 95 % CI: 0·65, 0·99) (P < 0·05). No significant differences were observed among women who only used Ca supplements before conception (OR: 0·38, 95 % CI: 0·10, 1·44) (P > 0·05). After stratifying by parity, nulliparous women with Ca supplementation were associated with a significantly reduced risk of LBW infants (OR: 0·75, 95 % CI: 0·58, 0·98) when compared with non-users (P < 0·05), while there was no significant difference between multiparous women with and without Ca supplementation (P > 0·05).
Table 2 Association between Ca supplementation and LBW and its subtypes by parity
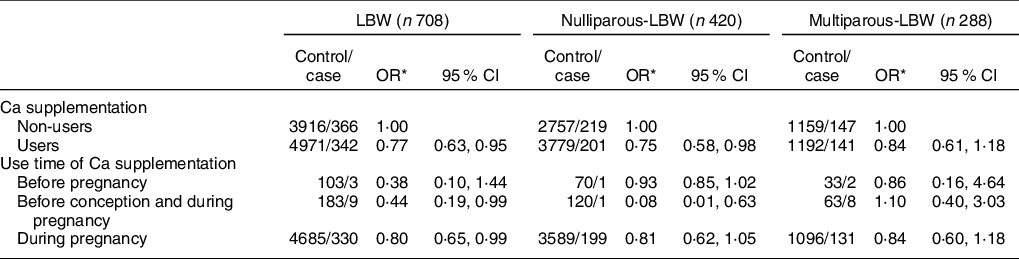
LBW, low birth weight.
* Adjusted for maternal age, pre-pregnancy BMI, gestation weeks, weight gain during pregnancy, or parity, education level, maternal employment (yes, no), family monthly income per capita and dietary Ca intake.
Reduced risk of LBW was also associated with higher estimated dietary Ca intake from food (Table 3). During pregnancy, significant protective effect against LBW was found for the higher quartile of dietary Ca intake (Q2 OR: 0·72, 95 % CI: 0·55, 0·94; Q3 OR: 0·68, 95 % CI: 0·50, 0·92). After stratification by parity, significantly reduced risks of LBW were only observed among nulliparous women with higher quartile intake of dietary Ca during pregnancy (Q2 OR: 0·68, 95 % CI: 0·48, 0·98; Q3 OR: 0·67, 95 % CI: 0·46, 0·98). No differences were found among multiparous women who delivered LBW infants and took Ca supplements either before or during pregnancy.
Table 3 Association between dietary Ca intake and LBW and its subtypes by parity
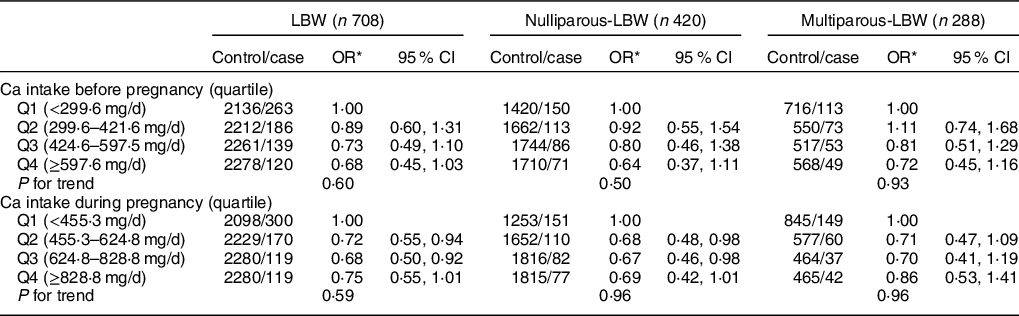
LBW, low birth weight.
* Adjusted for maternal age, pre-pregnancy BMI, gestation weeks, weight gain during pregnancy, parity, education level, maternal employment, family monthly income per capita and Ca supplementation.
Association between Ca intake and risk of small for gestational age
Compared with non-users, Ca supplementation users were associated with a reduced risk of SGA infants (OR: 0·87, 95 % CI: 0·75, 1·01), although results were not statistically significant (Table 4) (P > 0·05). However, there was a significant relation between SGA and Ca supplementation in women who used Ca supplementation only during pregnancy (OR: 0·86, 95 % CI: 0·75, 0·99) (P < 0·05). More specifically, after stratification by gestational age, reduced risk of SGA was only observed among women who took Ca supplementation only during pregnancy (OR: 0·85, 95 % CI: 0·73, 0·99) (P < 0·05).
Table 4 Association between Ca supplementation and SGA and its subtypes by gestational age
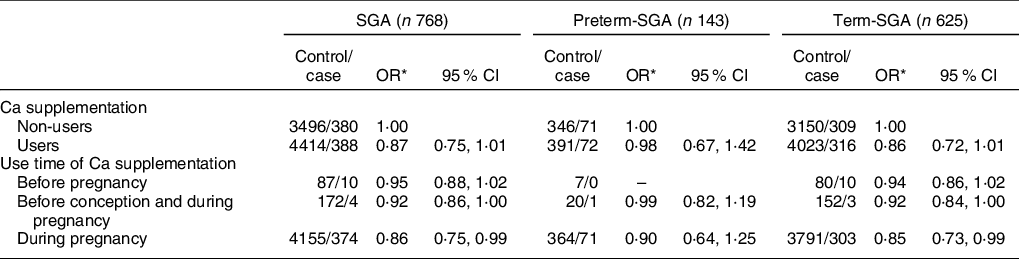
SGA, small for gestational age.
* Adjusted for maternal age, pre-pregnancy BMI, weight gain during pregnancy, parity, education level, maternal employment, family monthly income per capita and dietary Ca intake.
As shown in Table 5, reduced risk of SGA was observed among women in the highest quartile of dietary Ca intake before pregnancy (OR: 0·78, 95 % CI: 0·63, 0·97), which was mainly attributed to reduced risk found for term-SGA (OR: 0·77, 95 % CI: 0·61, 0·98) (P < 0·05). Higher quartiles of dietary Ca intake during pregnancy were all associated with reduced risk of SGA (Q2 OR: 0·77, 95 % CI: 0·63, 0·95; Q3 OR: 0·71, 95 % CI: 0·57, 0·88; Q4 OR: 0·71, 95 % CI: 0·57, 0·88) (P < 0·05). After stratification by gestational age, similarly, association between Ca intake and term-SGA was observed for highest quartile before pregnancy (OR: 0·77, 95 % CI: 0·61, 0·98) and higher or highest quartile during pregnancy (Q3 OR: 0·73, 95 % CI: 0·57, 0·93; Q4 OR: 0·74, 95 % CI: 0·58, 0·94) (P < 0·05). No reduction in SGA risk was found in women independent of Ca intake before pregnancy or during pregnancy (Table 6).
Table 5 Association between dietary Ca intake and SGA and its subtypes by gestational age
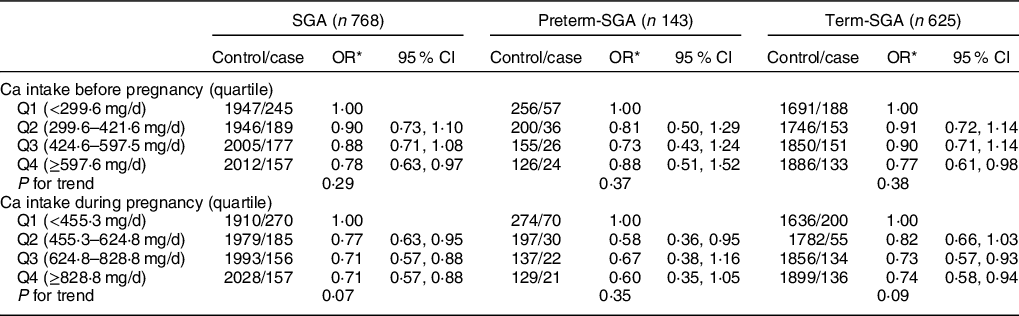
SGA, small for gestational age.
* Adjusted for maternal age, pre-pregnancy BMI, weight gain during pregnancy, parity, education level, maternal employment, family monthly income per capita and Ca supplementation.
Table 6 Association between Ca supplementation and SGA and its subtypes by parity
SGA, small for gestational age.
* Adjusted for maternal age, pre-pregnancy BMI, gestation weeks, weight gain during pregnancy, parity, education level, maternal employment, family monthly income per capita and dietary Ca intake.
In terms of dietary Ca intake associated with SGA, we observed a borderline significant reduced risk of SGA in the highest quartile of dietary Ca intake before pregnancy (Q4 OR: 0·80, 95 % CI: 0·66, 0·97) (P < 0·05) (Table 7). In contrast, higher dietary Ca intake during pregnancy significantly associated with reduced risk of overall SGA (Q2 OR: 0·77, 95 % CI: 0·63, 0·97; Q3 OR: 0·71, 95 % CI: 0·57, 0·88; Q4 OR: 0·71, 95 % CI: 0·57, 0·88) (P < 0·05).
Table 7 Association between dietary Ca intake and SGA and its subtypes by parity
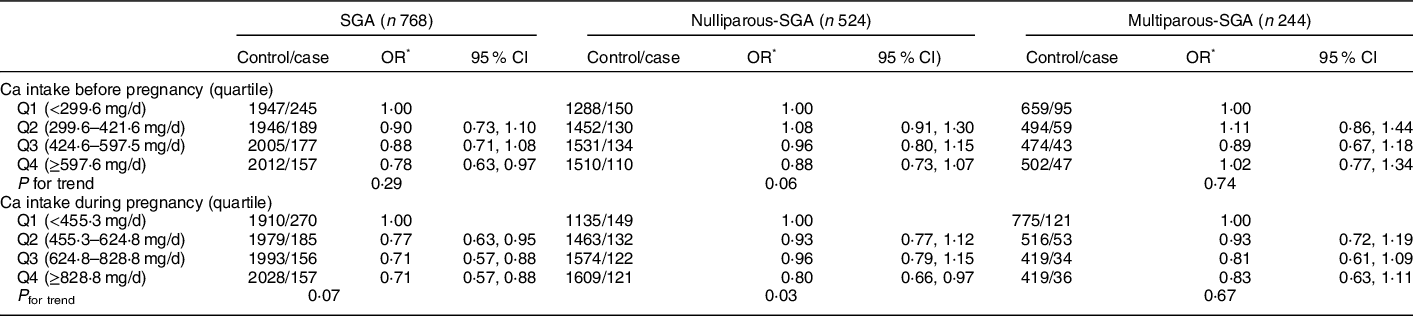
SGA, small for gestational age.
* Adjusted for maternal age, pre-pregnancy BMI, gestation weeks, weight gain during pregnancy, parity, education level, maternal employment, family monthly income per capita and Ca supplementation.
Discussion
This is the first study to investigate the association of Ca intake in the development of LBW and SGA in the Chinese population. The current study demonstrated that high consumption of Ca supplementation and dietary Ca intake during pregnancy were associated with reduced risk of nulliparous women giving birth to LBW infants. Higher dietary Ca intake from food during pregnancy can significantly reduce the risk of SGA, especially the term-SGA infants. Ca supplementation had no apparent association with risk of SGA.
In the present study, Ca supplementation during pregnancy was associated with a 23 % risk reduction of LBW and a 25 % risk reduction of nulliparous-LBW. Our results supported the finding that Ca supplementation during pregnancy reduced the risk of LBW in previous individual clinical trials and systematic reviews(Reference Villar and Repke15–Reference Ashok, Salam Gyaneshwori and Swaraj17,Reference Hofmeyr, Roodt and Atallah31,Reference Hofmeyr, Beliz and Von Dadelszen32) . Among these supporting studies, 2 g of Ca per day to reduce the risk of LBW (risk ratio (RR), 0·45 (0·22, 0·95)(Reference Villar and Repke15), 0·36 (0·14, 0·89)(Reference Crowther, Hiller and Pridmore16), 0·55 (0·32, 0·94)(Reference Ashok, Salam Gyaneshwori and Swaraj17)), respectively. Previous reviews also reported that Ca supplementation reduced the risk of LBW (RR 0·83, 95 % CI: 0·71, 0·98)(Reference Hofmeyr, Roodt and Atallah31) and low dose of Ca supplementation plus linoleic acid reduced the risk of LBW (RR 0·20, 95 % CI: 0·05, 0·88)(Reference Hofmeyr, Beliz and Von Dadelszen32–Reference Villar, Abdel-Aleem and Merialdi37). However, the outcomes of Ca supplementation on the risk of LBW in newborns are inconsistent. Hofmeyr et al. had reported that the risk of LBW in newborns reported in eight studies showed no association with Ca supplementation when compared with women who did not have Ca supplementation (RR 0·84, 95 % CI: 0·68, 1·03)(Reference Hofmeyr, Duley and Atallah38). Furthermore, Imdad et al. had reported that the risk of LBW in newborn reported in three trials showed no association with Ca supplementation in the developing countries (RR 0·81, 95 % CI: 0·58, 1·12)(Reference Aamer, Afshan and Zulfiqar39,Reference Aamer and Zulfiqar40) . A meta-analysis of multi-centre randomised controlled trials showed no significant reduction on risk of LBW in 6560 women who received Ca supplementation as compared with 6565 women who received placebo (RR 0·91, 95 % CI: 0·72, 1·16)(Reference An, Li and Xie41). In a recent Cochrane review(Reference Buppasiri, Lumbiganon and Thinkhamrop14) , there was no significant difference in LBW infant births between the Ca supplementation group and placebo group (RR 0·93, 95 % CI: 0·81, 1·07, in six trials, 14 162 infants; random-effects model). However, higher birth weights occurred with Ca supplementation when compared with placebo controls (mean difference 56·40 g; 95 % CI: 13·55, 99·25) in 21 trials with 9202 women. It is worth emphasising that the subject population in the current study are largely from inland cities; thus, the average Ca intake was low due to the limited accessibility to Ca-rich seafood. In contrast, high intake of dairy products in Western countries ensure a higher intake of Ca. The discrepancy of Ca levels in different populations might contribute to the different results found between Ca intake and the risk of disease.
We found that Ca supplementation could increase birth weight. Julian A Herrera et al. reported that birth weight was significantly higher in the supplemented group when compared with placebo group(Reference Herrera, Arévalo-Herrera and Shahabuddin18). No significant differences in the risk of LBW infant births were observed between the two groups, which might be attributed to the small sample size and selected age groups of women (<20 and >30 years old). Similarly, Niromanesh et al. reported that infants born to Ca-supplemented women, on average, were 552 g heavier than infants born to the placebo group(Reference Niromanesh, Laghaii and Mosavi-Jarrahi19). A few studies also found similar results(Reference Lopez-Jaramillo, Narvaez and Weigel20–Reference Purwar, Kulkarni and Motghare22,Reference Lopez-Jaramillo, Delgado and Jacome33,Reference Taherian, Taherian and Shirvani34) , while other studies failed(Reference Wanchu, Malhotra and Khullar25,Reference Levine, Hauth and Curet35,Reference Villar, Abdel-Aleem and Merialdi37) .
In our study, we found that women using Ca supplements had a significant association with LBW infants when taken both before conception and during pregnancy and during pregnancy only in comparison with non-users. After stratification by parity, a significant association between Ca supplement use and risk of LBW infant births was found in nulliparous women, while no association was found for multiparous women who delivered LBW infants. Our results were in contrast to a previous study(Reference Sara Rizvi, Rubeena and Muhammad42), which found that the intake of Ca supplements during pregnancy was associated with the reduced risk of LBW among women with at least one child born in the last 5 years.
We also found that dietary Ca intake was significantly associated with LBW infants and LBW infants born to nulliparous women in the higher quartile of Ca intake during pregnancy, while no differences were observed in the number of LBW infants born to multiparous women who took Ca supplements before or during pregnancy. From our dietary Ca intake data, we found that population in our study had low Ca intake, even in the highest category (< 900 mg)(Reference Hofmeyr, Duley and Atallah38). Some studies had reported that Ca supplementation might be of greater benefit to women with lower dietary Ca intakes(Reference Hofmeyr, Lawrie and Atallah43). Hence, the association was more prominent in women with low Ca intake in comparison with those with adequate Ca intake. Ca supplementation and dietary Ca intake were significantly associated with LBW infants born to nulliparous women. However, the association between Ca supplementation and dietary Ca intake and multiparous-LBW was unclear. Therefore, other factors may come into play affecting LBW infants born to nulliparous and multiparous women.
There was no overall association of Ca supplementation in the development of SGA. A few studies observed that nulliparous women with eclampsia showed a significant association with increased risk of preterm-SGA compared with preterm-AGA births. Sociodemographic factors have been reported as higher risk factors for term-SGA births(Reference Fairley and Leyland44–Reference Ota, Ganchimeg and Morisaki46). Term-SGA births may be more significantly relevant to lifestyle factors, such as sociodemographic status and malnutrition(Reference Ota, Ganchimeg and Morisaki46). Previous studies have found that maternal nutrition and diet during pregnancy could be beneficial to term-SGA(Reference Ota, Ganchimeg and Morisaki46,Reference Mitchell, Robinson and Clark47) . A few studies reported that nulliparous women had an increased risk of SGA by population centiles when compared with multiparous women(Reference Meis, Michielutte and Peters48–Reference Maureen, Dawn and Beverley54). In our study, after stratification by parity, we did not find an association between Ca supplement use by nulliparous women who gave birth to SGA infants or in the multiparous-SGA group. Only women in the nulliparous-SGA group showed significant association with the highest dietary Ca intake category that was consumed during pregnancy.
There are some strengths and limitations in the study. Although our study had a relatively large sample size, our statistical power was limited in stratified analyses. Even though many important confounding factors have been adjusted, we cannot rule out the potential of residual confounding. Because information on dietary Ca and Ca supplementation was collected through in-person interview at delivery, potential recall bias might exist. However, if there was any recall bias, it was likely to be non-differential and result in an underestimation of the observed association, as the association between Ca supplementation, dietary Ca and LBW risk was unclear. As our study was hospital-based in a single location, generalisability to the entire Chinese population is limited.
Conclusion
In conclusion, our study suggests that both Ca supplementation and dietary Ca intake during pregnancy are associated with a reduced risk of nulliparous-LBW infant births, while only higher dietary Ca intakes during pregnancy are significantly associated with risk of SGA infant births. Future studies are needed to confirm these associations and explore the mechanism and to identify that infants and women would have significant benefits from adequate Ca intake.
Acknowledgements
Acknowledgements: This study was sponsored by BiosTime Maternal and Child Nutrition Health Research Projects of China CDC Maternal and Child Health Center (No. 2018FYH007); Gansu provincial health research projects (No. GSWSKY2018-54); Internal funding from the Gansu Provincial Maternity and Child Care Hospital, and the Gansu Provincial Science and Technology Department Grant (1204WCGA021). Financial support: The authors thank BiosTime Maternal and Child Nutrition Health Research Projects of China CDC Maternal and Child Health Center (No. 2018FYH007); Gansu provincial health research projects (No. GSWSKY2018–54); Internal funding from the Gansu Provincial Maternity and Child Care Hospital, and the Gansu Provincial Science and Technology Department Grant (1204WCGA021). Conflict of interest: The authors declare that they have no conflict of interest. Authorship: The authors declare that all the listed authors have participated actively in the study and all meet the requirements of the authorship. Dr W.D. and X.H.D. designed the study and wrote the protocol, Dr J.Q., B.H.M. and L.L.L. performed research/study, Dr Y.W.S., S.J.X. and T.Y. contributed important reagents, Dr X.C.H., H.M.C. and X.J.L. managed the literature searches and analyses, Dr L.L., Z.F.T. and Q.L. undertook the statistical analysis, and Dr W.D. and Q.L. wrote the first draft of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Human Investigation Committee of the Gansu Provincial Maternity and Child Care hospital. Written informed consent was obtained from all patients.










