Fe deficiency (ID) is a worldwide nutritional problem, and includes Fe reduction and Fe deficiency anaemia (IDA). The prevalence of IDA is extremely high in reproductive-age women, especially in pregnant women, and preschool-age children. The WHO estimated that >700 million people have IDA(Reference Gupta, Venkateswaran and Gorenflo1), most of whom are in developing countries. The Fourth National Nutritional and Health Survey of China indicated that the average prevalence of anaemia was 15·2 % (24·2 % in infants; 21·5 % in the elderly; 20·6 % in gravidas)(Reference Li, Rao and Kong2). Puberty is the primary time for growth and development. Due to the high amounts of Fe needed to sustain growth and menstrual blood loss(Reference Hallberg, Hultén and Lindstedt3), adolescent girls are particularly susceptible to ID. It has been reported that the prevalence of anaemia in Shanghai students aged 6–17 years is 21·6 %(Reference Sun, Sheng and Wang4); however, there have been no data on IDA in Chinese adolescent girls. In addition, there is a need to determine why the prevalence of anaemia is high despite sufficient dietary intake of Fe (>25 mg/d). A possible explanation is that in addition to physiological factors affecting absorption and utilisation of Fe, infection with certain bacteria and some environmental factors may also be contributory factors. Specifically, Helicobacter pylori infection has been proposed to be one of the factors contributing to ID(Reference Peach, Bath and Farish5). H. pylori has been reported to possibly affect Fe absorption and metabolism in the stomach and exacerbate Fe deficit in adolescents, especially girls, with anaemia ensuing promptly. The relationship between H. pylori infection and IDA in adolescent female athletes has been verified(Reference Choe, Kwon and Jung6), although there have been no data on the relationship between H. pylori infection and IDA in Chinese adolescent girls.
In the present study, adolescent girls aged 12–18 years in northeast China were selected using a multi-level cluster sampling method and their Fe nutritional status was evaluated. The relationships among H. pylori infection and IDA, anaemia and development were investigated. Moreover, we conducted a double-blind controlled trial to determine the role of H. pylori infection in Chinese adolescent girls with IDA and to establish a treatment modality for IDA with co-existing H. pylori infection.
Materials and methods
Screening for anaemia
Adolescent girls 12–18 years of age were enrolled in the study through a multi-level cluster sampling method from October to November 2007. Participants were recruited from three academies, which represent different levels of socio-economic environment, in the Suihua district in Heilongjiang Province, China. Blood samples (10 ml) were obtained from the participants by venepuncture. Approximately 1 ml of blood was collected into an EDTA-coated tube for the determination of Hb (automatic blood cell analyser; Sysmex Corporation, Hyogo, Japan). Anaemia was defined as Hb < 120 g/l. The remaining blood was centrifuged and the serum was separated and stored at − 20°C for ID analysis.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the Research and Ethics Committee of Harbin Medical University. Before enrolment of the participants in the study, written informed consent was obtained from each parent of participants < 18 years and each adolescent 18 years of age.
Determination of serum ferritin and serum transferrin receptor values
Serum ferritin (SF) and serum transferrin receptor (sTfR) of participants were measured using a microparticle enzyme immunoassay method on an IMx Analyzer machine (Abbott Laboratories, Abbott Park, IL, USA)(Reference Choe, Kwon and Jung6) and an ELISA (TfR ELISA kit; WuHan Uscn Sciences Company Limited, Wuhan, China) according to the manufacturers' instructions. Levels of sTfR in non-anaemic participants (range 0·6–60·5 μmol/l; mean 13·1 (sd 12·8) μmol/l) were used to construct reference intervals for the subjects (1·6–24·5 μmol/l).
Evaluation of iron nutritional status
Fe sufficiency was defined as an Hb value>120·0 g/l and an SF value>12·0 μg/l. Borderline anaemia was defined as 110·0 g/l < Hb < 120·0 g/l. ID was defined as SF < 12·0 μg/l(Reference Siti-Noor, Wan-Maziah and Narazah7, Reference Leenstra, Kariuki and Kurtis8). IDA was defined as Hb < 120·0 g/l, SF < 12·0 μg/l or Hb < 120·0 g/l, SF>12·0 μg/l and sTfR>24·5 μmol/l.
Diagnosis of current Helicobacter pylori infection
Participants were tested for H. pylori IgG antibody (ASSURE H. pylori Rapid Test; Genelabs Company, Singapore, Singapore) and H. pylori stool antigen (H. pylori Antigen ELISA Diagnostic Kit; PUMC Pharmaceutical Company Limited, Beijing, China)(Reference Ni, Lin and Huang9–Reference Wang, Lin and Zhou10). A current H. pylori infection was defined as H. pylori IgG antibody seropositivity and H. pylori stool antigen-positivity.
All tests were performed according to the manufacturers' instructions. As recommended by the manufacturers, positive, equivocal and negative results were defined as an OD ≥ 0·120, 0·100–0·120 and ≤ 0·100, respectively. Cases with equivocal results were recalculated and those with equivocal recalculated results were excluded from the study.
Questionnaire on developmental and general physiological indices
A structured questionnaire for information on consumption of Fe supplements, age of menarche, volume of menstruation, length of flow in days, numbers of sanitary pad usage/d of menses and past history of illness was personally administered by a trained investigator. Light menstrual blood volume was indicated by menstrual flow duration of 1–5 d or usage of 1–2 pads/d, while 6–10 d of flow or usage of 3–5 pads was defined as heavy volume. Spot measurements of height and weight were performed at the time of blood collection. Atrophy was defined as BMI (BMI = body weight (kg)/height (m)2) < 15·0 kg/m2 in participants 11–13 years old, < 16·5 kg/m2 in those 14–17 years old and < 18·5 kg/m2 in those 18 years old. Overweight and obese subjects were screened according to the ‘BMI reference for screening overweight and obese Chinese children and adolescents’ established by the Working Group on Obesity in Chinese(11). To ensure the reliability and accuracy of the questionnaire, two pre-investigations were conducted.
Survey on dietary iron intake
Daily Fe intake of subjects with anaemia was measured using a 24-h recall method for 3 d, including a weekend day. This method exhibited good reliability and validity, confirmed by other members of our group(Reference Xia, Sun and Zhang12). Food portions were estimated by pictures and models. Data were calculated with a nutrient calculator software (Fei Hua V2·3; The Institute for Nutrition and Food Security, Chinese Center for Disease Control and Prevention, Beijing, China). Sufficient Fe intake for adolescent girls was defined as an average Fe intake >25 mg/d, as recommended by the Chinese Nutrition Society(Reference Kun13).
Randomisation and treatment
Criteria for enrolment in the study included the following: no history of severe diseases (e.g. kidney disease, hepatic disease, asthma and musculoskeletal disorders); and no recent use of drugs (e.g. antibiotics or antacids) during the preceding 6 months. Subjects who exhibited IDA in combination with H. pylori infection and had an intake of Fe>25 mg/d were randomly assigned to Fe-supplemented intervention and Fe-supplemented control groups. Subjects in the intervention group received oral Fe supplementation (EDTA–Na–Fe, 60 mg Fe/dose, three times a week) for 12 weeks and a 2-week course of triple therapy with colloidal bismuth subcitrate (440 mg of bismuth/d), amoxicillin (1000 mg/d) and metronidazole (800 mg/d). Subjects in the control group received oral Fe supplementation (EDTA–Na–Fe, 60 mg Fe/dose, three times a week) alone for 12 weeks. Follow-up assessments of H. pylori infection were conducted 4 weeks after completion of the 2-week triple therapy regimen using an H. pylori stool antigen test. Fe status was reassessed 3 months after conclusion of the 12-week regimen through determination of SF, Hb and sTfR.
Statistical analysis
Data were entered into EPIDATA3.02 in duplicate. The χ2 test was used to assess the association between the prevalence of H. pylori infection and IDA. The relationships between the risk factors (e.g. BMI, age, menstrual blood volume, dietary Fe intake, Fe supplementation in the past 6 months and residence) and anaemia were analysed by multiple logistic regression modelling. These models facilitated the assessment of the relative importance of H. pylori infection on IDA while controlling for other risk factors. Outcomes after the intervention trial were assessed using t test. Due to the side effects of the anti-H. pylori drugs, more subjects (five) in the intervention group were lost to follow-up, compared with the control group (one). This made it impossible to process the intent-to-treat analysis, so that results were based on per protocol. All statistical analyses were performed using SPSS statistics software (version 13.0; Stats Data Mining, Beijing, China) and Excel 2003. All data were expressed as means and standard deviations, unless stated otherwise. A P value < 0·05 was considered significant.
Results
Prevalence of anaemia and iron deficiency
Among 1037 adolescent girls aged 12–18 years, 202 (19·5 %) were anaemic, with borderline anaemia accounting for 84·2 % of patients with anaemia. A total of 177 (17·1 %) adolescent girls had IDA and 419 (40·4 %) had ID. IDA accounted for 87·6 % of patients with anaemia.
Status of Helicobacter pylori infection
In the H. pylori stool antigen test, four cases with equivocal results were duplicated again. Of these, one girl was validated as a positive result and three girls with still equivocal results were excluded (as negative). In combination with the results of the serum H. pylori IgG antibody test, the prevalence of H. pylori in adolescent girls was 31·2 % (324/1037). Among 177 IDA cases, twenty-five subjects with non-IDA anaemia, and 835 non-anaemic subjects, eighty-three (46·9 %), six (24·0 %) and 235 (28·1 %), respectively, were positive for H. pylori (P < 0·01; Fig. 1).

Fig. 1 Prevalence of Helicobacter pylori infection in adolescent girls in the Suihua area of China. Iron deficiency anaemia (IDA, n 177), non-IDA (n 25), non-anaemic (n 835), total (n 1037). ** Mean values were significantly different compared with the non-anaemic group (P < 0·01). □, Subjects without H. pylori infection; ![]() , subjects with H. pylori infection.
, subjects with H. pylori infection.
Analysis of risk factors for anaemia
Multiple logistic regression analysis, using BMI, age, menstrual blood volume, dietary Fe intake, Fe supplements in the past 6 months and residence as independent variables, and anaemia as a dependent variable, was performed. We found that normal BMI was a protective factor for anaemia (r − 0·537, OR = 0·584, 95 % CI 0·417, 0·819). Large volume of menstruation and H. pylori infection were the risk factors for anaemia (r 0·239, OR = 1·270, 95 % CI 1·059, 1·523; and r 0·347, OR = 1·360, 95 % CI 1·212, 1·638, respectively; Table 1).
Table 1 Logistic regression predicting for risk factors of anaemia

* Parameter of score test.
Randomisation and treatment
Excluding one adolescent girl with kidney disease and two who were unwilling to participate, the eighty subjects were randomly assigned to two groups: intervention group (n 37) and control group (n 43). Baseline measurements of Hb, SF, sTfR, volume of menstruation and BMI were performed. There were no significant differences in these values between the two groups (P>0·05). A total of six participants (five in the intervention group and one in the control group) did not return at the end of the trial.
Follow-up determination of H. pylori infection was performed 4 weeks after completion of the 2-week regimen in both groups. H. pylori was eradicated in twenty-nine of thirty-two subjects (90·6 %) in the intervention group and two of forty-two (4·7 %) in the control group. After the intervention trial, all subjects (both in the intervention and control groups) were re-divided into two groups according to H. pylori eradication status, i.e. H. pylori-positive group and H. pylori-negative group. Hb, SF and sTfR values of the thirty-one H. pylori-negative subjects before the intervention trial were compared with those after the intervention trial; the same was done for the forty-three H. pylori-positive subjects. Fe status was reassessed 3 months after completion of the 12-week regimen. The number of participants in every step of the Fe intervention trial is presented in Fig. 2.

Fig. 2 Trial participants flow chart.
Hb and SF values were increased and sTfR was decreased significantly after treatment compared with those before treatment in the intervention group (P < 0·01). Although Hb and SF were not statistically different before and after treatment in the control group, sTfR was significantly decreased (P < 0·01; Table 2). After re-grouping according to H. pylori eradication status, Hb and SF were increased and sTfR was decreased after treatment compared with those before treatment in the H. pylori-negative group (P < 0·01). All values of Hb, SF and sTfR were not different before and after treatment in the H. pylori-positive group (Table 3).
Table 2 Values of Hb, serum ferritin (SF) and serum transferrin receptor (sTfR) before and after iron intervention in adolescent girls† from the Suihua area of China
(Number of participants, mean values and standard deviations)
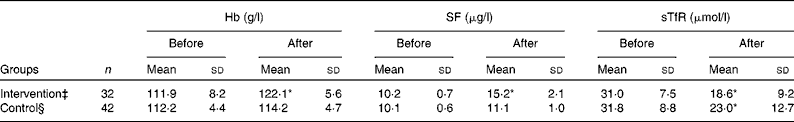
* Mean values were significantly different compared with the before treatment (P < 0·01).
† Adolescent girls with Fe deficiency anaemia and co-existing Helicobacter pylori infection who had an Fe intake of >25 mg/d.
‡ Subjects received oral Fe supplementation (EDTA–Na–Fe, 60 mg Fe/dose, three times a week) for 12 weeks and a 2-week course of triple therapy with colloidal bismuth subcitrate (440 mg of bismuth/d), amoxicillin (1000 mg/d) and metronidazole (800 mg/d).
§ Subjects received oral Fe supplementation (EDTA–Na–Fe, 60 mg Fe/dose, three times a week) for 12 weeks.
Table 3 Values of Hb, serum ferritin (SF) and serum transferrin receptor (sTfR) in the Helicobacter pylori-negative and H. pylori-positive groups according to H. pylori eradication status after an iron intervention trial† in adolescent girls‡
(Number of participants, mean values and standard deviations)

* Mean values were significantly different compared with the before treatment (P < 0·01).
† Subjects in the intervention group received oral Fe supplementation for 12 weeks and a 2-week course of triple therapy with colloidal bismuth subcitrate, amoxicillin and metronidazole. Subjects in the control group received oral Fe supplementation for 12 weeks.
‡ Adolescent girls with Fe deficiency anaemia and co-existing H. pylori infection who had an Fe intake of >25 mg/d.
§ All H. pylori-negative participants from either the intervention or control group upon completion of Fe intervention trial.
¶ All H. pylori-positive participants from either the intervention or control group upon completion of Fe intervention trial.
Discussion
The present study involved 1037 adolescent girls from northeast China, with an anaemia prevalence of 19·2 %, most of which was IDA (84·2 %). This indicates that anaemia, especially IDA, is still a prominent problem in adolescent girls of northeast China. Both the family and society should pay more attention to the physiological health problems of adolescent girls, reinforce Fe supplementation and control anaemia-associated factors to prevent the development of anaemia.
The common laboratory tests for evaluating Fe status are SF, serum Fe and free erythropoiesis protoporphyrin. However, the concentration of serum Fe may fluctuate widely in an individual due to diurnal and other factors(Reference Dallman, Barr and Allen14), while SF concentration is influenced by various clinical conditions(Reference Mast, Blinder and Gronowski15); the use of either test will decrease the efficiency of IDA diagnosis. sTfR is assumed to reflect the degree of tissue ID and the speed of erythropoiesis in the bone marrow(Reference Cazzola and Beguin16–Reference Thorstensen and Romslo21), so that its value in the evaluation of Fe nutritional status has received much attention. Researchers have found that there are age- and race-related differences in sTfR concentrations(Reference Allen, Backstrom and Cooper22, Reference Choi, Pai and Im23), indicating that the average level of sTfR in the community should be determined when using this measurement to assess Fe nutritional status. Reference intervals for sTfR in Chinese adolescent girls have not been established. In the present study, we found that the average concentration of sTfR in Chinese adolescent girls was 13·1 μmol/l, with a reference interval of 1·6–24·5 μmol/l. The sTfR concentration is not influenced by inflammation(Reference Cook, Skikne and Baynes17), whereas SF is one of the acute-phase reactants, so that even mild upper respiratory infections are associated with an increase in SF(Reference Choi24, Reference Reeves, Yip and Kiley25). Thus, the sensitivity of SF for IDA diagnosis is conspicuously decreased when there is co-existing infection, especially in adolescent girls who have severe H. pylori infection. Based on these information, SF combined with sTfR was deemed to be a reliable criterion for IDA diagnosis in our study.
H. pylori is one of the most common bacterial causes of chronic infection. The prevalence of H. pylori infection varies widely by geographic area, age, race, ethnicity and socio-economic status, and seropositivity increases with age(Reference Brown26, Reference Jafri, Yakoob and Abid27). The prevalence rate of H. pylori infection is 50 % in adults in developed countries and 60–90 % in developing countries(Reference Wan, Xu and Xue28). A study reported that the prevalence rate of H. pylori infection in Chinese adults is >50 %(Reference Wang and Wang29); however, there has been a paucity of data on Chinese adolescent girls. In the present study, we found the prevalence of H. pylori infection in Chinese adolescent girls to be 31·2 %, which is lower than that in adults. H. pylori infection has not only been implicated in the aetiology of a variety of upper gastrointestinal diseases, but has also been correlated with many external gastrointestinal diseases. H. pylori causation or aggravation of ID and contribution to IDA development mainly involve three mechanisms: (1) the bacteria may exert a negative effect on body Fe balance by chronic blood loss from the gastrointestinal tract, thus increasing Fe loss(Reference Milman, Rosenstock and Andersen30); (2) H. pylori gastritis can reduce gastric acid secretion and ascorbic acid levels, thus reducing Fe absorption(Reference Annibale, Capurso and Martino31); (3) Fe sequestration in lactoferrin in the gastric mucosa and Fe uptake by H. pylori increase Fe need(Reference Dhaenens, Szczebara and Husson32). In our interventional study, the Fe intake by all the adolescent girls meets the Chinese recommended nutrient intake of Fe for this population (25 mg/d). Considering the aforementioned three mechanisms, the most possible reason about causing or aggravating ID by H. pylori infection was the decrease in Fe absorption. No direct evidence was exhibited for the other two mechanisms in the present study, although they might also produce the effects.
A large study of Alaskan natives (n 2080)(Reference Parkinson, Gold and Bulkow33) found lower levels of SF in persons seropositive for H. pylori. A study in Germany found significantly lower levels of Hb in pregnant women suffering from H. pylori infection(Reference Weyermann, Rothenbacher and Gayer34). In a population-based study of healthy Americans aged >3 years (n 7462)(Reference Cardenas, Mulla and Ortiz35), persons seropositive for H. pylori infection had significantly lower SF levels, compared with seronegative individuals. After adjustment for confounding factors, H. pylori infection was still significantly correlated with IDA. A double-blind, randomly controlled trial in children with IDA(Reference Choe, Kim and Son36) found that individuals who underwent H. pylori treatment with or without Fe had significantly higher Hb levels compared with those who received Fe only, thus providing more evidence that H. pylori infection is closely related to IDA. However, studies on the relationship between H. pylori infection and IDA in adolescent girls are rare. In our study, we found that the prevalence of H. pylori infection was significantly higher in adolescent girls with IDA than in non-anaemic adolescent girls. Female adolescents who exhibited IDA in combination with H. pylori infection and had an Fe intake >25 mg/d were randomly assigned to Fe-supplemented intervention and Fe-supplemented control groups. The intervention group received a 2-week course of triple therapy with 12-week Fe supplementation, whereas the control group received 12-week Fe supplementation only. We found that subjects who underwent H. pylori treatment had significantly higher improvement in Hb and SF values and significantly decreased sTfR levels, compared with subjects who were untreated for H. pylori. sTfR levels in both groups were significantly decreased after therapy.
Findings of the present study suggest that there is an association between H. pylori infection and IDA in Chinese adolescent girls, which is consistent with results in South Korea(Reference Choe, Kwon and Jung6, Reference Choe, Kim and Hong37, Reference Seo, Ko and Choi38). Results indicate that H. pylori infection may cause ID and contribute to IDA development in Chinese adolescent girls. In cases of IDA and co-existing H. pylori infection, IDA can be treated by the eradication of H. pylori in combination with Fe supplementation.
The high prevalence of infection in the population indicates that a measure of infection is essential (e.g. C-reactive protein), although this will considerably increase the workload. This is one of the limitations of our present study. In addition, our study is also limited by the small population. Every step of the sampling was randomised in order to make the participants as representative of all the teenage girls in the district as possible.
Acknowledgements
L. W. was involved in designing the trial and writing the trial protocol, calculating the sample size, analysing the data and finalising the manuscript. W. X. was involved in writing the trial protocol, supervising subjects' recruitment, data collection and drafting the manuscript. X. Z. was involved in data collection, analysing the data and revising the manuscript. J. W. and C. S. initiated and supervised the trial as principal investigators. All authors approved the final version of the manuscript. None of the authors had a personal or financial conflict of interest. The source of funding for this study was from the Science and Technology Agency of Heilongjiang Province, China (Youth Fund QC06C059).







