Rabies is a fatal disease that affects the central nervous system of humans and other mammals. The rabies virus belongs to the genus Lyssavirus, within the family Rhabdoviridae in the order Mononegavirales. Eleven Lyssavirus genotypes are known, ten of which have been isolated from bats. The main genotypes of interest in Europe are 1, 5 and 6, corresponding to classical rabies virus (RABV), European bat lyssavirus types 1 and 2 (EBLV-1, -2). Genotype 1 viruses have a worldwide distribution and are generally found in foxes, raccoon dogs, dogs, cats and cattle and other terrestrial animals, but have never been isolated from European bats. In North America, bat rabies is caused by genotype 1. Genotypes 5 and 6, commonly known as EBLV-1 and -2, are restricted to Europe and EBLV-1 is frequently isolated from European bats, which are important reservoir hosts of these emerging viruses [Reference Fooks1]. Both EBLV-1 and EBLV-2 have been subdivided into two phylogenetic lineages, EBLV-1a, EBLV-1b, and EBLV-2a, EBLV-2b, respectively. Four new genotypes, Khujand virus, Aravan virus, Irkut virus and West Caucasian bat virus, have also been accepted among lyssaviruses recently isolated from bats in Russia and in Central Asia.
In Europe, nearly 900 EBLV cases in bats had been reported by the end of 2008, mainly in Denmark, The Netherlands, Germany, Poland, France and Spain [2]. Most (>95%) of these cases were EBLV-1 and predominantly associated with the serotine bat (Eptesicus serotinus). The numbers of EBLV-2 cases in bats confirmed by genomic sequence analysis have remained low. There have now been more than 20 reported cases of EBLV-2, which have occurred in only five countries: Switzerland, The Netherlands, UK, Germany [2] and now Finland. EBLV-2 is typically detected in Myotis daubentonii (Daubenton's bat) and M. dasycneme (Pond bat) [Reference Fooks1]. Two cases of EBLV-2 infection in humans have been confirmed, both in individuals working with bats [Reference Lumio3, Reference Fooks4].
In 1985, a bat researcher in Finland died of rabies encephalitis caused by EBLV-2b [Reference Davis5]. However, the researcher had handled bats in several countries during the previous year and it could not be concluded where the researcher had become infected. In 1986, active systematic and targeted bat samplings were executed in order to determine whether bat rabies occurs in Southern Finland [Reference Hagner, Hanák, Horácek and Gaisler6]. Specimens sent by the public extended the sampled area to Central Finland. This study focused on sampling of the four most common species in Finland, i.e. Eptesicus nilssonii, M. daubentonii, M. mystacinus and M. brandtii. These were also the species that the deceased bat researcher had worked with. A sick Daubenton's bat had bitten him, but the bat was set free and never investigated for rabies. A total of 183 bats were analysed and found to be free from rabies [Reference Hagner, Hanák, Horácek and Gaisler6]. The results did not explain where the deceased bat scientist had become infected.
Here, we describe the northernmost case of bat rabies ever detected in Europe, and the first encounter of bat rabies in a bat in Finland, as well as the relationship between the rabies virus isolated from the human case around 25 years previously and that from the Daubenton's bat of the present study, including the possibility that bat rabies is permanently established in Finland.
The rabid male Daubenton's bat was caught among other conspecifics in a mist net placed adjacent to a pond used by Daubenton's bats for feeding near the town of Turku (60° 22′ N, WGS84). We used a harp trap with two 6-m mist nets on either side to catch bats as they commuted along a forest corridor from their day roosts to their foraging areas. A Sussex Autobat was used as an acoustic lure to attract bats to the traps. The rabid bat appears to have flown straight towards the acoustic lure, as it was caught very close to the ultra-sound loudspeaker, although much lower than the other bats caught. The bat was highly tangled in the net, more so than the normally placid Daubenton's bats. During removal, the bat's behaviour was very aggressive and the bat convulsed strongly. After the bat was released from the net and placed on the examination table, it became extremely passive. When touched the bat began to scream and convulse. The peculiar behaviour of the bat suggested that it was rabid. The bat weighed 8·4 g and had a forearm length of 38·5 mm. Thus, its body condition index (mass g/forearm mm) was almost 0·22, which indicates good health for a male [Reference Senior, Butlin and Altringham7]. The bat did not salivate excessively and the mouth was clean of debris.
Daubenton's bat is one of the most common bat species found in Finland. The range of the species extends up to 67° N in the north of Finland [Reference Siivonen and Wermundsen8]. However, due to limited sampling, accurate range or population size estimates are as yet unavailable. Daubenton's bats feed almost entirely on non-biting midges (Chironomidae), which are caught when emerging from water bodies. During the summer months, Daubenton's bats mainly roost in abandoned cavities excavated by woodpeckers and in bat boxes or bird nest boxes. Daubenton's bats hibernate in moist underground caves, deep rock crevices and occasionally in cellars.
The suspected bat was transported to the University of Turku, where it was euthanized with CO2 gas and sent to the Finnish Food Safety Authority (Evira) for further examination. Routine rabies laboratory diagnosis was performed according to the OIE Manual of Standards for Diagnostic Tests and Vaccines, 2009. The sample was studied with a standard fluorescent antibody test (FAT) on the day it was received by the laboratory. Two conjugates [rabies conjugate anti-nucleocapsid (Bio-Rad, USA) and FITC anti-rabies monoclonal globulin (Fujirebio Diagnostics Inc., USA)] were used. Conjugates were diluted according to the manufacturers' instructions. The virus was isolated in mouse neuroblastoma cells (MNA) and stained with both conjugates after 72 h.
The RNA for PCR studies was extracted from 10% brain suspension supernatant with QIAamp Viral RNA Mini kit (Qiagen, Germany) according to the manufacturer's instructions. Other tissues were not studied. The preliminary typing of the strain and confirmation of the result was performed using a real-time reverse transcriptase–polymerase chain reaction (rRT–PCR) test specific for EBLV-1 and EBLV-2 [Reference Wakeley9]. In order to produce material for sequencing and genetic typing of the strain, primers JW12 (5′-atg taa cac cyc tac aat g-3′) and JW6 (5′-cag ttg gcg cac atc ttg tg-3′) (modified from Heaton et al. [Reference Heaton10]) were used in OneStep RT–PCR (Qiagen). These primers amplify a 606-bp fragment of the nucleoprotein gene of most lyssaviruses. The following thermal profile was used: a single cycle of reverse transcription for 30 min at 50°C, 15 min at 94°C for reverse transcriptase inactivation and DNA polymerase activation followed by 40 amplification cycles of 1 min at 94°C, 1 min at 50°C and 1 min at 72°C.
Mini gels, DNA recovery kit and BandPick™ (Elchrom Scientific, Switzerland) were used to prepare the PCR product for sequencing. The sequencing of both strands was performed with the Applied Biosystems 3100 Genetic Analyzer and the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, USA). Reaction products were purified using the DyeEx 2.0 Spin kit (Qiagen).
Database sequence homology searches were performed using the program BLAST (http://www.ncbi.nlm.nih.gov/BLAST). Before sequence analysis, the primer sequences were excluded. All EBLV-2 sequences at the GenBank sharing this 567-bp-long stretch were included in the sequence analysis. The sequences were edited with programs of the EMBOSS package, aligned with the ClustalW program and analysed using the neighbour-joining method and the MEGA 4 program. The data were bootstrapped with 1000 replicates.
Brain smears of the Daubenton's bat were strongly positive with the FAT, showing distinct specific fluorescence. The first and second MNA cell culture passages gave weak positive results with both conjugates. The real-time RT–PCR test specific for EBLV-2 was clearly positive, with a Ct value of 20, while the corresponding test for EBLV-1 was negative. The modified RT–PCR test gave a strong positive band. The subsequent sequence analysis revealed that the isolated strain was EBLV-2 (Fig. 1). It shared 99·1% nucleotide identity with the EBLV-2b strain isolated from the human rabies case in Finland in 1985 [Reference Lumio3, Reference Davis5], while the nucleotide identities with all the other EBLV-2 strains available in the database, represented by the Dutch, Swiss and Scottish isolates, were 95·8–96·3%, 95·4–95·6% and 95·6%, respectively. The sequence of the nucleoprotein gene region has been deposited in the EMBL sequence data bank under accession number GU002399.
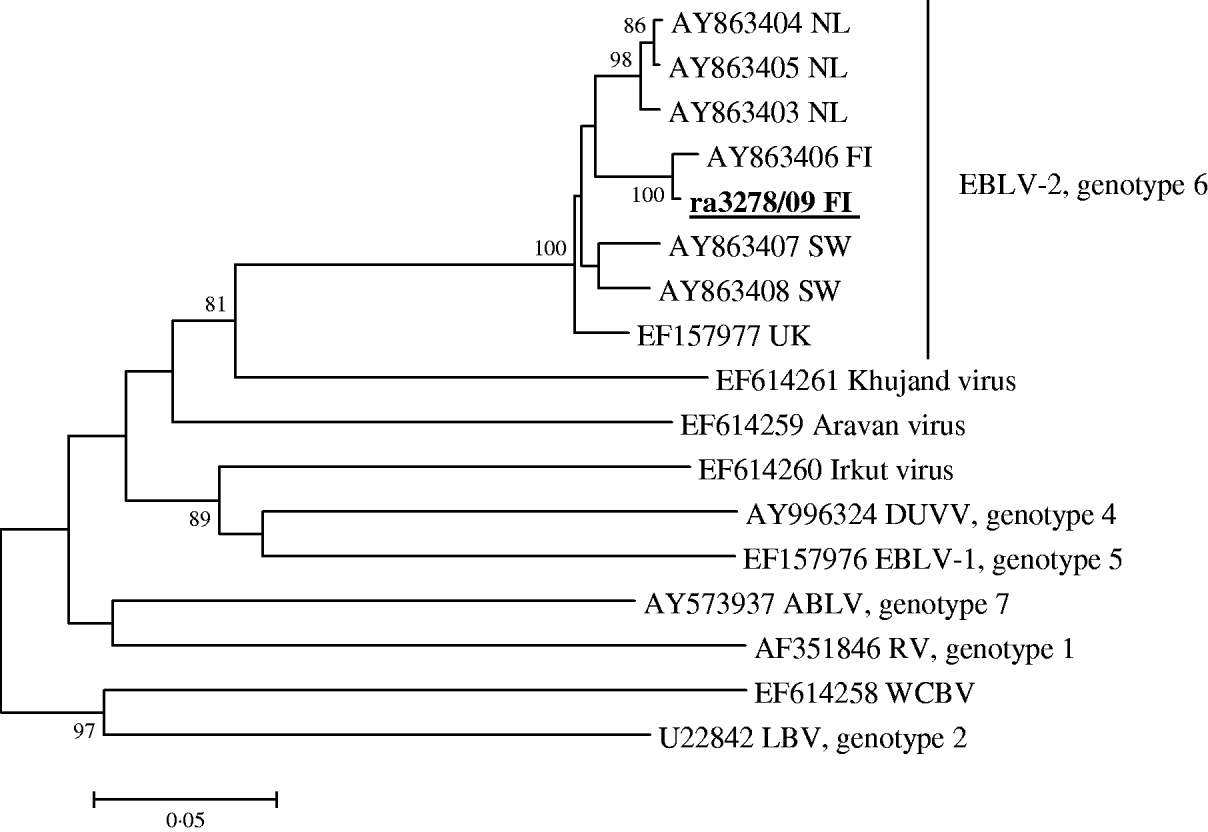
Fig. 1. Lyssaviruses associated with bats. The phylogenetic tree is based on 567 nucleotides of the nucleoprotein gene of the current Finnish strain ra3278/09 FI and representatives of all other Lyssavirus genotypes known to be associated with bats: RV, rabies virus; LBV, Lagos bat virus; DUVV, Duvenhage virus; EBLV-1, European bat lyssavirus 1; EBLV-2, European bat lyssavirus 2; ABLV, Australian bat lyssavirus; WCBV, West Caucasian bat virus and Khujan virus, Aravan virus, Irkut virus. Bootstrap values lower than 80 are removed. The GenBank accession number of the ra3278/09 FI strain is GU002399.
The currently low number of EBLV-2 isolations in Europe makes the phylogenetic subgrouping into 2a and 2b types uncertain. Nevertheless, the high bootstrap values indicate the existence of several geographical EBLV-2 clusters in Europe. More sequence data are needed to create a reliable classification, and it would be most informative if all the sequences from isolated strains were available in the databases.
Finland has been declared rabies-free since 1991. No rabies cases have been found, except for imported cases in a horse in 2003 and a dog in 2007. A bat rabies case was diagnosed in a human in 1985, but the origin of infection was not identified because the researcher had been bitten by bats in several countries. Our study confirms the presence of European rabies virus of genotype EBLV-2 for the first time in Finland. It is also the northernmost confirmed bat rabies case ever detected in Europe. The further characterization of the virus revealed that it was genetically closely related and clearly clustered together with the Finnish EBLV-2b isolate from the human case isolated around 25 years earlier. Thus, it can be concluded that EBLV-2 may have been circulating undetected in Finland for many years. The rabid bat reported here was not found in any of the study areas where the deceased researcher had been working and which were sampled after he died in 1986 [Reference Hagner, Hanák, Horácek and Gaisler6]. The case reported here was found about a further 100 km towards the south-west.
Little information is available on the duration of the estimated incubation period in naturally infected Daubenton's bats. It has been suggested that EBLV-2 might have an extended incubation period compared to other strains of rabies virus before being intermittently excreted in saliva. In one case of a naturally infected Daubenton's bat, an incubation period >9 months was documented [Reference Pajamo11]. A captive Daubenton's bat infected by subdermal inoculation developed disease after 32 days [Reference Johnson12]. This also supports the conclusion that exposure of the bat worker to EBLV-2b in Finland in 1985 was due to the bite of an infected bat in Finland. However, it is known that the length of incubation is inversely related to the amount of virus transmitted during infection, and depends on the virus variant [Reference Johnson12]. The time interval between clinical signs in Daubenton's bats and the excretion of live EBLV-2 in saliva has been studied and it has been estimated to be up to 3 days before the development of rabies [Reference Johnson12].
The numbers of confirmed EBLV-2 cases in bats has remained low. As rabies in bats is coming under greater scrutiny, lyssavirus infections are more frequently being found in Europe. Due to the protected status of bats and the small number of bat studies conducted in Finland, knowledge of the prevalence and epidemiology of EBLV in the country is limited. It is possible that EBLV is under-reported and that the recorded cases of EBLV represent only a small proportion of the actual number of infected bats. So far, no infected bats have been reported from the neighbouring countries of Sweden and Norway, or the Baltic countries [2], even though Daubenton's bat is also common in these countries. Active sampling of bats has been recently conducted in Sweden with no positive results so far (M. Nedinge, personal communication).
In Finland, the health risk to the general public, which has little contact with bats, is low. The fact that Daubenton's bats do not usually roost in buildings reduces the likelihood of property owners finding grounded bats and having to move or help them. This might also lead to lower passive sampling of this species. Specimens of Daubenton's bats sent by the public to natural history museums are less numerous compared to species such as Brandt's bats (M. brandtii) or Northern bats (E. nilssonii), which commonly roost in house attics or other sheltered structures in buildings.
In order to assess the prevalence of EBLVs in Finnish bat populations and to gain a better understanding of the public health risk that EBLV-infected bats pose, a targeted active surveillance project on suspect bat cases has been initiated. These studies will be performed in close collaboration with bat conservationists in order to support public health issues whilst protecting the welfare of the bats.
ACKNOWLEDGEMENTS
We thank Tiina Peltonen, Merja Hautala, Tanja Rajamäki and other laboratory technicians at Evira for excellent technical assistance. We also thank Hanna Tuominen for assistance in the field.
DECLARATION OF INTEREST
None.



