Cervical cancer is the second most common cancer among women worldwide, with the highest rates occurring in developing countries(Reference Parkin, Bray and Ferlay1). The high incidence in these regions is attributed to inadequate screening and other factors strongly correlated with socio-economic and demographic characteristics(Reference Kogevinas, Susser and Boffetta2, Reference Parikh, Brennan and Boffetta3). Human papillomavirus (HPV) infection is considered a necessary but not sufficient cause of cervical cancer, since most women infected with HPV do not develop cervical cancer, while many cofactors such as tobacco smoking, oral contraceptive use and high parity play ancillary roles in the disease process(Reference Walboomers, Jacobs and Manos4–Reference Appleby, Beral and Berrington de Gonzalez8). Tobacco smoking has been associated with the early stages of carcinogenesis, from the acquisition of HPV infection to cervical cancer, increasing the risk twofold relative to non-smokers(Reference Plummer, Herrero and Franceschi7–Reference Vaccarella, Herrero and Snijders9). The impaired immunological response and increased free radicals produced by cigarette smoking provide the biological plausibility for a link between tobacco and cervical cancer(Reference Poppe, Ide and Drijkoningen10, Reference Eiserich, van der Vliet and Handelman11).
Nutritional factors, mainly antioxidants such as carotenoids and tocopherols, have been associated with the cervical carcinogenic process: from the clearance of HPV infection, persistence of the virus causing the early stages of carcinogenesis to the late stages of cervical neoplasia and invasive cancer through immunological deprivation(Reference Goodman, Shvetsov and McDuffie12–Reference Tomita, Logatto Filho and Costa22). A diet rich in vegetables and fruits has been recommended for the prevention of cancer due to their antioxidant properties, which reduce the toxic effects of reactive oxygen species (ROS) and possible enhancement of the immune response(23, Reference Chew and Park24). ROS can affect fluidity and integrity of the membrane of immunological cells causing changes in the distribution and function of cellular receptors(Reference Chew and Park24). A slightly lower consumption of carotenoids and vitamin C, particularly from fruits, has been observed among smokers compared with among non-smokers(Reference Stryker, Kaplan and Stein25–Reference Chow, Thacker and Changchit29). Lower serum concentrations of antioxidants, especially carotenoids, have been observed among smokers independently of dietary intake, which further increases the effect of a low intake of fruits(Reference Stryker, Kaplan and Stein25–Reference Tomita, Almeira and Roteli-Martins30). Thus, it is unknown whether the smoking effect on the risk of cervical neoplasia is modified by a low intake of fruits and vegetables.
The present study aimed to investigate the joint effect of fruit and vegetable intake, serum concentrations of carotenoids and tocopherols and smoking on the risk of cervical intraepithelial neoplasia grade 3 (CIN3, the lesion grade accepted as a cervical cancer precursor) in a hospital-based case–control study.
Subjects and methods
Study setting
Participants in the present study were selected from the Brazilian Investigation into Nutrition and Cervical Cancer Prevention (BRINCA) study, a hospital-based case–control study designed to investigate the relationship between diet and cervical cancer in São Paulo, Brazil. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the São Paulo University School of Public Health Ethics Committee. Written informed consent was obtained from all subjects. The BRINCA study included women attending two major hospitals (Instituto Brasileiro de Controle do Câncer and Hospital Perola Byington) in São Paulo, Brazil, for cervical cytological screening. We prospectively recruited newly diagnosed cases of CIN and invasive cancer. Eligible women were residents of São Paulo aged 21–65 years and had no prior hysterectomy, no previous treatment for CIN and no cancer history. Women who were positive for HIV or who had been pregnant or breastfeeding within 6 months of enrolment were ineligible.
Cases included women with a histological diagnosis of CIN grades 1 (CIN1), 2 (CIN2) or 3 (CIN3) or invasive adenocarcinoma, adenosquamous or squamous cell carcinoma of the cervix, reviewed by two pathologists. During the same period, control women were selected from among those attending screening in the same clinics where cases were diagnosed. To be eligible, control women had to have a cytological diagnosis within normal limits, in addition to meeting the above-mentioned criteria for cases.
Sampling strategies, participation rates and initial results from the BRINCA study have been reported elsewhere(Reference Tomita, Logatto Filho and Costa22, Reference Tomita, Almeira and Roteli-Martins30). A total of 1729 women were contacted between 2003 and 2005. Among these women, fifty-three (3·1 %) refused participation (thirty-four potential cases and nineteen potential controls) and 1676 were interviewed. Among them, 1394 (83·2 %) completed the study protocol. Among those enrolled, 1179 women were eligible for the present study. Overall, 121 participants were excluded due to incomplete data, and the final study groups included 453 controls and four case groups (140 CIN1, 126 CIN2, 231 CIN3 and 108 cervical cancer cases). The present analysis included 453 controls and 231 women diagnosed with CIN3, an acceptable precancerous cervical lesion, due to considerable sample size for comparisons. We did not include invasive cancer cases because of the possible effects of the disease process on the nutritional measurements.
Data collection
Participants underwent a personal interview by trained dietitians blinded to the group assignment. A standardised questionnaire was used to elicit information on socio-economic and demographic characteristics, physical activity (including both occupational and leisure activities, expressed in metabolic equivalent tasks in h/week), tobacco use (current smoker, former smoker or non-smoker; individuals who had quit smoking at least 1 year before the interview were considered former smokers), alcohol consumption, reproductive and sexual histories and other risk factors for cervical neoplasia. Self-reported race/ethnicity was classified as white and non-white (black women and mulatto).
BMI was expressed as kg/m2 and calculated based on weight and height measurements with subjects wearing light clothes and no shoes, according to standard protocols and cut-offs proposed by the WHO(31).
Food consumption was assessed using a validated FFQ(Reference Cardoso, Kida and Tomita32) and adapted to epidemiological studies on diet and chronic diseases in São Paulo(Reference Sartorelli, Sciarra and Franco33). Briefly, energy-adjusted, attenuation-corrected Pearson validity correlations between FFQ and three dietary recalls ranged mostly from 0·40 to 0·75. Bland–Altman plots indicated that the FFQ is accurate in assessing nutrient intake at a group level, and a small proportion of grossly misclassified nutrient intakes was observed(Reference Cardoso, Tomita and Laguna34). Intake of fruits and vegetables assessed by the FFQ was a good predictor of serum total carotene whose intakes contributed to about 5 % of the serum total carotene concentration, after adjusting for confounding variables(Reference Tomita, Almeira and Roteli-Martins30).
Women were asked about their usual frequency of consumption of seventy-six food items and their portion sizes, an open-ended food section, and vitamin and mineral supplements during the previous year. A validation study with good accuracy for the FFQ was also observed in a random sub-sample of ninety-six cases and controls from the BRINCA study, using three 24 h dietary recalls obtained during a year, as reported previously(Reference Tomita, Almeira and Roteli-Martins30). Nutrient and food groups were analysed using the DietSys software version 4.01 (National Cancer Institute, Bethesda, MD, USA)(Reference Block, Coyle and Hartman35). The nutrient database was based primarily on the US Department of Agriculture publications supplied by DietSys and supplemented by the Brazilian Standard Food Composition table. Questionnaires were excluded from further analysis if energy intake was implausible < 2929 kJ/d (700 kcal/d) or >25 104 kJ/d (6000 kcal/d), corresponding to the percentiles < 2·5 or >97·5, respectively. In the present study, two controls and one woman with diagnosis of CIN3 reported implausible values of dietary intake. For the present study, we investigated five food groups: (1) dark-green and deep-yellow vegetables and fruits (green salad, kale, broccoli, spinach, pumpkin, carrot, sweet potatoes, papaya and mango); (2) total fruit and fruit juices (orange, papaya, banana, apple, pear, watermelon, melon, grape, pineapple, avocado, mango, persimmon, orange juice and fruit juices); (3) only citrus fruit and citrus fruit juices (orange); (4) total vegetables (lettuce, chicory, watercress, cabbage, Chinese cabbage, cauliflower, kale, broccoli, spinach, pumpkin, sweet potatoes, carrot, tomato, aubergine, peas, beans, green been, beetroot, summer squash, mixed vegetables and vegetable soup); (5) total fruit and vegetables (vegetables and fruits reported earlier).
Serum micronutrient analyses
All participants were scheduled to provide a fasting blood sample within 1 week after recruitment in the study. Whole blood was collected, protected from light, centrifuged within 1 h of collection and frozen at − 70°C until being analysed. Serum samples were analysed for total carotene (β-, α- and γ-carotene), lycopene, α- and γ-tocopherols and retinol by HPLC (HP-1100 HPLC system; Hewlett Packard, Palo Alto, CA, USA), as described elsewhere(Reference Gomes, Alves and Sevanian36). Peaks for carotenoids that were under the quantification limits were set to zero. There were two samples for total carotene (one control and one diagnosed with CIN3), and one sample for lycopene (diagnosed with CIN3) that were below the limit of quantification (the detectable level of total carotene, lycopene and γ-tocopherol were 0·5, 0·2 and 0·2 μmol/l, respectively). Serum total cholesterol was measured enzymatically using an automatic device (ADVIA 1650; Bayer, East Walpole, MA, USA). All samples were analysed within 6 months after collection. The laboratory assayed internal and external blinded quality-control specimens in every run. From the control specimens, the accuracy and inter-assay CV for these analytes were within 8 %.
Human papillomavirus testing
Exfoliated cervical cells were collected from each woman using the DNA-Cytoliq® (Digene Brazil, São Paulo, Brazil) liquid-based system. The ectocervical and endocervical samples were collected using the brush provided with the kits and immersed in Universal Collecting Medium™ vials. The cervical specimen was stored at 4°C until processing for cytology and HPV testing.
Laboratory personnel were blinded to the case–control status of the participants. The cervical specimens were centrifuged at 5000 rpm for 10 min at 22–24°C. Cells were digested with 0·2 mg/ml proteinase K in 50 μl 100 mm-NaCl, 10 mm-Tris–HCl (pH 8·0), 25 mm-EDTA buffer (pH 8·0), 0·5 % SDS between 2 and 16 h at 60°C and purified. Extracted DNA was tested for the presence of human DNA using a PCR protocol for β-globin and for HPV DNA by a PCR protocol amplifying a highly conserved 450 bp segment in the L1 viral gene (flanked by primers MY09/11), as described previously(Reference Tomita, Logatto Filho and Costa22). Typing of amplified products was performed by hybridisation with individual oligonucleotide probes specific for twenty-seven HPV genital types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 56, 57, 58, 59, 66, 68, 73, 82, 83 and 84.
HPV types were classified as having either high-risk oncogenic potential or low-risk oncogenic potential. The high-risk types included HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82(Reference Munoz, Bosch and de Sanjose37). HPV infection status was classified as per the following four hierarchical mutually exclusive categories: (1) HPV negative; (2) only positive for low-risk HPV types; (3) positive for at least one high-risk HPV type except HPV-16 and HPV-18; (4) positive for HPV-16 and/or HPV-18.
Statistical analysis
The Pearson χ2 test was used to examine differences in proportions between the CIN3 and control groups stratified by smoking habit (non-smoker or former and current smoker). Median values and interquartile ranges were calculated for serum micronutrients and dietary intakes according to the outcome status. The Mann–Whitney test was used for comparison between groups for continuous variables.
Serum tocopherols were adjusted for serum total cholesterol(Reference Willett38) and classified according to the nutritional adequacy of α-tocopherol (normal values ≥ 2·5 μmol/mmol v. inadequacy)(Reference Haller, Weggemans and Lammi-Keefe39). Serum retinol concentrations were dichotomised according to adequacy ( ≥ 1·50 v. ≤ 1·49 μmol/l), serum lycopene and total carotene were categorised according to a cut-off point of the median concentrations of 0·41 and 0·28 μmol/l (below v. above the median concentration), respectively(Reference Wei, Kim and Boudreau40), measured in the National Health and Nutrition Examination Survey III among healthy non-smoker women. Serum γ-tocopherol adjusted for serum total cholesterol was categorised according to a cut-off point of the median concentration of 1·03 μmol/mmol (below v. above the median concentration) observed in the National Health and Nutrition Examination Survey 1999–2000 among the healthy non-institutionalised US population(Reference Ford, Schleicher and Mokdad41). Vegetable and fruit intakes were dichotomised (below v. above daily intake of one portion of fruits or half portion of vegetables) based on the daily intake of 80 g total fruit and fruit juices, 80 g total citrus fruits and citrus fruit juices, 40 g total vegetables, 40 g dark-green and deep-yellow vegetables and fruits and 320 g or four portions of total vegetable and fruit group intakes. Unconditional logistic regression models were used to calculate OR for CIN3 according to combinations of tobacco smoking status and dietary or nutritional variables, with individuals in the first category of the exposure of interest as the reference group.
To assess interaction, all participants were categorised in four groups stratified by tobacco smoking status (non-smokers v. former and current smokers) and dietary intakes or serum micronutrients (same categories reported earlier)(Reference Rothman, Greenland and Walker42, Reference Szklo and Nieto43). Former and current smokers were combined due to similarity in the micronutrients concentrations and fruit and vegetable intake(Reference Tomita, Almeira and Roteli-Martins30). All analyses were adjusted for age (21–30, 31–40, 41–50 and 51–65 years), hospital (Instituto Brasileiro de Controle do Câncer and Hospital Perola Byington), race/ethnicity (white and non-white), schooling ( ≥ 6 and ≤ 5 years) and potential confounders or mediators if their inclusion in any of the models caused a change in the OR estimate of 10 % or more: sexual debut ( ≥ 20, 17–19 and ≤ 16 years), lifetime sexual partner (1, 2 and ≥ 3 partners), parity (0, 1–3 and ≥ 4 pregnancies) and HPV status (as defined above). A restricted analysis was performed selecting only women infected with high-risk HPV types.
Interaction was identified by comparing the observed OR for the joint effect of smoking and dietary intake and the expected OR using a simple mathematical operation(Reference Szklo and Nieto43). The Wald test and likelihood ratio test were used to test for the statistical significance of the estimated interaction term(Reference Kleimbaum44, Reference Knol, van der Tweel and Grobbee45). In the likelihood ratio test, the difference of log-likelihood statistics for two models, one model with and the other model without interaction terms, and in the Wald test only one parameter, the interaction term, were tested. In the adjusted model, effect modification was assessed by including the interaction terms between the two variables of interest (tobacco smoking status and dietary intake or serum micronutrients, for example: 40 g vegetables × smoking status)(Reference Kleimbaum44). Additional analyses were conducted with six categorical variables considering three tobacco smoking status (non-smoker, former smoker, current smoker) and dietary intakes and serum micronutrients. Statistical significance was set at 0·05, and 95 % CI around the OR are presented. All P values were derived from two-sided statistical tests. All analyses were done in STATA 9.0 (StataCorp, College Station, TX, USA).
Results
The study population reported a low intake of fruits and vegetables (median intake about 250 g/d) far below the WHO recommendation of 400 g/d for this food group(23). The primary reported fruits and vegetables were orange, banana, kale and tomato available during all seasons in São Paulo. Median values of fruit and fruit juices were 144 (interquartile range 61–299) and 99 (interquartile range 62–150) g/d of total vegetables. Significantly reduced consumption of vegetables and fruit groups and serum micronutrient concentrations were observed among the CIN3 cases when compared with those among the controls (P < 0·005). Former and current smokers reported a lower intake of fruits and serum total carotene, lycopene and tocopherols (P < 0·05) among both the controls and CIN3 cases (data not shown).
Table 1 shows the distribution of the CIN3 cases and the controls according to the selected covariates and stratified by smoking status according to selected covariates. Differences were observed between the cases and controls among former and current smokers: cases tended to be younger, non-white, less educated, on lower incomes and to have had an earlier sexual debut than the controls. However, lifetime number of sexual partners, higher parity and higher proportion of oncogenic type of HPV infection were observed among CIN3 women for both smoking status groups. Lower dietary intakes of vegetables and fruits and serum micronutrient concentrations were observed among the CIN3 cases than among the controls for both smoking status groups (P < 0·05) (data not shown).
Table 1 Characteristics of the study subjects stratified by smoking habit and outcome status†
(Number of study subjects and percentages)

CIN3, cervical intraepithelial neoplasia grade 3; USD, US dollar; HPV, human papillomavirus.
* Proportions were significantly different from those of the control group (χ2 test): P ≤ 0·001.
† Totals do not coincide due to missing values for some variables.
Table 2 shows the age- and hospital-adjusted OR for CIN3 by dietary intake and serum micronutrients, overall and stratified according to tobacco smoking status. Estimated risks differed slightly among lifelong non-users of tobacco, and no consistent associations emerged, apart from the finding that increases in risk were invariably associated with lower consumption or concentration categories. CI were wide, except for serum tocopherols. However, an increased risk of lower intake of dark-green and deep-yellow vegetables and fruits and lower concentration of serum α-tocopherol was identified among former and current smokers.
Table 2 Cervical intraepithelial neoplasia grade 3 (CIN3) by daily dietary intake and serum micronutrient concentrations stratified by tobacco smoking status
(Odds ratios and 95 % confidence intervals)
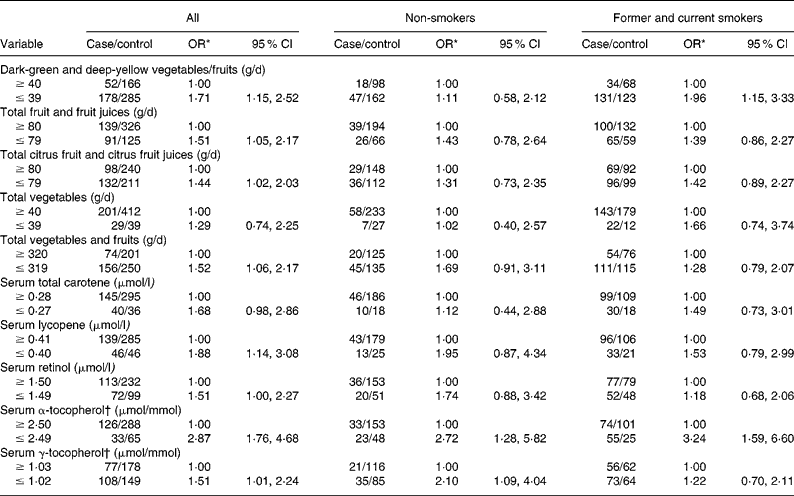
* OR adjusted for age (21–30, 31–40, 41–50 and 51–65 years) and hospital (Instituto Brasileiro de Controle do Câncer and Hospital Perola Byington).
† Adjusted for serum total cholesterol.
Table 3 presents the adjusted joint effects of dietary intake and tobacco smoking on the risk of developing CIN3. The observed OR for the joint effect of smoking and intake of dark-green and deep-yellow vegetables and fruits (adjusted OR 3·86; 95 % CI 1·74, 8·57) was slightly greater than the expected OR in the additive model (1·14+1·82 − 1·00 = 1·96), suggesting an interaction. A similar additive interaction was found between smoking status and total fruit and fruit juice intake. However, the expected joint OR for total citrus fruit and citrus fruit juices and total vegetables were fairly close to the observed OR, so interaction if any was weak. The additive modification effect observed in fruit intake and smoking was not observed for total vegetable and fruit intake. Compared with never-exposed women, joint exposure to lower consumption of dark-green and deep-yellow vegetables and fruits, total fruit and fruit juices yielded about a fourfold risk for CIN3 after adjusting for confounding variables and HPV status. A strong and significant dose–response relationship was observed, although the interaction term was not statistically significant under any of the models.
Table 3 Combined effect of daily dietary intakes and tobacco smoking status on cervical intraepithelial neoplasia grade 3 (CIN3) risk
(Odds ratios and 95 % confidence intervals)
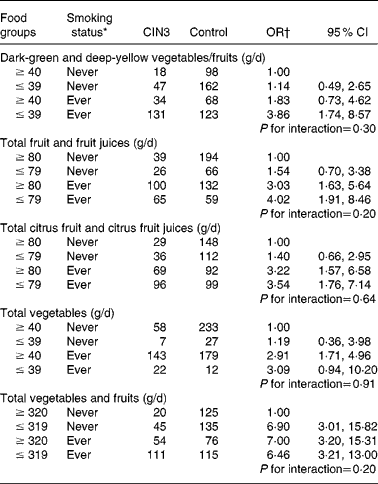
* Ever smoker: former smoker and current smoker.
† OR adjusted for age (21–30, 31–40, 41–50 and 51–65 years), hospital (Instituto Brasileiro de Controle do Câncer and Hospital Perola Byington), race/ethnicity (white and non-white), schooling ( ≥ 6 and ≤ 5 years), sexual debut ( ≥ 20, 17–19 and ≤ 16 years), lifetime sexual partners (1, 2 and ≥ 3 partners), parity (0, 1–3 and ≥ 4 pregnancies), human papillomavirus (HPV) status (negative for HPV, positive only for low-risk HPV, positive for at least one high-risk type except HPV-16/HPV-18 and positive for HPV-16 and/or HPV-18).
With regard to the joint effect of serum micronutrients and smoking habits (Table 4), although the interaction was not statistically significant, our data suggest that adequate concentrations of α-tocopherol carried a lower risk (adjusted OR 2·73; 95 % CI 1·26, 5·92) than in women with a deficiency (adjusted OR 5·86; 95 % CI 2·30, 14·96) among former and current smokers. A similar effect was observed for high concentrations of serum total carotene and γ-tocopherol.
Table 4 Combined effect of serum micronutrients and tobacco smoking status on cervical intraepithelial neoplasia grade 3 (CIN3) risk (Odds ratios and 95 % confidence intervals)
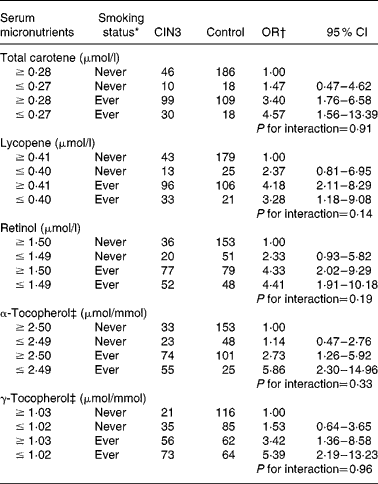
* Ever smoker: former smoker and current smoker.
† OR adjusted for age (21–30, 31–40, 41–50 and 51–65 years), hospital (Instituto Brasileiro de Controle do Câncer and Hospital Perola Byington), race/ethnicity (white and non-white), schooling ( ≥ 6 and ≤ 5 years), sexual debut ( ≥ 20, 17–19 and ≤ 16 years), lifetime sexual partners (1, 2 and ≥ 3 partners), parity (0, 1–3 and ≥ 4 pregnancies), human papillomavirus (HPV) status (negative for HPV, positive only for low-risk HPV, positive for at least one high-risk type except HPV-16/HPV-18 and positive for HPV-16 and/or HPV-18).
‡ Adjusted for serum total cholesterol.
An additional analysis of the joint effects of dietary intakes and smoking was conducted only among women positive for high-risk HPV infection. A lower intake of dark-green and deep-yellow vegetables and fruits without tobacco smoking had a small effect on CIN3 individuals (OR 1·16; 95 % CI 0·47, 2·85) than among smokers with higher intake (OR 2·14; 95 % CI 0·77, 5·91). Joint exposure of tobacco smoking and lower intake was OR 2·73 (95 % CI 1·16, 6·38), after adjusting for confounding variables, although the interaction term was not statistically significant. Infection with oncogenic HPV types is a prerequisite for cervical cancer, and our data suggest that a diet poor in fruits and vegetables, especially in dark-green and deep-yellow types, among smokers increases the risk for CIN3 about threefold compared with women who have a healthy diet and are not tobacco users. When we conducted an analysis restricted to women with oncogenic HPV infection for serum micronutrients, we observed an additive interaction effect of the low concentration of serum total carotene (OR 9·28; 95 % CI 1·83, 46·96; P for interaction = 0·64), retinol (OR 6·60; 95 % CI 2·31, 18·85; P for interaction = 0·99) and γ-tocopherol and smoking habits (OR 4·28; 95 % CI 1·55, 11·80; P for interaction = 0·31) on the risk of CIN3. Additional interaction was not observed for other serum micronutrients.
We conducted an analysis investigating the joint effect of dietary intake and tobacco smoking in three categories (non-smoker, former smoker and current smoker) on the risk of CIN3. Considering non-smokers with higher intake as the reference category, former smokers presented intermediary risk followed by smokers with low and high intake for all investigated food groups and blood nutrients (data not shown).
Discussion
To our knowledge, this is the first study that focused on the effect of smoking on CIN3 as modified by diet. It is worrying since cervical cancer is the most common in developing countries and data from the WHO World Health Survey, a large cross-sectional study conducted in seventy low and medium-income countries in 2002–3 (including Brazil), showed a high prevalence of low fruit and vegetable intake ( < 400 g/d) by 78 % (about 58 % in Brazil), with the highest prevalence of low fruit and vegetable intake (82 %) in the poorest income quintile(Reference Parkin, Bray and Ferlay1, Reference Hall, Spencer and Harper46).
Our findings suggest a synergistic interaction between tobacco smoking and dietary intake of fruits and vegetables, in particular dark-green and deep-yellow vegetables and fruits, total fruit and fruit juices. However, since we found no additive interaction for total vegetables and fruits, it is likely that the effect on CIN3 is associated with specific nutrients found particularly in dark-green and deep-yellow vegetables and fruits rich in β-carotene. These associations were confirmed by serum total carotene, considered a good biomarker for fruit and vegetable intake, and serum vitamin E, a potent antioxidant(Reference Tomita, Almeira and Roteli-Martins30, Reference Campbell, Gross and Martini47, 48).
The present results were consistent with the findings of previous studies: three cohorts and four case–control studies that have reported the synergistic effect of low intake of total fruit, total vegetables, total β-carotene, as well as a study on low plasma carotenoids and smoking habit for many cancers (oral, head and neck, oesophageal, gastric, ovarian and cervical, bladder and colorectal adenoma) that ranged from a 50 % greater risk to a thirteenfold risk(Reference Hansson, Baron and Nyren49–Reference Boccia, Cadoni and Sayed-Tabatabaei55). All these studies showed statistically non-significant results for the interaction term, except for the study investigating blood β-carotene(Reference Hung, Zhang and Rao51).
As suggested by Szklo & Nieto(Reference Szklo and Nieto43), interaction can be assessed by the comparison of an observed risk and their expected joint effects. Many discussions about concepts of interaction have been conducted in the previous decades based on statistical interaction, biological, public health and individual decision-making(Reference Rothman, Greenland and Walker42). For public health interest, the proportion of the population at risk according to the exposed variable and risk with both exposures is important to inform policy regardless of statistical significance for judging whether two variables are synergistic(Reference Rothman, Greenland and Walker42, Reference de Gonzalez and Cox56). Although precision of the estimated risk was affected by reduced sample size in some categories, the highest risk for CIN3 among smoking women who reported lower intake of fruits and vegetables is sufficient to suggest that CIN3 is modified by nutritional factors.
Many possible explanations for the biological plausibility of intake of fruits and vegetables, rich in β-carotene, associated with cancer have been reported: (1) antioxidant activity that mitigates the damaging effects of endogenous and exogenous ROS on cellular membranes, protein and nucleic acids; (2) conversion to vitamin A; (3) gap junction communication; (4) immunological function since carotenoids are important to maintain membrane receptors; (5) cell growth regulation; (6) modulation of gene expression; (7) regeneration of oxidised vitamin E important for the protection of lipids in membranes(Reference Chew and Park24, Reference Bendich and Olson57–Reference Ames and Wakimoto62). It is important to remember that smoking is one of the causes of ROS. In our previous analysis conducted in the total study population, lower intake of fruit and fruit juices and reduced total carotene concentrations independent of dietary intake were observed among smokers(Reference Tomita, Almeira and Roteli-Martins30). A significant linear trend between serum total carotene and quartiles of total fruit and fruit juices and quartiles of dark-green and deep-yellow vegetables and fruits was observed for non-smokers and smokers (P for trend < 0·001)(Reference Tomita, Almeira and Roteli-Martins30). A possible explanation would be the enhanced turnover rate in response to the oxidant load(Reference Stryker, Kaplan and Stein25–Reference Galan, Viteri and Bertrais28, Reference Ames and Wakimoto62).
Vitamin E or α-tocopherol, the major compound with highest biological activity, is considered to be the major lipid-soluble antioxidant present in cellular membranes(Reference McCullough and Giovannucci63). The anti-carcinogenic activity of α-tocopherol is to reduce ROS damage of DNA and avoid oxidation of immunological cellular membranes rich in fatty acids(Reference Calder and Grimble64). Changes in fatty acid composition of immune cell membranes showed an effect on fluidity of the membrane, distribution and function of cellular receptors, and changed the affinity of immune cell, thus reducing the immunological response(Reference Calder and Grimble64). In previous studies, serum α-tocopherol concentrations in smokers compared with non-smokers have been controversial(Reference Marangon, Herbeth and Artur65–Reference Hosomi, Arita and Sato68). Previous research on serum γ-tocopherol has showed slightly increased concentrations among smokers(Reference Dietrich, Block and Norkus26, Reference Sobczak, Golka and Szoltysek-Boldys66); however, further studies are necessary to identify differential metabolism of tocopherols and activity of other forms of vitamin E in the body.
Several limitations of the present study should be noted. First, the design of the study is prone to selection and recall bias, while residual influences of dietary intakes could have distorted the findings. However, the participation rate in the main study was very high, 97 % (thirty-four potential cases and nineteen potential controls refused), thereby minimising the selection bias. Recall bias is another weakness of case–control studies. Regarding this possible limitation, subject recruitment and interviews were conducted before diagnosis. The FFQ used reflects the usual intake over a previous year, and the biomarker for fruit and vegetable intake is sensitive for recent intakes. Although biochemical indicators can be considered an objective measure of exposure when compared with dietary intake reports, they are also prone to misclassification due to within-person variability and can result in random error that can attenuate estimates of association(Reference Block, Dietrich and Norkus69). A similar magnitude of the effect on CIN3 was also observed on fruit and vegetable intake and on the best biomarker for fruit consumption (total carotene), reflecting that long-time and recent exposures were associated with CIN3. Second, the findings of the present study are prone to residual confounding by sexual activity, strongly correlated with smoking habits. Although we used a sensitive method for HPV infection diagnosis, it is possible that some unmeasured factor associated with sexual behaviour could be present, thus distorting the results(Reference Franco and Spence70). However, we tried to minimise the effect of residual confounding of sexual lifestyle by conducting an HPV-specific analysis only among high-risk HPV-positive women. This analysis confirmed the findings with the entire sample.
The present results suggest that there is an additive interaction of low intake of fruits and vegetables, especially dark-green and deep-yellow vegetables and fruits, and smoking habits on CIN3 risk. These results were confirmed using biomarkers of fruit intake and also from restricted analyses conducted among women infected by oncogenic HPV type. A higher risk of low intake of these food groups among smokers v. non-smokers may reflect the relative deficit of β-carotene intake, and thus a higher benefit achieved among smokers increasing its intake. These findings emphasise the public health importance of higher intake of vegetables and fruits, especially dark-green and deep-yellow vegetables and fruits, and, more importantly, of quitting smoking. Further studies on the interaction between dietary intake and smoking on HPV clearance are needed.
Acknowledgements
The present work was supported by research funding from Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP/03/03013-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq/473043/03-3, 300167/97-0). L. Y. T. received PhD scholarships from FAPESP (02/11184-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/BEX3775/05-4). There are no conflicts of interest to declare. All authors made contributions to the study design and the interpretation of results. C. M. R.-M. and L. L. V. assisted in the development and implementation of the study protocols and contributed to the writing of the final manuscript. L. Y. T., E. L. F. and M. A. C. designed the overall study protocols, analysed the data and were responsible for the writing of the manuscript. All authors read and approved the final manuscript.






