Food insecurity is an important issue in the USA: 14·3 % of households experienced food insecurity in 2013( Reference Coleman-Jensen, Gregory and Singh 1 ). Food insecurity exists ‘whenever the availability of nutritionally adequate and safe foods or the ability to acquire acceptable food in socially acceptable ways is limited or uncertain’( 2 ). Food insecurity has been associated with poor overall health, poor mental health, obesity and chronic diseases( Reference Lee and Frongillo 3 – Reference Haering and Syed 5 ). Among those with diabetes, studies have shown an association between food insecurity and higher risk of poor glucose control( Reference Gucciardi, Vogt and DeMelo 6 – Reference Berkowitz, Meigs and DeWalt 11 ).
Food-insecure households may employ coping strategies to manage economic stress that in turn may cause poor control of type 2 diabetes. Coping strategies as defined by the WHO refer to ‘remedial actions undertaken by people whose survival and livelihood are compromised or threatened’( 12 ). These include consumption of low-cost, energy-dense sugars, fats and grains( Reference Drewnowski and Specter 13 , Reference Drewnowski and Darmon 14 ); decreased consumption of high-cost foods such as fruits and vegetables( Reference Kendall, Olson and Frongillo 15 , Reference Rehm, Monsivais and Drewnowski 16 ); overconsumption in times of adequacy alternating with meal reduction and skipping in times of inadequacy( Reference Tarasuk, McIntyre and Li 17 ); use of food assistance and emergency food services (which may be associated with poorer-quality diets)( Reference Dinour, Bergen and Yeh 18 – Reference Nguyen, Shuval and Njike 22 ); and prioritizing food purchasing over other competing demands, including medications and medical care. Food insecurity in patients with diabetes has previously been associated with foregoing medications( Reference Seligman, Davis and Schillinger 8 , Reference Billimek and Sorkin 23 ). However, few studies have examined the role of coping strategies other than foregoing medications specifically among individuals with diabetes and their association with glucose control.
Greater understanding of the relationship between food insecurity and type 2 diabetes can potentially allow physicians and policy makers to direct interventions towards modifiable determinants of disease control. In order to further explore the mechanisms by which food insecurity impacts diabetes control, we performed a cross-sectional study of likely low-income patients with diabetes to examine the use of coping strategies and to determine if those strategies are associated with food insecurity and poor glycaemic control. Because of the important role of diet in glucose control, we focused primarily on food-related coping strategies. We hypothesized that greater use of coping strategies would be associated with worse glycaemic control.
Methods
We used electronic health records to identify potential participants who were between 30 and 80 years old, had at least one diagnosis code for type 2 diabetes in the past year and had a laboratory result for glycated Hb (HbA1c) in the previous 7 d. Potential participants were seen at the University of Pennsylvania Health System in Philadelphia, PA, USA, an urban health system that includes primary care and specialty providers throughout the city. We selected patients likely to be low income by including only those insured by Medicaid or uninsured, and/or residing in a zip code where over 30 % of the population is below the federal poverty level( 24 ). In order to ensure that our sample included only patients with type 2 diabetes, we excluded from our analyses all patients who were diagnosed before age 20 years, assuming those individuals were likely to have type 1 diabetes.
We mailed potential participants a letter about the study and within one month called and invited them to participate. We made up to six call attempts. Those who agreed to participate were read a verbal consent form. We excluded participants who were non-English speaking, as our instrument was not translated into other languages. After survey completion, we mailed participants a $US 10 gift card in appreciation along with area resource information from the Greater Philadelphia Coalition Against Hunger. We collected data between June and December 2013. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Pennsylvania Institutional Review Board. Verbal consent was obtained from all participants and formally recorded.
Glycaemic control
Our primary outcome was glycaemic control, measured by HbA1c. This is the American Diabetes Association’s recommended metric by which to guide medical treatment and is strongly associated with important clinical outcomes( Reference Inzucchi, Bergenstal and Buse 25 ). We defined poor glycaemic control as HbA1c≥8·0 %, as this goal meets the American Diabetes Association’s guidelines for the majority of patients( 26 ).
Survey instrument
The survey contained seventy items (see online Supplementary Appendix A). We measured food insecurity using the US Department of Agriculture’s eighteen-item Adult Food Security Survey – Core Module (FSS), a validated and commonly used tool in the USA( Reference Bickel, Nord and Price 27 ). Each respondent is classified as ‘food secure’ (0–2 affirmative responses) or ‘food insecure’ (>2 affirmative responses). The FSS asks about food budget, food supply and food quality. The FSS contains skip patterns and only individuals with children under 18 years of age in the household are asked eight of the questions. Only those who respond affirmatively to initial questions about food insecurity are asked questions about more severe manifestations of food insecurity.
Other variables included coping strategies proposed in the literature as probable mechanisms for the relationship between food insecurity and glucose control, including: cost-related medication non-adherence; foregone medical care; use of emergency food programmes; receipt of food assistance in the form of the Supplemental Nutrition Assistance Program (SNAP; formerly known as Food Stamps); fruit, vegetable and added sugar intake; and food management practices.
We used a five-question measure of cost-related medication non-adherence developed by Pierre-Jacques et al. and adapted by Ngo-Metzger et al.( Reference Pierre-Jacques, Safran and Zhang 28 , Reference Ngo-Metzger, Sorkin and Billimek 29 ). We asked one question from the National Health Interview Survey to assess delayed or foregone medical care( 30 ).
To assess use of emergency food programmes and food assistance programmes we employed questions from the Food Security Supplement of the Current Population Survey, conducted by the US Census Bureau and the Bureau of Labor Statistics( 31 ).
To assess diet we focused on fruit, vegetable and added sugar intake, rather than total dietary intake, for several reasons. Greater fruit and vegetable intake has been associated with lower HbA1c and is an important component of diet recommendations for patients with diabetes( Reference Sargeant, Khaw and Bingham 32 , Reference Evert, Boucher and Cypress 33 ). Added sugars, on the other hand, contribute to overconsumption of energy, without providing nutritional benefit( Reference Johnson, Appel and Brands 34 ). However, fruits and vegetables cost more than many others foods, including those with added sugars( Reference Aggarwal, Monsivais and Drewnowski 35 ). Given the limited survey time, we chose the Dietary Screener in the California Health Interview Survey from 2009, a ten-item instrument that measures intake over the previous month of fruits, vegetables and added sugars( 36 ). We used scoring algorithms developed by the National Center for Health Statistics to convert responses to estimates of daily intake and calculate variance-adjusted aggregate estimates. We calculated two variance-adjusted aggregate estimates for fruits and vegetables excluding beans (one including fried potatoes and the other excluding fried potatoes), and used each in separate regression models. Validation results from the National Cancer Institute indicate that this screener underestimates fruit and vegetable intake by 0 to 2/3 cup equivalents/d, while misestimates (over and under) of added sugar intake range from 0·7 to 1·6 teaspoons/d. However, the National Cancer Institute concludes that because misestimates are small, screener data can still be used to compare intake between different groups( 36 ).
After review of the literature, we did not identify any standard survey items addressing food management practices; that is, behavioural modifications of eating and shopping patterns that individuals may utilize in the face of hardship. Given the possible effects on health of such behaviours, we developed ten questions based on findings from qualitative studies( Reference Kempson, Keenan and Sadani 37 – Reference Smith and Richards 41 ). The questions ask how often participants overeat in times of adequacy and to avoid hunger, purchase fresh foods in times when funds are adequate, and purchase processed foods in times of shortage. The questions also ask how often participants follow a food budget and plan meals. Finally, the questions ask how often participants eat with relatives, friends or neighbours in times of need, or eat food that is not fresh when necessary. We employed five categorical answer options from ‘never true’ to ‘always true’ (see online Supplementary Appendix B). We piloted these questions with 100 respondents from primary-care waiting rooms. Each question demonstrated adequate spread (no variable had more than 80 % of answers in one category). Internal consistency was indicated by a Cronbach’s α of 0·73. Validity was indicated by correlation between more frequent use of the food management practices examined and food insecurity. All ten questions were included in the main survey instrument. Using the results from the main survey, we performed an exploratory factor analysis (using principal factor analysis with varimax rotation) and maximum likelihood methods as a sensitivity analysis.
We included questions on age, race, ethnicity, marital status, number of children in the household, education, employment and income. We also asked patients their age at diagnosis of diabetes and whether they take insulin. Finally, we abstracted height and weight from the medical record.
Analyses
We performed comparisons of sociodemographic and clinical characteristics between those with well-controlled and poorly controlled diabetes, using χ 2 tests and t tests. We also evaluated the association of each coping strategy with glucose control and with food insecurity. We then examined the role of coping strategies as mediators of the relationship between food insecurity and glucose control. Next, we developed logistic regression models to examine the association of food insecurity with glucose control, after adjusting for sociodemographic factors, clinical covariates and coping strategies. We then examined potential interactions between food insecurity and coping strategies (by including a multiplicative term in the logistic regression model), hypothesizing that coping strategies may modify the relationship between food insecurity and glucose control. Finally, we performed a stratified analysis to further examine the association between coping strategies and glucose control within the food-insecure and food-secure groups. All analyses were performed using the statistical software package Stata version 12.
Results
The team attempted to contact 1247 patients. Thirty-four per cent completed the survey (n 427), 33 % refused (n 408) and 33 % were not successfully contacted (n 412). We excluded patients likely to have type 1 diabetes (diagnosed with diabetes before age 20 years; n 7) and those missing data on key variables (including food security, any coping strategy or insulin status; n 13). Our final analyses included 407 participants.
Participants were largely non-Hispanic Black (82·8 %), female (73·7 %) and low income (Table 1). The mean BMI was in the obese range (38·7 kg/m2). The majority of patients (59·2 %) had HbA1c≥8 %. Those we were unable to contact were similar with regard to race/ethnicity (84 % Black), BMI (mean 36·6 kg/m2) and glucose control (57·8 % had HbA1c≥8 %). A smaller proportion of the group we were unable to contact were female (63·3 %).
Table 1 Sample characteristics, overall and by food security status, among low-income patients with diabetes from an urban US medical centre, June–December 2013

FPL, federal poverty level;.VA, Veterans Affairs; HbA1c, glycated Hb
* For comparison between food-insecure and food-secure groups.
Of participants, 40·5 % were food insecure. In comparison to food-secure participants, those who were food insecure were younger (53·5 v. 59·0 years, P<0·001), more likely to be below 100 % of the federal poverty level (67·9 % v. 52·5 %, P<0·001), more likely to be disabled (70·3 % v. 49·6 %, P<0·001), more likely to be on Medicaid (50·9 % v. 30·3 %, P=0·001), more likely to be on insulin (50·9 % v. 40·5 %, P=0·04) and had a higher BMI (40·3 v. 37·7 kg/m2, P=0·004; Table 1). Those in the food-insecure group were more likely to have poorly controlled glucose (68·5 % v. 52·9 %, P=0·002). The mean and interquartile range (IQR) for HbA1c in the food-insecure group was 8·5 % (IQR 7·3–9·6 %) v. 8·2 % (IQR 6·9–9·4 %) in the food-secure group.
Food management practices items showed adequate spread (no variable had more than 80 % of answers in one category; see online Supplementary Appendix B). Principal factor analysis showed a one-factor solution with an eigenvalue >1 (1·79), indicating one underlying construct accounting for 0·87 of overall variance. Only seven of the ten items demonstrated adequate factor loading (≥0·4; unrotated and rotated). These seven items had a Cronbach’s α of 0·68. The other three items (meal planning, budgeting and buying food in bulk) were thus excluded from all analyses and we combined the remaining items into a Food Management Practices Scale, with a range from 7 to 35, with higher scores indicating more frequent use of these strategies. Confirmatory factor analysis supported our findings: all scale items had adequate loading onto one factor (≥0·4).
Many of the coping strategies were employed significantly more frequently among those who were food insecure (Table 2), including cost-related medication non-adherence (60·6 % of food insecure v. 37·5 % of food secure, P<0·001), foregone medical care (35·2 % v. 9·1 %, P<0·001), use of emergency food programmes (53·3 % v. 24·0 %, P<0·001), receipt of SNAP (76·4 % v. 59·1 %, P<0·001) and frequency of food management practices (mean score 18·8 v. 13·1, P<0·001). Mean daily intakes of fruits, vegetables and added sugar were not significantly different between the two groups.
Table 2 Coping strategies and food insecurity among low-income patients with diabetes from an urban US medical centre, June–December 2013

IQR, interquartile range; SNAP, Supplemental Nutrition Assistance Program.
* For comparison between food-insecure and food-secure groups.
Table 3 shows unadjusted comparisons between those with well-controlled glucose and with poorly controlled glucose, and odds ratios from the fully adjusted logistic regression for each coping strategy, along with the sociodemographic and clinical factors included in the model. In unadjusted analysis, those with poorly controlled glucose were more likely to be food insecure (46·9 % of poorly controlled v. 31·3 % of controlled group, P=0·002), younger in age (55·2 v. 58·9 years, P=0·001), disabled (62·7 % v. 51·2 %, P=0·04), insulin users (61·8 % v. 19·9 %, P=<0·001), and to eat fewer fruits (0·5 v. 0·8 cup equivalents/d, P=0·005) and vegetables (0·4 v. 0·5 cup equivalents/d, P=0·01). Aside from fruit and vegetable intake, none of the coping strategies differed significantly by glucose control. As none of the coping strategies were associated with both food insecurity and glucose control, they did not meet the definition of mediator and we did not proceed through further steps in a mediation analysis( Reference Baron and Kenny 42 ).
Table 3 Associations between food insecurity, coping strategies and glucose control among low-income patients with diabetes from an urban US medical centre, June–December 2013

IQR, interquartile range; FPL, federal poverty level; SNAP, Supplemental Nutrition Assistance Program; Ref., referent group;
* For unadjusted comparison of well-controlled and poorly controlled groups.
† Odds ratio for poor glucose control, from fully adjusted logistic regression analysis, all covariates shown.
‡ From fully adjusted logistic regression analysis.
§ Using variance-adjusted aggregate estimates; fruit and vegetable intake combined (excluding beans and fried potatoes).
In the adjusted model, those who were food insecure were more likely to have poorly controlled glucose (OR=2·23; 95 % CI 1·22, 4·10, P=0·01). Use of insulin was also significantly associated with poor glucose control (OR=6·44; 95 % CI 3·95, 10·50, P<0·001), while a greater intake of fruits and vegetables was associated with lower risk of poor glucose control (OR=0·51; 95 % CI 0·32, 0·82, P=0·005). Models using a variance-adjusted aggregate estimate for fruits and vegetables excluding beans and excluding (shown in Table 3) or including (not shown) fried potatoes yielded similar results.
We found a statistically significant interaction between SNAP and food insecurity, indicating the association between food insecurity and glucose control is modified by receipt of SNAP (Table 4). Compared with food-insecure participants not receiving SNAP, food-insecure participants receiving SNAP had lower risk of poor glucose control (OR=0·35; 95 % CI 0·13, 0·91, P=0·03). Compared with those who were food secure and receiving SNAP, food-insecure participants receiving SNAP had a greater risk of poor glucose control (OR=1·68; 95 % CI 0·87, 3·25, P=0·12); however, this difference was not statistically significant at P<0·05. In the adjusted stratified model (Table 5), among those who were food insecure, those who received SNAP were at lower risk of poor glucose control than those not receiving SNAP (OR=0·27; 95 % CI 0·09, 0·80, P=0·02).
Table 4 Interaction between food insecurity and SNAP receipt on the risk of poor glucose control among low-income patients with diabetes from an urban US medical centre, June–December 2013
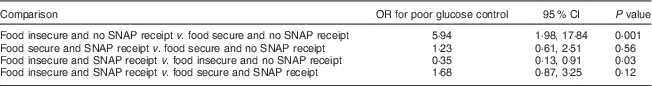
SNAP, Supplemental Nutrition Assistance Program.
The interaction term food insecurity×SNAP receipt in our multivariable logistic model had OR=0·28 (95 % CI 0·09, 0·89); P=0·03.
Results are adjusted for age, income, employment status, use of insulin, BMI and other coping strategies.
Table 5 Stratified analysis: coping strategies and poor glucose control by food security status among low-income patients with diabetes from an urban US medical centre, June–December 2013
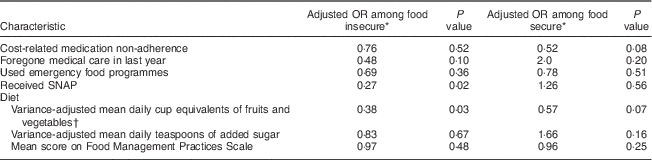
SNAP, Supplemental Nutrition Assistance Program.
* Odds ratio for poor glucose control from stratified, adjusted logistic regression analysis, including the following covariates: age, income, employment status, use of insulin and BMI.
† Excluding beans and fried potatoes.
Discussion
Among this population of largely low-income patients with diabetes, food insecurity was associated with greater risk of poor glucose control, after adjusting for sociodemographic and clinical characteristics. The use of coping strategies was more common among food-insecure participants, apart from intakes of fruits, vegetables and added sugars, which were similar between the two groups. However, intake of fruits and vegetables was the only coping strategy associated with glucose control in the full sample.
SNAP receipt was associated with lower risk of poor glucose control among those who were food insecure. It is possible that individuals who are food insecure but receiving SNAP have more funds to spend on food and therefore may have: (i) less stress; (ii) more ability to purchase healthier foods shown in previous studies to be more expensive (although not assessed in our study)( Reference Aggarwal, Monsivais and Drewnowski 35 ); or (iii) more ability to spend funds on non-food items with the potential to improve diabetes control. Alternatively, our results may be attributable to non-random entry into SNAP. SNAP receipt may be a marker for characteristics associated with better disease control, such as skills in navigating complex public systems. This association was noted only among food-insecure participants. This suggests that SNAP may play a different role in individuals who remain food insecure even while receiving SNAP, likely a more vulnerable population than those who are food secure after receiving SNAP. Many individuals who receive SNAP remain food insecure (54 % in 2013, according to the US Department of Agriculture)( Reference Coleman-Jensen, Gregory and Singh 1 ).
The present study is the first one we are aware of to look at the role of food assistance receipt in glucose control among elderly and non-elderly adults. Literature examining food assistance receipt and health has focused on obesity and child health outcomes( Reference Currie 43 , Reference Ver Ploeg and Ralston 44 ). SNAP recipients can spend their benefits on most food items (excluding hot food and alcohol). Research evaluating what SNAP recipients purchase v. eligible non-participating counterparts (none of which has focused on individuals with diabetes) has yielded mixed results, from worse diet quality to no difference( Reference Leung, Ding and Catalano 21 , Reference Nguyen, Shuval and Njike 22 , Reference Todd and Ploeg 45 ). In the only study we are aware of focusing on food assistance receipt and diabetes, Nicholas examined glucose control in older Americans and found no difference between the risk of poorly controlled glucose in those receiving Food Stamps and likely eligible non-recipients( Reference Nicholas 46 ). One explanation for our contrasting finding is our inclusion of non-elderly adults, who face different financial pressures from the elderly. Our study suggests that further research should explore how low-income patients with diabetes who are receiving SNAP differ from those who are not receiving SNAP.
The coping strategies we examined did not account for the difference in glycaemic control observed between food-secure and food-insecure patients (i.e. there are remaining differences even taking into account coping strategies). One interpretation of these findings is that these coping strategies may be adaptive, rather than harmful – that these strategies lead to better control for patients. Second, other unmeasured factors may account for the relationship between food insecurity and glucose control, such as emotional distress related to food insecurity, which in a previous study partially mediated the relationship between food insecurity and control( Reference Seligman, Jacobs and Lopez 9 ). Third, our cross-sectional study does not let us examine the longitudinal effects of the measured coping strategies. For example, a patient classified in our study as food secure and skipping medications because of cost may have been food insecure before deciding to use medication funds for food. If as a consequence of skipping medications their glucose control became worse, it would appear as though this coping mechanism was detrimental to those who were food secure not insecure.
While we found that greater intake of fruits and vegetables was associated with better glucose control, we found no difference between the food-secure and food-insecure groups. One interpretation of this finding is that fruit and vegetable intake was similar between the two groups and other unmeasured factors associated with food insecurity account for differences in glucose control. It is also possible that we did not detect differences due to our measurement tool, a screener limited to assessment of fruit, vegetable and added sugar intake, rather than a measure of full dietary intake. Screeners are subject to systematic error. They can be used to compare different populations as to higher or lower intake, or to examine associations between intake of certain dietary components and other variables, but they cannot be reliably used to characterize individual intake( 47 ). Our findings contrast with a recent study of Puerto Rican adults with diabetes in Boston that found lower overall diet quality and lower fruit and vegetable intake among food-insecure participants( Reference Berkowitz, Gao and Tucker 48 ). Our different findings may relate to differences in dietary patterns between the study populations or to differences in measurement. Further research can more fully characterize the diets of low-income patients with diabetes in additional populations and further examine the relative roles of dietary patterns and other factors important to glucose control.
The present study has several additional limitations. Given our cross-sectional study design, we cannot draw conclusions regarding longitudinal phenomena, including the causal role of food insecurity in glucose control. We examined patients attending a single medical centre in one US city and thus our findings may not be nationally generalizable. In addition, the patients in our study had all recently received medical care and thus may represent a population with relatively greater access to medical care. These patients may experience different social contexts and utilize different coping strategies from those without regular access, also limiting the generalizability of our findings. Another limitation of the study is our response rate (34 % of the sample, 51 % of those contacted). Those who refused to participate and those with whom we were unable to make contact may differ from those who completed the survey. This also limits our generalizability, as the sample represents a limited subset of all low-income patients with diabetes who are seen in this health system.
Our findings have several important implications. Multiple studies have shown a relationship between food insecurity and glucose control; physicians and health systems should consider screening and addressing food insecurity in patients with diabetes. The coping strategies we studied, apart from SNAP receipt, did not act as modifiers of the relationship between food insecurity and diabetes control, and further research is warranted to elucidate what factors do mediate the relationship, such as diabetes distress or self-efficacy, other known predictors of diabetes control which may be particularly salient in low-income populations( Reference Aikens 49 – Reference Gao, Wang and Zheng 51 ). The association of SNAP receipt with lower risk of poor glucose control in food-insecure individuals suggests that programmes aimed at ameliorating food insecurity are avenues for further research and intervention. Understanding how food assistance affects diabetes self-care, including food purchasing and diet, could help elucidate this relationship. Physicians and health systems can pilot programmes to increase food assistance coverage among patients with diabetes. Finally, our findings indicate that recent cuts to SNAP benefits( Reference Rampel 52 , Reference Shear 53 ) may have unintended consequences, such as worse chronic disease control among low-income patients with diabetes.
Acknowledgements
Acknowledgements: The authors would like to acknowledge Esther Kim and Elizabeth Wall for their important work in data collection for this study. They thank Steven Honeywell and Marie Synnestvedt for their work developing and executing electronic tools for patient selection. Also, they thank Steve Schachterle for additional statistical consultation. Financial support: V.L.M. was supported with funding from the Division of General Internal Medicine Matt Slap Award from the Perelman School of Medicine at the University of Pennsylvania and the National Institutes of Health Institutional Training Grant 5-T32-HP-100296-20-00. She is currently supported by the Empire Clinical Research Investigator Program (Principal Investigator Dr Carol Horowitz). The funders had no role in the design, analysis, or writing of this article. Conflict of interest: None. Authorship: V.L.M conceived of and designed the study, researched data and wrote the manuscript. K.M. researched data and reviewed/edited the manuscript. H.S. contributed to study design and reviewed/edited the manuscript. N.M. contributed to researching data and reviewed/edited the manuscript. J.A.L. contributed to study design, researching data and reviewed/edited the manuscript. Ethics of human subject participation: The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Pennsylvania Institutional Review Board. Verbal consent was obtained from all participants and formally recorded.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015002323








