Introduction
Cacti are important elements of the vegetation structure in arid and semiarid ecosystems in the Americas and are used for traditional and economic purposes (Agra, Reference Agra1996; Oldfield, Reference Oldfield1997; Anderson, Reference Anderson2001; Hernández et al., Reference Hernández, Gómez-Hinostrosa and Bárcenas2001; Boyle & Anderson, Reference Boyle, Anderson and Nobel2002; Ortega-Baes & Godínez-Alvarez, Reference Ortega-Baes and Godínez-Alvarez2006; Andrade, Reference Andrade2008; Lucena et al., Reference Lucena, Lucena, Costa, Carvalho, Costa, Alves, Pereira, Ribeiro, Alves, Quirino and Nunes2013; Alanís-Rodríguez et al., Reference Alanís-Rodríguez, Mora-Olivo, Jimenez-Pérez, Gonzalez-Tagle, Yamallel, Martínez-Avalos and Gonzalez-Rodriguez2015). The role of environmental factors as determinants of the distribution patterns of cactus species has been evaluated previously (Mourelle & Ezcurra, Reference Mourelle and Ezcurra1996, Reference Mourelle and Ezcurra1997; Godínez-Alvarez & Ortega-Baes, Reference Godínez-Alvarez and Ortega-Baes2007; Guerrero et al., Reference Guerrero, Duran and Walter2011; Gurvich et al., Reference Gurvich, Zeballos and Demaio2014). Differences that are related to altitude, topography and climatic factors, such as temperature and precipitation, are the determinants of species distributions at regional scale (Mourelle & Ezcurra, Reference Mourelle and Ezcurra1997; Pavón et al., Reference Pavón, Hernández-Trejo and Rico-Gray2000; Guerrero et al., Reference Guerrero, Duran and Walter2011; Gurvich et al., Reference Gurvich, Zeballos and Demaio2014). The chemical and physical properties of soils are also primary predictors of the distribution of cactus species, primarily at local scale (Hernández et al., Reference Hernández, Gómez-Hinostrosa and Bárcenas2001, Reference Hernández, Goettsch, Gómez-Hinostrosa and Arita2008; Alanís-Rodríguez et al., Reference Alanís-Rodríguez, Mora-Olivo, Jimenez-Pérez, Gonzalez-Tagle, Yamallel, Martínez-Avalos and Gonzalez-Rodriguez2015). In addition to these determinants, patterns of cactus distribution are linked to reproductive outputs and dispersal strategies (Taylor & Zappi, Reference Taylor and Zappi2004).
Four centres of diversity are identified for the Cactaceae (Taylor & Zappi, Reference Taylor and Zappi2004). The first centre is in central Mexico and south-western USA. The second centre contains approximately 18% of the genera and is in the Andes, including parts of Peru, Bolivia, southern Ecuador and north-eastern Chile. The third centre of diversity is located in Campos Rupestres in south-eastern Brazil and in north-eastern Brazil in the Caatinga phytogeographic domain, the largest and most isolated of the 21 core areas of seasonal dry forests in the Neotropical region (Queiroz, Reference Queiroz, Pennington, Lewis and Ratter2006; Linares-Palomino et al., Reference Linares-Palomino, Kvist, Aguirre-Mendoza and Gonzales-Inca2010). These semiarid caatingas are hotspots of Cactaceae, with high levels of species endemism (Taylor & Zappi, Reference Taylor and Zappi2004) in the driest climates in Brazil, characterised by 5–6 months of precipitation of < 100 mm and by a large proportion of deciduous species (Pennington et al., Reference Pennington, Prado and Pendry2000).
Despite these data, the determinants of distribution of Cactaceae species have not been adequately addressed (Dutra Saraiva & Souza, Reference Dutra Saraiva and Souza2012), particularly at the local scale. A central question to define conservation strategies is how distribution patterns of species vary as a function of geographical space and the environment (Mourelle & Ezcurra, Reference Mourelle and Ezcurra1997). Because of the vulnerability of the Caatinga biome to the unceasing impoverishment of the vegetation caused by human disturbance, such as agriculture and extractive activities, this question is a major concern (Leal et al., Reference Leal, Silva, Tabarelli and Lacher2005; Ribeiro et al., Reference Ribeiro, Arroyo-Rodriguez, Santos, Tabarello and Leal2015). In the Caatinga biome, 4956 km2 are included in protected areas, but the integral protected areas, such as national or state parks, ecological stations and biological reserves, cover < 1% of the entire region (Leal et al., Reference Leal, Silva, Tabarelli and Lacher2005).
Ribeiro-Silva et al. (Reference Ribeiro-Silva, Zappi, Taylor and Machado2011) proposed a number of studies and actions primarily related to ecological and botanical field assessments to determine more accurately the conservation status and to reduce the extinction risk of the Cactaceae species in Brazil. This article describes the distribution patterns of the Cactaceae community in a protected area in the Brazilian semiarid north-east in the Contendas do Sincorá National Forest (CSNF). This protected area is located in a hotspot region in north-eastern Brazil that was selected as a conservation priority area for Cactaceae (Taylor & Zappi, Reference Taylor and Zappi2004). In particular, our aim in doing this study was to answer the following questions. What are the determinants of species distribution patterns of Cactaceae at a local scale within a protected area in a hotspot of cactus diversity? What is the floristic composition and structure of the Cactaceae community in this region? Are the environments that encompass the floristic variation of the Cactaceae within the protected area?
Materials and Methods
Study area
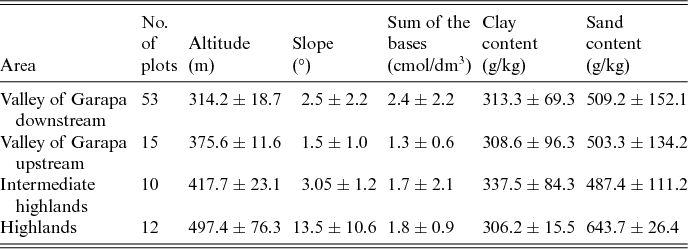
The study was conducted in the CSNF, in a semiarid region in north-eastern Brazil (Fig. 1). The CSNF was established in 1999 as a conservation unit for sustainable use (SNUC, 2002) in the Caatinga biome in the state of Bahia (13°57′30′′ S, 41°5′0′′ W). This protected area covers 11,034 ha in the valley of the Garapa stream. The elevation varies between 220 and 550 m a.s.l. A small part of the CSNF (270 ha) is located in the Grotas highlands, with altitudes up to 750 m (Fig. 1). The climate is semiarid, with a mean annual temperature of 23°C.

Fig. 1. Plots within, and in the surrounding areas of, the Contendas do Sincorá National Forest, Bahia, Brazil.
The annual precipitation ranges from 500 to 700 mm (Ibama, 2006), and the rainy period is from November to January. The primary soil types are lithosols, podzols and latosols (Embrapa, 2015). The vegetation is composed of the physiognomies of the Caatinga, including the forested steppe savannah and the woody steppe savannah (Veloso et al., Reference Veloso, Rangel-Filho and Lima1992).
Before the establishment of the protected area, the CSNF area was characterised by a history of anthropogenic disturbances that were primarily related to the deforestation of woody and shrubby caatingas for timber and charcoal production (Ibama, 2006).
Data collection
The biodiversity monitoring programme of the CSNF was established by the Brazilian government environmental agency the Chico Mendes Institute for Biodiversity Conservation. This monitoring programme is composed of permanent trails and transects that are located in sites that range from 290 to 457 m. We used these trails to locate additional plots to those that were previously established to reach altitudes above 500 m within the CSNF and in the surrounding areas. The locations of the additional trails were dependent on accessibility, and were based on an altitude map that was produced using a digital terrain model with 90 m of resolution in SIG ArcGis 9.2 (Esri, 2004). These additional trails were located between the altitudes of 290 and 596 m. Each trail was a minimum of 2 km long (Fig. 1), and along these trails, 91 plots were established, with a minimum distance of 100 m between plots. In each 200 m2 (20 × 10 m2) plot, all individuals of the family Cactaceae were recorded and identified in the dry and rainy seasons from 2012 to 2014. Most of the plots were within the CSNF, at altitudes ranging from 290 to 450 m. The higher elevation areas up to 596 m were sampled within, and in the surrounding areas of, the Grotas highlands (Table 1 and Fig. 1).
Table 1. Mean values and standard deviations of environmental factors in the Contendas do Sincorá National Forest, Bahia, Brazil

To provide additional information on the floristic composition within, and in the surrounding areas of, the CSNF, the floristic data were also recorded on the identical trails but not within the plots, in accordance with the ‘wide patrolling’ method (Filgueiras et al., Reference Filgueiras, Nogueira, Brochado and Guala II1994). In this method, the linear trails were walked to record and collect new species until no further new species were recorded. The walking of linear trails ideally covered the different types of vegetation and also required estimates of species abundance (Filgueiras et al., Reference Filgueiras, Nogueira, Brochado and Guala II1994). The identifications were performed in the field by taxonomists, with the aid of specialised literature (Taylor & Zappi, Reference Taylor and Zappi2004). Voucher specimens were deposited in the herbarium of Embrapa Genetic Resources and Biotechnology, in accordance with the standard methodology (Mori et al., Reference Mori, Silva, Lisboa and Coradin1985). The scientific names followed the criteria of the Brazilian Flora Checklist (Zappi et al., Reference Zappi, Taylor, Machado and Santos2015; Rio de Janeiro Botanical Garden, no date).
Three environmental factors were selected as important predictors of plant distribution patterns of the Cactaceae in arid and semiarid ecosystems at local geographical scales: soil physical–chemical parameters, altitude and slope (Mourelle & Ezcurra, Reference Mourelle and Ezcurra1996; Hernández et al., Reference Hernández, Gómez-Hinostrosa and Bárcenas2001; Guerrero et al., Reference Guerrero, Duran and Walter2011; Gurvich et al., Reference Gurvich, Zeballos and Demaio2014; Alanís-Rodríguez et al., Reference Alanís-Rodríguez, Mora-Olivo, Jimenez-Pérez, Gonzalez-Tagle, Yamallel, Martínez-Avalos and Gonzalez-Rodriguez2015). The soil variables included sand, clay and silt, pH (H2O), the sum of the bases (Ca2+, Mg2+, K+ and Na+), the concentrations of Al3+, N and P+, and the carbon:nitrogen ratio. The soil samples were collected at two points within each plot at 0–20 cm depth. The soil samples were dried at room temperature and aggregated in one sample for physical and chemical analyses.
The slope of the terrain in each plot was measured using a Suunto PM-5/360 clinometer (Suunto, Vantaa, Finland) through measures perpendicular to the central axis. The coordinates and altitude were recorded using the GPS model GPSMap 60csx (Garmin, Olathe, Kansas, USA).
Data analyses
Principal component analysis (PCA) was used to analyse the 11 environmental variables: nine physical–chemical parameters of soil, and altitude and slope. The data were previously standardised using the standard deviation of each variable.
The floristic composition was analysed using non-metric multidimensional scaling (NMS) and two types of ordination: the Jaccard index (the presence or absence of species) and the Bray–Curtis index (quantitative).
The PCA and NMS analyses were performed with PC Ord version 5.15 software (McCune & Mefford, Reference McCune and Mefford2006).
We performed a variance partitioning procedure to assess the contribution of each of the following fractions to floristic variance: a, environment alone; b, shared contribution between environment and space; and c, space alone. The routine followed the implementation of Eisenlohr (Reference Eisenlohr2014), with Moran's eigenvector maps (Dray et al., Reference Dray, Legendre and Peres-Neto2006) as spatial variables, using the ‘spacemakeR’ package of R environment (R Development Core Team, 2012). Both environmental and spatial variables were forward selected (Blanchet et al., Reference Blanchet, Legendre and Borcard2008) using the ‘packfor’ package in R.
Results
Composition and structure of Cactaceae
The floristic survey using the wide patrolling method recorded 18 taxa within the CSNF and in the surrounding areas: Arrojadoa penicillata (Gürke) Britton & Rose, Arrojadoa rhodantha (Gürke) Britton & Rose, Brasilicereus phaeacanthus (Gürke) Backeb., Brasiliopuntia brasiliensis (Willd.) A.Berger, Cereus jamacaru DC., Pereskia aureiflora F.Ritter, Pereskia bahiensis Gürke, Stephanocereus leucostele (Gürke) A.Berger, Tacinga funalis Britton & Rose, Tacinga palmadora (Britton & Rose) N.P.Taylor & Stuppy, Tacinga inamoena (K.Schum.) N.P.Taylor & Stuppy, Pilosocereus catingicola (Gürke) Byles & G.D.Rowley, Pilosocereus pentaedrophorus (Labour.) Byles & G.D.Rowley subsp. robustus Zappi, Pilosocereus gounellei (F.A.C.Weber) Byles & G.D.Rowley, Melocactus concinnus Buining & Brederoo, Melocactus inconcinnus Buining & Brederoo, Espostoopsis dybowskii (Rol.-Goss.) Buxb. and Hylocereus setaceus (Salm-Dyck) R.Bauer. These taxa were used in an identification guide for the Cactaceae surveyed in this region of the Caatinga (Peixoto et al., Reference Peixoto, Lima, Ribeiro-Silva, Medeiros, Zappi and Aona2015).
The quantitative surveys within the plots sampled 1135 individuals comprising 10 species (eight genera), and Cactaceae were recorded in 76 of the 91 plots. The most abundant species was Tacinga palmadora, with 534 individuals, or 47% of the total individuals in the Cactaceae community (Table 2). The other abundant species were Tacinga funalis (201 individuals) and Brasilicereus phaeacanthus (189 individuals), which combined with Tacinga palmadora accounted for 924 individuals, or 81.4% of the total number sampled. The least abundant species was Pereskia aureiflora, with only one individual recorded.
Table 2. Number of individuals of Cactaceae recorded in 76 plots in the Contendas do Sincorá National Forest, Bahia, Brazil

Most of the species that were recorded within these plots were endemic to the Caatinga biome (i.e. Arrojadoa penicillata, Brasilicereus phaeacanthus, Pereskia aureiflora, Pereskia bahiensis, Stephanocereus leucostele, Tacinga funalis and Tacinga palmadora), in addition to two endemic species from the restricted distribution areas in the caatingas from Bahia (Pereskia bahiensis and Stephanocereus leucostele) (Taylor & Zappi, Reference Taylor and Zappi2004; Rio de Janeiro Botanical Garden, no date). The abundance varied from 1 to 34 individuals per plot.
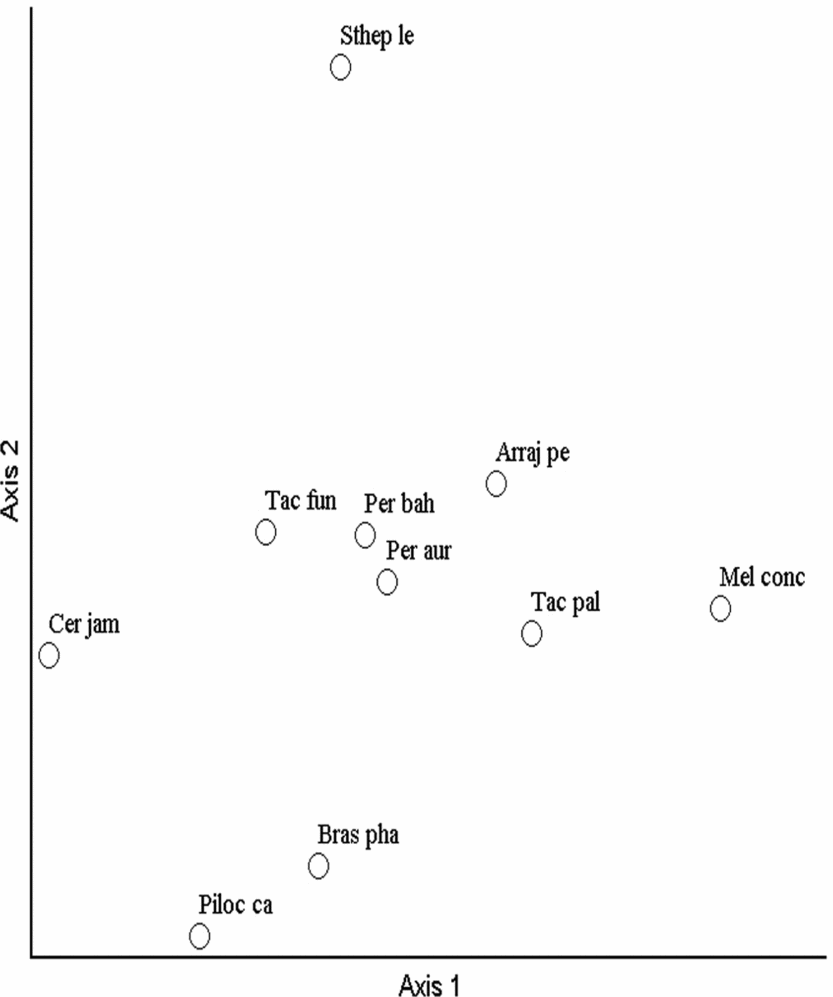
The ordination axes of the NMS for the Bray–Curtis index indicated distinct groups in the Cactaceae community (Fig. 2). In general, the pattern of species distribution was different between the Grotas highlands in the surrounding areas of the CSNF and the Cactaceae community at low altitudes in the valley. In both types of ordination, the distribution pattern of the Cactaceae community was more restricted in the areas of low altitude in the Garapa valley in which most of the species and individuals were recorded, primarily Tacinga palmadora, Tacinga funalis, Pereskia bahiensis and Arrojadoa penicillata. Some species, such as Stephanocereus leucostele, Cereus jamacaru and Pereskia aureiflora, were restricted to specific sites with fewer individuals.

Fig. 2. Non-metric multidimensional scaling ordination analysis of 76 plots based on the floristic composition and structure of the Cactaceae with the Bray–Curtis index in the Contendas do Sincorá National Forest in Bahia, Brazil. Arraj pe, Arrojadoa penicillata; Bras pha, Brasilicereus phaeacanthus; Cer jam, Cereus jamacaru; Mel conc, Melocactus concinnus; Per aur, Pereskia aureiflora; Per bah, Pereskia bahiensis; Piloc ca, Pilosocereus catingicola; Sthep le, Stephanocereus leucostele; Tac fun, Tacinga funalis; Tac pal, Tacinga palmadora.
Soils and slope
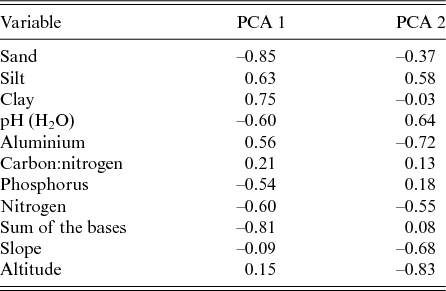
The first two axes of the PCA explained 33.8% and 26.5% of the total variance related to the nine physical–chemical soil variables and the slope. The axis PCA 1 described a gradient that was positively correlated with the clay and aluminium contents and that was negatively correlated with the sum of the bases (Mg2+, Ca2+, Na+ and K+), the sand content and the pH. The axis PCA 2 described a gradient that was positively correlated with the silt and the pH and that was negatively correlated with the slope, the altitude and the aluminium (Al3+; Table 3). Therefore the soils at lower altitudes in the Garapa valley within the CSNF had higher fertility than those in areas at higher altitudes.
Table 3. Correlation values of the nine physical–chemical soil variables, slope and altitude with the two first axes produced by principal component analysis (PCA) of the Contendas do Sincorá National Forest, Bahia, Brazil
Variance partitioning
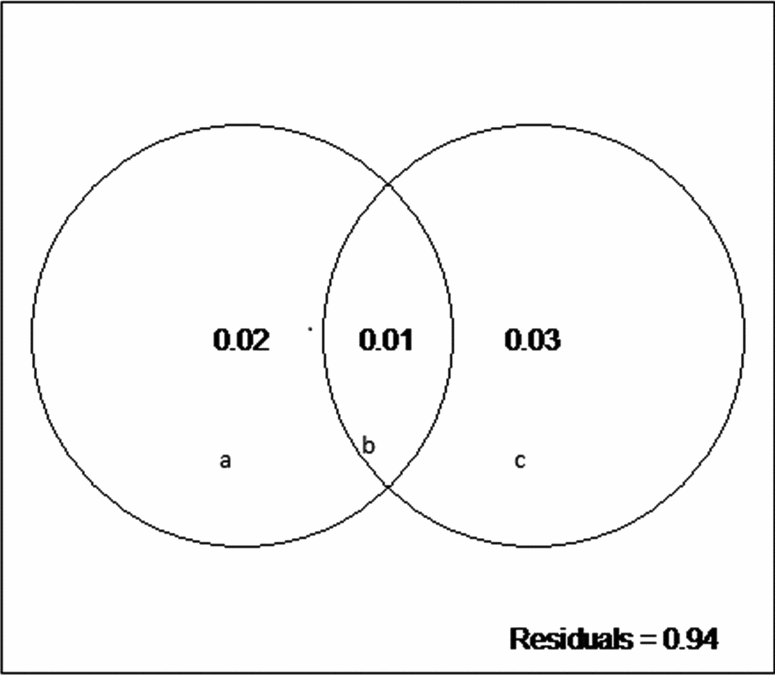
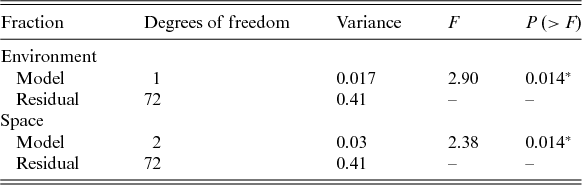
We did not detect a primary role of the spatially structured environmental factors on the floristic patterns. The model resulted in low explanation values (fractions a, b and c), and 94% of the species distributions remained undetermined (residuals) (Fig. 3). However, the individual fractions were significant (Table 4).

Fig. 3. Partitioning variance: a, the fraction explained solely by environmental variables; b, the fraction explained by spatial and environmental components; and c, the fraction explained solely by space.
Table 4. Performance of the selected models used to explain the floristic variation
*P < 0.05.
Discussion
The floristic survey that identified 18 species provided essential information additional to the quantitative data from the plots in which only 10 species were recorded. These differences were associated with the intermittent distribution patterns of the Cactaceae species (Taylor & Zappi, Reference Taylor and Zappi2004; Hernández et al., Reference Hernández, Goettsch, Gómez-Hinostrosa and Arita2008) and the rare individuals of eight species (Arrojadoa rhodantha, Brasiliopuntia brasiliensis, Tacinga inamoena, Pilosocereus pentaedrophorus subsp. robustus, Pilocereus gounellei, Melocactus inconcinnus, Espostoopsis dybowskii and Hylocereus setaceus) that were restricted to specific sites within, and in the surrounding areas of, the CSNF.
The high levels of endemic species were expected because north-eastern Brazil is a priority area for conservation efforts for endemism and species richness of Cactaceae (Taylor & Zappi, Reference Taylor and Zappi2004). The occurrence of two endemic species that were restricted to the caatingas of Bahia, namely Pereskia bahiensis and Stephanocereus leucostele (Taylor & Zappi, Reference Taylor and Zappi2004), emphasised the importance of the CSNF as a protected area for the conservation of Cactaceae species in the Caatinga biome.
A few species of Cactaceae are associated with anthropogenic disturbances, and this plant family might be an effective indicator group to establish protected areas (Taylor & Zappi, Reference Taylor and Zappi2004). Of the species sampled in the CSNF, only Cereus jamacaru, with a few individuals, was associated with human-disturbed environments.
The species that was the most abundant in this study, Tacinga palmadora, has a wide distribution pattern in the north-eastern Brazilian caatingas, ranging from the state of Rio Grande do Norte to southern Bahia, in addition to being endemic in this region (Taylor & Zappi, Reference Taylor and Zappi2004). This species, in association with the other endemic species that were typical of the caatingas, such as Tacinga funalis, Arrojadoa penicillata, Brasilicereus phaeacanthus, Pereskia aureiflora, Pilosocereus catingicola and Stephanocereus leucostele, occur in highly fragmented areas in the north-eastern Brazilian caatingas; therefore, to avoid inclusion in the category ‘Endangered’ in the International Union for Conservation of Nature Red List (IUCN, 2015), monitoring and protection are required (Taylor & Zappi, Reference Taylor and Zappi2004). Furthermore, some of the species deserve special attention because of anthropogenic threats; examples are Pereskia aureiflora, with only one individual recorded in the CSNF and classified as an ‘Endangered’ species; Espostoopsis dybowskii, a vulnerable species recorded only in the floristic survey in the sandstones in the northern Grotas highlands; and Pilosocereus pentaedrophorus subsp. robustus, a ‘Near Threatened’ species. Although more abundant than the other species requiring special attention that are also recorded in the quantitative survey, Brasilicereus phaeacanthus is also classified as an ‘Endangered’ species (IUCN, 2015).
Although environmental factors are important as predictors of the composition and abundance of Cactaceae species (Hernández et al., Reference Hernández, Gómez-Hinostrosa and Bárcenas2001; Reference Hernández, Goettsch, Gómez-Hinostrosa and Arita2008; Alanís-Rodríguez et al., Reference Alanís-Rodríguez, Mora-Olivo, Jimenez-Pérez, Gonzalez-Tagle, Yamallel, Martínez-Avalos and Gonzalez-Rodriguez2015), the largest fraction of the floristic variation was not explained by environment or space. This unexplained fraction may be related to climatic alterations in the past (ter Steege, Reference Ter Steege2010), evolutionary processes, natural and anthropic disturbances and other unmeasured environmental variables (Terborgh & Andresen, Reference Terborgh and Andresen1998; Bohlman et al., Reference Bohlman, Laurance, Laurance, Nascimento, Fearnside and Andrade2008; Higgins et al., Reference Higgins, Ruokolainen, Tuomisto, Llerena, Cardenas, Phillips, Vásquez and Räsänen2011), or even biotic factors such as herbivory (Fine et al., Reference Fine, Mesones and Coley2004; Reference Fine, Miller, Mesones, Irazuzta, Appel, Stevens, Sääksjärvi, Schultz and Coley2006). The taxa with only a few individuals, such as Pereskia aureiflora, Pilosocereus catingicola and Stephanocereus leucostele and the eight species sampled in the floristic survey, require further studies on their areas of distribution and relationships to the environment or space. Although no unique Cactaceae community was identified in the Grotas highlands, the quantitative data and the floristic survey recorded essential data relating to some species that occurred in restricted sandstone environments in the area surrounding the CSNF, i.e. Espostoopsis dybowskii and Pilocereus gounellei.
Although seed dispersal of Cactaceae is typically through transport by bats, birds, lizards and ants, a few species are dispersed vegetatively by stem segments, such as the species of Opuntioideae (Taylor & Zappi, Reference Taylor and Zappi2004). This dispersal mechanism could explain the high abundance of Tacinga palmadora in comparison with the other species. This feature of dispersal could also explain the unexplained fraction of the floristic variation.
Given the endemic, vulnerable and endangered species of the caatingas from Bahia, in addition to the intermittent floristic patterns, our results indicate that broader protection areas for the Cactaceae species in the region of the CSNF are necessary. These data indicate the need specifically for a broader conservation area that would include the areas in the Grotas highlands that were associated with threatened species. Furthermore, the category of this protected area must be changed from sustainable use to integral protection to improve the outcomes of the conservation actions that are required in this region.
Acknowledgements
We thank the Fundação Flora and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior for scholarships to Victor Ferreira Lima and Mônica Peixoto, respectively; PROBIO II–ICMBio and Embrapa Genetic Resources and Biotechnology for financial support; Pedro V. Eisenlohr for support in spatial statistics; Marianna Rodrigues de Souza, Daniela Zappi and Nigel Taylor for reviewing the species list; Geraldo Machado Pereira, Head of CSNF, for logistics and technical support; and Valdeci Ferreira (Dudu), Valdemar Souza (Camarão) and Glocimar Pereira Silva for support in field activities. L.Y.S.A. acknowledges support from the Foundation for Research Support, Bahia, and the National Research Council (CNPq) in financing the project (grants RED0034/2014 and 442604/2014-9, respectively). S.R.-S. acknowledges the support of PROBIO II–ICMBio in financing the project (2012–2014).