Many birds, particularly passerines, visualise light in the 300–400-nm ultraviolet (UV) spectrum, wavelengths that are undetectable to the human eye (Hart et al. Reference Hart, Partridge, Cuthill and Bennett2000). Although birds have been shown to use UV reflectance (output of UV wavelengths when material is excited by light in the UV spectrum) in foraging (Church et al. Reference Church, Bennett, Cuthill and Partridge1998; Siitari et al. Reference Siitari, Honkavaara and Viitala1999; Lyytinen et al. Reference Lyytinen, Lindström and Mappes2004; Yang et al. Reference Yang, Wang and Liang2016), the role that UV fluorescence (the output of human-visible light when material is excited by UV wavelengths) plays is unclear (Lagorio et al. Reference Lagorio, Cordon and Iriel2015). Lepidopteran larvae across a phylogenetically diverse array of species exhibit various patterns of UV fluorescence (Moskowitz Reference Moskowitz2017). It is unknown what effect these patterns have on predation of caterpillars by insectivorous birds.
Caterpillars are critical in the diet of many insectivorous birds, and many lepidopteran species experience intense predation pressure (Bereczki et al. Reference Bereczki, Ódor, Csóka, Mag and Báldi2014). Birds are primarily visual foragers, and their advanced colour perception has undoubtedly contributed to the selection of cryptic patterns in caterpillars (Church et al. Reference Church, Bennett, Cuthill and Partridge1998). Quantifying predation on caterpillars is fraught with experimental difficulties in natural environments and the potential confounding effects of phylogeny in interspecific comparisons. An alternative approach incorporates replica caterpillar models deployed in life-like poses. This “dummy model” method of assessing predation is increasingly common in situations where other techniques are prohibitively difficult (Lövei and Ferrante Reference Lövei and Ferrante2017; Aslam et al. Reference Aslam, Nedvěd and Sam2020). Life-like models are formed from impressionable materials that retain marks from attempted predation events. These dummies are deployed in the field for a set amount of time, recovered, and then examined for imprints (Low et al. Reference Low, Sam, McArthur, Posa and Hochuli2014; Leuenberger et al. Reference Leuenberger, Larsen, Leuenberger and Parry2019). Caterpillars are the most common taxa modelled in predation assessments (Posa et al. Reference Posa, Sodhi and Koh2007; Howe et al. Reference Howe, Lövei and Nachman2009; Ferrante et al. Reference Ferrante, Barone, Kiss, Bozóné-Borbáth and Lövei2017; Roslin et al. Reference Roslin, Hardwick, Novotny, Petry, Andrew and Asmus2017). Although using dummies to estimate real predation rates should be done with caution (Nagy et al. Reference Nagy, Schellhorn and Zalucki2020), they can measure relative predation intensity among sites or other treatments (Lövei and Ferrante Reference Lövei and Ferrante2017).
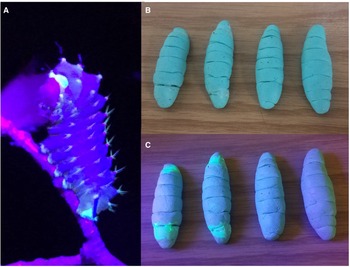
We investigated the effect of UV fluorescence in caterpillars on avian predation. Field experiments were conducted in 2017 and 2018 using plasticine larvae modelled after a fluorescing species of giant silk moth (Saturniidae), Antheraea polyphemus (Fig. 1A). In 2017, as a component of a larger study examining mortality in A. polyphemus caterpillars at densities of three per tree, 240 dummy caterpillars were made using moulded plasticine (Plastalina, 10128 Mint, Van Aken International, Alpharetta, Georgia, United States of America) and weighed using an electronic OHAUS balance (0.01g; OHAUS Corporation, Parsippany, New Jersey, United States of America). We applied transparent UV-fluorescent water-based paint (GLO Effex Liquid F/X Paint: 365 nm; GLO Effex, Murrieta, California, United States of America) to 80 dummies, emulating patterns exhibited on living larvae. Painted dummy caterpillars displayed little change in appearance under daylight but fluoresced noticeably under a UV flashlight (Fig. 1B and C). Dried paint did not interfere with the impressionability of the plasticine. Dummies were allowed to air-dry over a period of several days to allow for the dissipation of any potential repellent airborne compounds emitted by the paint. Dummies were stored in individual lidded containers before transportation into the field and after collection.

Fig. 1. A, Antheraea polyphemus fluorescence under blacklight illumination (photo courtesy of S. Jaffe, The Caterpillar Lab); B, paint treatments used on dummy caterpillars under daylight: (left to right) bird UV, human UV, clear paint, and no paint; and C, the same paint treatments under blacklight illumination.
In 2017, sampling was conducted in 16 sites in the southeastern region of the Adirondack Park, New York, United States of America (from 43° 4' 57.3594" to 43° 59' 45.6" N; from –73° 33' 48.9594" to –74° 24' 47.16" W). Elevation ranged from 190 to 657 m, representing a gradient of climate-driven deciduous–hardwood forest types of both old growth and secondary growth. Insectivorous foliage-gleaning passerine birds are abundant in this region (Able and Noon Reference Able and Noon1976). Dummies were deployed from 12 to 15 July 2017, for six days of exposure to ambient predation. At each site, five understorey American beech, Fagus grandifolia (Fagaceae), trees, at least 60 m apart, were randomly chosen as dummy locations. On each tree, one painted and two unpainted dummies were attached using acrylate superglue to the undersides of leaves on the chosen tree, for a total of 15 dummies per site. Leaf undersides were used as attachment sites to mimic the daytime resting behaviour of A. polyphemus larvae. The height above ground and the distance between dummies were measured.
In 2018, we added two treatments – a clear water-based, nonfluorescent paint (“clear paint”; Createx Colors Illustration Base Paint, Transparent; Createx Colors, Granby, Connecticut, United States of America) and UV-fluorescent paint layered with a UV-reflective clear paint (“bird UV”; Flock Free Flock Off! UV Paint “clear”; Flock Free Bird Control, Lakewood, New Jersey) – in addition to the unpainted (“no paint”) and UV-fluorescent (“human UV”) cohorts. We made 30 dummies per treatment, for a total of 120 dummies. Sampling was conducted in another mixed northern hardwood forest (Heiberg Memorial Forest, Tully, New York, United States of America, 42° 46' 11.3" N; –76° 5' 2.5" W). This area contains similar bird communities to those in the Adirondack Park site. Dummies were deployed on 60 understorey American beech trees, with each tree separated by at least 40 m, and were exposed for six days (19–25 July 2018). A single paint treatment was assigned randomly to each tree. Dummies were affixed in pairs – one to a leaf underside and one to an exposed section of branch on each tree – to test for the effect of background contrast. During retrieval, one “bird UV” tree and one “no paint” tree were unable to be relocated.
Recovered dummies were examined and categorised as “bird strike” or “no strike” by consensus of two independent observers. Dummies with strikes by mammals (clear incisor marking) were removed from the data sets; invertebrate predation was not determined because putative marks were difficult to score conclusively as attacks. In 2017, the effects of paint treatment (“no paint” versus “human UV”), mass (g), height above ground (cm), and distance from nearest dummy (cm) on bird strikes were modelled in generalised logistic regression (glm, family = binomial) and mixed-effects logistical regression (glmer, family = binomial, lme4 1.1–26) models in R, version 3.6.0 (R Core Team 2019). The interaction of paint and mass was also considered. Site was retained as the random effect for all 2017 mixed models after finding no significant difference with a mixed-effects model using both tree and site as random effects (analysis of variance,
![]() $$\chi _1^2$$
= 0.0009, P = 0.98). Analyses of 2018 data considered the effect of paint treatment (“no paint”, “clear”, “human UV”, “bird UV”), position (bark versus foliage), and the interaction of those variables on bird strikes in a mixed-effects logistical regression. To address convergence failures, control intercept glmer model optimisers were selected by the allFit function (lme4; Chung et al. Reference Chung, Rabe-Hesketh, Dorie, Gelman and Liu2013). Mixed models with fixed effects that failed to converge were run with a maximum penalised likelihood (bglmer, family = binomial, blme 1.0–5). Models from each analysed data set were compared by Akaike information criterion, and best models were determined by a difference of 2.
$$\chi _1^2$$
= 0.0009, P = 0.98). Analyses of 2018 data considered the effect of paint treatment (“no paint”, “clear”, “human UV”, “bird UV”), position (bark versus foliage), and the interaction of those variables on bird strikes in a mixed-effects logistical regression. To address convergence failures, control intercept glmer model optimisers were selected by the allFit function (lme4; Chung et al. Reference Chung, Rabe-Hesketh, Dorie, Gelman and Liu2013). Mixed models with fixed effects that failed to converge were run with a maximum penalised likelihood (bglmer, family = binomial, blme 1.0–5). Models from each analysed data set were compared by Akaike information criterion, and best models were determined by a difference of 2.
We recovered 240 dummies in 2017 and observed 26 dummies with bird strikes: 22 strikes occurred on unpainted dummies, and four strikes occurred on painted dummies (Fig. 2A). Three dummies featured mammal strikes. Two dummies with missing height-above-ground and distance-to-nearest-neighbour measurements were dropped from initial analyses, but ultimately these variables did not appear in any of the top models using this partial data set. As one of the dummies dropped from the initial analysis included a bird strike, the full data set was analysed examining only paint treatment and mass. The strongest models from the full data set indicate an effect of paint and mass on bird strikes (Table 1A). The mean mass of bird-struck dummies was heavier, although the difference was small (3.6%) and may not be biologically significant. Control models never exceeded a 23% probability of minimising information loss compared to the strongest model.

Fig. 2. A, Mean number of bird strikes per painted and unpainted dummies across sites in 2017; B, mean number of bird strikes per paint treatments in 2018; and C, mean number of bird strikes per background treatment in 2018. Sample size located beneath marker. Error bars represent 95% bootstrapped confidence intervals.
Table 1. Summary AIC table comparing fixed and mixed models for each year. P represents the probability that the model minimises information loss relative to the strongest model. AIC, Akaike information criteria.

In 2018, birds struck 10 of 116 recovered dummies, with nearly all strikes on nonfluorescent caterpillars (Fig. 2B) located on foliage (Fig. 2C). Small mammals struck two dummies. Position of the dummy, testing the effect of background contrast, and the random intercept control produced the strongest models (Table 1B). A posthoc analysis grouping the paint treatments by fluorescence improved the models over the multiple-paint treatment models but did not surpass the intercept control. Despite the proportional dominance of nonfluorescing dummies in the struck category, the intercept model indicated that the overall number of strikes in 2018 was too low to meaningfully test.
Our results suggest that birds select non-UV fluorescing caterpillar dummies over those that fluoresce. A higher number of strikes on the “no paint” and “clear paint” treatments suggest paint itself did not impact predation, whereas no difference between “human UV” and “bird UV” treatments suggests that UV fluorescence, not UV reflectance, is responsible for the observed effect.
Many lepidopteran taxa fluoresce in multiple life stages (Sourakov Reference Sourakov2017), although its function has yet to be clarified (Marshall and Johnsen Reference Marshall and Johnsen2017). Bird-attracting UV reflectance is more common in nocturnal than diurnal adult Lepidoptera (Lyytinen et al. Reference Lyytinen, Lindström and Mappes2004), but some butterflies have conspicuous UV-fluorescent patches (Welch et al. Reference Welch, Van Hooijdonk, Intrater and Vigneron2012). UV fluorescence may act aposematically, deterring bird attacks, but these patterns are not consistent for aposematic Lepidopteran larvae (Sourakov Reference Sourakov2017). Conversely, larval UV fluorescence could be related to some other morphological or physiological function, as has been hypothesised for scorpions (Gaffin et al. Reference Gaffin, Bumm, Taylor, Popokina and Mann2012) and tardigrades (Suma et al. Reference Suma, Prakash and Eswarappa2020).
Birds struck 10% of dummy caterpillars in both years, 1.8% per day in 2017 and 1.4% per day in 2018. These treatment-incorporated rates are lower than the median daily vertebrate predation rate of 3.9% in a review of 61 studies of insect models (Lövei and Ferrante Reference Lövei and Ferrante2017), although the mean exposure period for their rate was three days versus our six days. Using geometrid dummies, 7.5% bird predation was measured in a similar forest type over a six-day exposure interval (Leuenberger et al. Reference Leuenberger, Cohen, Rustad, Wallin and Parry2021). Although longer exposures do increase overall predation levels, daily attack rates decay as birds presumably learn over time that model larvae are inedible. Maximising the absolute number of strikes, however, is necessary for complex analyses. Our sample size in 2017 more adequately captured predation attempts than did our sample size in 2018, suggesting sample sizes below the median of 216 found by Lövei and Ferrante (Reference Lövei and Ferrante2017) may not suffice for complex comparisons using this approach.
To our knowledge, this study is the first to assess the role of UV fluorescence in the susceptibility of caterpillars to bird predation. Given the number of birds that visualise in this spectral range and the number of insects that have fluorescent properties, we urge further research in this poorly understood area of predator–prey interactions.
Acknowledgements
This work was conducted in compliance with ESF IACUC protocol #180701. The authors thank Emma Livingston and George Laribee, Jr. for field assistance. They are grateful for funding from the Northeast States Research Cooperative to D.P.