Introduction
Acute basilar artery occlusion (BAO) is associated with a significant rate of death and disability.Reference Lindsberg, Soinne and Tatlisumak1 Large prospective observational studies did not support the superiority of endovascular thrombectomy (EVT) over intravenous thrombolysis (IVT) for patients with acute BAO and others demonstrated poor clinical outcome despite successful recanalization.Reference Schonewille, Wijman and Michel2,Reference Singer, Berkefeld and Nolte3 The Acute Basilar Artery Occlusion: Endovascular Interventions versus Standard Medical Treatment (BEST) trial was the first randomized controlled trial designed to evaluate the efficacy of EVT for patients with BAO.Reference Liu, Xu and Liu4 The trial was terminated early due to progressive loss of equipoise among recruiting physicians resulting in high crossover rates. The only other randomized trial for acute basilar occlusion assessed the safety and efficacy of intra-arterial urokinase in 16 patients and was halted early due to poor recruitment.Reference Macleod, Davis and Mitchell5 Collectively, these studies highlight the clinical challenges pertaining to the management of BAO.6
Given the heterogeneous group of patients undergoing EVT for BAO, stroke societies currently describe the benefits of EVT in BAO as uncertain.Reference Powers, Rabinstein and Ackerson7,Reference Boulanger, Lindsay and Gubitz8 The aim of this study is to audit our local experience for patients with acute BAO undergoing EVT with modern endovascular devices, as part of a quality improvement initiative for our regional stroke center. The descriptive analysis explored predictors of good functional outcome.
Methods
Patient Characteristics
Research ethics board approval was obtained for this single-center retrospective observational study. All consecutive patients undergoing EVT for BAO at our center, a high EVT volume (>125 cases/year) regional stroke center serving the north and east Greater Toronto Area, from January 1, 2013 to March 1, 2019 were included. There were no exclusion criteria. During this period, as anterior circulation large vessel occlusion EVT went from clinical trials’ phase to standard of care, our institution did not have specific EVT inclusion/exclusion criteria for patients with BAO, which was not an uncommon scenario in many centers. Patient selection for EVT was made on an individual basis by the consulting stroke neurologist and neurointerventionalist. Generally, EVT was considered in patients > 18 years, pre-stroke modified Rankin Scale (mRS) score 0–2, with no intracranial hemorrhage (ICH) or evidence of extensive bilateral brainstem ischemia. The authors do not use a time window cutoff and considered patients >24 hours from ictus in certain situations.
Patient baseline characteristics retrieved included age, sex, stroke risk factors (hypertension, smoking, dyslipidemia, coronary heart disease, diabetes, and atrial fibrillation), and antithrombotic therapy. Administration of intravenous tissue plasminogen activator (tPA), symptom onset to puncture time, and stroke etiology according to the trial of ORG 10172 in acute stroke treatment classification were recorded.Reference Adams, Bendixen and Kappelle9 Baseline National Institutes of Health Stroke Scale (NIHSS) score was calculated on arrival by the stroke team.
All patients underwent computed tomography (CT) angiography (CTA) before and after EVT (within 24 hours). Imaging features retrospectively obtained include any evidence of ICH after EVT, posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS), length of thrombus, Basilar Artery on Computed Tomography Angiography (BATMAN) score, and early (<24 hour) reocclusion.Reference Puetz, Sylaja and Coutts10,Reference Alemseged, Shah and Diomedi11 pc-ASPECTS is a 10-point scale where points are lost for infarction of the thalami, occipital lobes, cerebellar hemispheres, midbrain, and pons as observed on non-contrast CT. The BATMAN score is a 10-point CTA-based grading system utilized to assess the extent of basilar occlusion and the presence of posterior communicating artery collateralization. Points are distributed as: one point if either vertebral artery is patent, one point for each proximal posterior cerebral artery, one point for each patent segment of the basilar artery (proximal, middle, and distal), and two points for each of the posterior communicating arteries.
Endovascular Thrombectomy
Procedures were performed at our regional stroke center with anesthesia coverage. The primary technique used was determined by the treating neurointerventionalist and included either a second-generation stent retriever or direct contact aspiration. Intra-arterial thrombolysis was utilized in certain instances if the clot could not be traversed or significant residual stenosis/thrombus remained. We recorded the primary device used as the treatment technique, along with the number of attempts before recanalization.
Outcome Measures
The primary outcome measure was functional outcome at 1 month. The mRS score was used to define dichotomized functional outcome, as measured by stroke physicians. A mRS of 0–3 was considered a good outcome, and mRS 4–6 was considered a poor outcome as defined in the BASICS registry.Reference Schonewille, Wijman and Michel2 Secondary outcome measures included reperfusion status as determined on the final angiographic run during EVT and the incidence of procedure-related complications. The modified treatment in cerebral infarction (mTICI) score was used, with 2B-3 defined as successful reperfusion and 0-2A defined as unsuccessful. Stroke-onset time was defined as the onset of symptoms as per the patient or witnesses and consistent with the clinical diagnosis of BAO by the treating stroke physician. Prodromal symptoms such as transient ischemic attack or minor stroke in the hours/days leading to the current event were not considered as the stroke-onset time. For example, if patients experienced episodes of dizziness/vertigo and diplopia one day prior and now presented with severe neurological deficit, the time of onset would be the severe deficit and subsequent imaging confirming BAO.
Statistical Analysis
All variables (patient baseline, clinical, imaging, and treatment) were compared between patients with good (mRS 0–3) and poor (mRS 4–6) functional outcomes. All continuous variables were recorded as means with interquartile range, and categorical variables were recorded as counts with percentages. For continuous variables, an independent sample t-test or Mann–Whitney U test was used to compare the two groups. The Chi-squared or Fishers’ exact tests were used to compare categorical variables depending on number of samples in each quadrant. The threshold for significance was defined as p < 0.05. For those values found to be significant, logistic regression analysis was performed with odds ratios (OR) and 95% confidence intervals (95% CI). Statistical analysis was done using SPSS version 25.
Results
A total of 43 consecutive patients underwent EVT for BAO with no patients excluded from the analysis. The average age (standard deviation) was 67 years (16) with 61% (26/43) male patients. Overall, 37% (16/43) of patients achieved good functional outcome (mRS 0–3) at 1 month (Table 1). The 1-month mortality rate was 33% (16/43). Successful reperfusion (mTICI 2B-3) was achieved in 72% (31/43) of cases. In patients with successful reperfusion, 39% (12/31) achieved good function outcome and in patients with unsuccessful reperfusion, 33% (4/12) achieved good functional outcome.
Table 1: Patient baseline characteristics, imaging findings, and treatment techniques dichotomized with respect to good (mRS 0–3) and poor (mRS 4–6) functional outcome. Univariate analysis was used to examine for differences between the groups

Abbreviations: pc-ASPECTS = Posterior circulation Alberta Stroke Program Early CT Score; BATMAN = Basilar Artery on Computed Tomography Angiography; NIHSS = National Institutes of Health Stroke Scale; T2DM = type 2 diabetes mellitus; AFIB = atrial fibrillation; CAD = coronary artery disease; IAT = intra-arterial thrombolysis.
The procedure-related complication rate was 9% (4/43). In three patients, intraoperative ICH occurred, as observed with active contrast extravasation (two subarachnoid hemorrhages with intraventricular extension and one intraparenchymal hemorrhage). All three patients had extensive CT evidence of established infarcts (pc-ASPECTS ≤ 6) before EVT and none received intravenous or intra-arterial thrombolysis. The fourth patient had a large midbrain infarct prior to EVT and developed hemorrhagic transformation on postoperative (24 hours) imaging. He received intravenous tPA with time to EVT puncture of 255 minutes.
The median (interquartile range) stroke onset to treatment time was 420 (270–639) minutes (7 h) for all patients. Good functional outcome was achieved in 33% (7/21) of those patients treated <6 hours of stroke onset and in 40% (9/22) of patients treated >6 hours of stroke onset. The mean onset to treatment time for all patients with good and poor functional outcomes was 674 and 624 minutes, respectively. The longest onset to treatment time was 48 hours in a patient with postural neurological changes that required ongoing blood pressure augmentation, who also achieved good functional outcome. The most common BAO etiology was basilar atherosclerotic disease (60%), followed by cardioembolism (26%) and undetermined (12%).
On univariate analysis, baseline NIHSS, pc-ASPECTS, and BATMAN scores were all associated with improved functional outcome (p < 0.05). Length of occlusion and stroke etiology were not found to be significantly associated with improved functional outcome. Stroke onset to puncture time, successful reperfusion, and the administration of tPA were also not found to be significantly associated with outcome (p = 0.68) (Figure 1). There was also no difference between patients treated with stent retrievers and contact aspiration. On multivariate analysis, only pc-ASPECTS and BATMAN scores were found to be significant (p < 0.05).

Figure 1: Scores on the modified Rankin Scale as a function of EVT reperfusion status. Successful reperfusion (mTICI 2B-3) was achieved in 72% patients (31/43). The presence of successful reperfusion was not found to be associated with dichotomized good functional outcome (dash line).
Discussion
Our single-center retrospective study of 43 patients observed good functional outcome in 37% of patients, successful recanalization achieved in 72% of patients, with a periprocedural complication rate of 9%. Despite functional improvement in a subset of patients, a majority of patients with successful recanalization did not achieve good outcomes, which is consistent with previously described series. Intriguingly, a portion of patients without recanalization also achieved good outcome in short-term follow-up highlighting challenges in predicting outcome based on measurable parameters in this fulminant type of stroke.
Our study has several limitations. First, this was a retrospective observational study, and patient selection criteria for EVT could be subject to selection biased. There was no standardization for patient and treatment selection.
The BASICS registry is the largest prospective observational study done to assess outcomes and treatment responses for 619 patients with BAO.Reference Schonewille, Wijman and Michel2 They observed that overall 32% of patients had favorable outcome (mRS 0–3) at 1 month, and that there was no difference between those patients receiving IVT and EVT. This study cast doubt on the assumption that IAT is superior to IVT in patients with BAO. More recently, ENDOSTROKE subsequently examined 148 patients with BAO undergoing EVT (84% second-generation stent retrievers) and observed 42% of patients had good outcome (mRS 0–3), with 35% mortality rate.Reference Singer, Berkefeld and Nolte3 They found however that the presence of successful reperfusion was not correlated to outcome, again underscoring the need to search for reasons of futility of recanalization in patients with BAO.Reference Lindsberg and Strbian12 Our findings are consistent with the notion that vessel recanalization alone is insufficient and that some patients will even have adequate recovery without vessel recanalization.
Two multicenter randomized controlled trials were undertaken comparing the safety and efficacy of EVT plus best medical management compared to best medical management alone for patients with acute BAO.Reference Liu, Xu and Liu4,Reference van der Hoeven, Schonewille and Vos13 The BEST trial was terminated early due to progressive loss of equipoise resulting in high crossover rates. This is not an uncommon scenario in EVT centers. Despite the early termination of the trial, those patients who were treated with EVT had significantly better outcomes.6 In the as-treated analysis, 47% of patients who underwent EVT had a good outcome (mRS 0–3) at 90 days compared to 24% in the standard medical therapy group (p = 0.008). Symptomatic ICH was reported in 8% of the intervention group.Reference Liu, Dai and Ye14 The BASICS trial recruitment continues.6
Our findings of good function outcome in 37% of patients and successful reperfusion in 72% are in agreement with more recent EVT series for patients with BAO.Reference Verstraete, Bounameaux, de Cock, Van de Werf and Collen15–Reference Giorgianni, Biraschi and Piano19 The authors report a complication rate of 9%, which is in line with that of the BEST trial which reported symptomatic ICH rate of 8% in the intervention arm.Reference Liu, Dai and Ye14 We observed that only baseline NIHSS score, pc-ASPECTS, and BATMAN score were significantly associated with good outcome on univariate analysis, and only the baseline pc-ASPECTS and BATMAN score were significant on multivariate logistic regression analysis. As observed in the ENDSTROKE study, we did not find that successful reperfusion or decreased symptom onset to treatment time was associated with improved functional outcome. We hypothesize several reasons for these findings.
With respect to stroke onset to treatment time, defining accurate symptom onset in BAO is challenging, as some patients experience a prodromal phase of symptoms before the basilar occludes.Reference Ferbert, Bruckmann and Drummen20 Thus, the standard anterior circulation definition of “last seen normal” time does not always convey the same implications for BAO patients. This may lead to prolonged recorded symptom-onset times. Furthermore, the posterior circulation's unique collateralization may allow for longer time interval for intervention. Lindsberg et al. hypothesized that a thrombus will cause a drop in pressure in the basilar artery, leading to backflow into the basilar via the posterior communicating arteries, maintaining vital brainstem perforators if the clot does not enlarge (Figure 2).Reference Lindsberg, Pekkola, Strbian, Sairanen, Mattle and Schroth21 The extent of brainstem ischemia and recovery after BAO depends on the integrity of vascular collateralization to vital brainstem structures not yet infarcted. However, any stepwise growth of thrombus will put these structures at risk. Intervention in these patients could be beneficial, within an extended time window after the onset of symptoms.
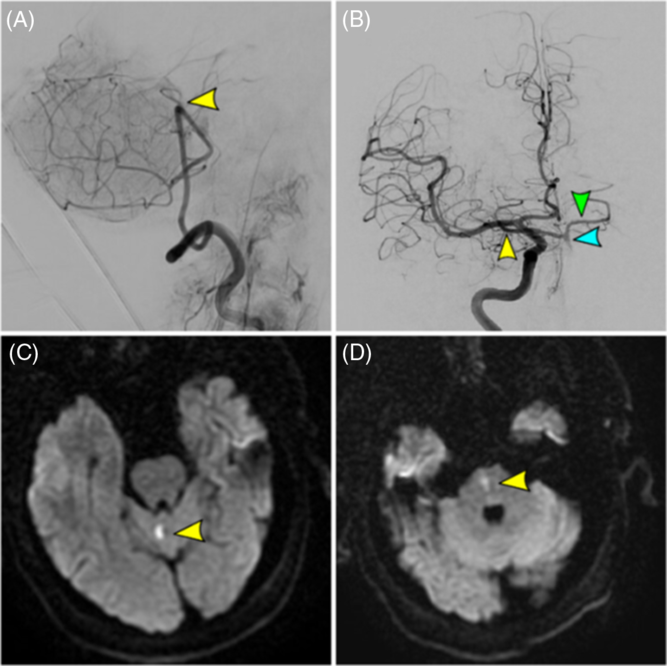
Figure 2: Acute basilar occlusion in a 67-year-old male with failed recanalization (TICI 0) and mRS 0 at 1 month. (A) Vertebral artery injection (final angiographic run) showing proximal basilar occlusion (yellow arrow) with failed recanalization. (B) Right internal carotid artery injection showing retrograde flow through the right posterior communicating artery (yellow arrow) into the basilar artery (blue arrow) and contralateral posterior cerebral artery (green arrow). (C/D) Small cerebellar and medulla infarcts as observed on DWI MRI.
Second, the finding that successful reperfusion was not associated with improved functional outcome could be due to treating a significant number of patients with already established disabling brainstem infarcts, in which revascularization is futile (Figure 3). We found that 40% of patients treated >6 hours of stroke onset had good functional outcome, compared with just 33% in those treated <6 hours of onset, which is contrary to established paradigms of early reperfusion improving outcome. Our finding that only pc-ASPECTS score on multivariable analysis was associated with outcome supports the hypothesis that patients with evidence of significant brainstem ischemic before EVT have poor outcome. Puetz et al. showed that pc-ASPECTS score independently predicted death and functional outcome from patients in the BASICS registry.Reference Puetz, Khomenko and Hill22 It is notable that pc-ASPECTS based on CTA source images may have further sensitivity in detecting already infarcted tissue.Reference Puetz, Sylaja and Hill23 Furthermore, hyperacute rapid magnetic resonance imaging (MRI) protocols may also be informative in-patient selection and/or hemorrhagic risk stratification in patients who are being revascularized. In the ENDOSTROKE study, they found that use of magnetic resonance before EVT also predicted clinical outcome. We speculate that it is unlikely there is a large ischemic penumbra in the brainstem, particularly to midbrain/pontine structures supplied by single perforators/circumflex arteries, and hence diffusion weighted imaging (DWI) changes observed will not be reversed. The basilar artery vascular territory has been largely excluded from the ischemic penumbra literature, and the limited data suggest that no penumbra can exist in the pons after occlusion of internal arteries.Reference Lindsberg, Pekkola, Strbian, Sairanen, Mattle and Schroth21 The anatomical location of posterior circulation infarcts also plays a role in outcome. Lee et al. showed that diffusion restriction in the pons was independently associated with early neurological deterioration, while other locations (midbrain, thalamus, cerebellum, and medulla) were not independent predictors.Reference Lee, Kim and Suh24

Figure 3: Acute basilar occlusion with successful recanalization (TICI 2B) in a 79-year-old female with time to puncture of 4.5 hours and discharge mRS 5. (A) Vertebral artery injection showing proximal basilar occlusion (yellow arrow). (B) Final angiographic run demonstrating TICI 2B reperfusion with significant ongoing mid-basilar stenosis (blue arrow). (C/D) Large diffuse cerebellar (green arrows) infarcts and extensive medullary infarcts (yellow arrows) as observed on DWI MRI.
Collectively, our finding and those from the literature demonstrate that patient selection for EVT for BAO remains an important challenge. We hypothesize that strict time of symptom-onset thresholds for EVT inclusion may exclude patients that would benefit from EVT. Sterbian et al. showed that in the absence of extensive baseline ischemia, EVT up to 48 hours was effective and pc-ASPECTS was the single most important prognostic factor and independent of time to treatment.Reference Strbian, Sairanen, Silvennoinen, Salonen, Kaste and Lindsberg25 It is likely that thrombus within the basilar artery will progress over time, and EVT could prevent clot propagation and eventual infarction of vital structures in patients with salvageable brainstem. Furthermore, mechanism of thrombus (cardioembolic vs. atherosclerotic) impacts on clinical progression and recognition, rate of tissue infarction, and EVT-related technical challenges. The paradigm of utilizing a tissue window as oppose to a time window for EVT patient selection was on display in the Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct and Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging trials, and this concept could also be expanded to the posterior circulation, especially given the posterior circulation collateralization.Reference Nogueira, Jadhav and Haussen26
The findings of the present study have solidified the authors opinion that hard time window cutoffs for EVT consideration in BAO may not be advantageous. Furthermore, patients with poor neurological examination and non-contrast CT evidence of extensive brainstem infarcts are typically not considered for EVT. At that point, discussions are held with the family regarding the observed irreversible brainstem injury and futility of revascularization. In cases where the clinical examination is poor and non-contrast CT imaging does not clearly show brainstem ischemia, the authors have started utilizing rapid MRI DWI imaging typically within 30 minutes to determine the extent of brainstem injury.
Conclusion
EVT is safe and feasible for patients with acute BAO. Patient selection for thrombectomy for basilar occlusion remains a challenge. Given the unique and variable collateral anatomy, the paradigm of utilizing a tissue window as oppose to a time window for patient selection could be advantageous. The authors finding (along with findings in the literature) that time to treatment and successful reperfusion were not associated with improved outcome is likely due to including patients with established infarcts, and MRI imaging may be a reasonable option to determine the extent of ischemia in certain situations.
Conflict of Interest
All authors declare no conflicts of interest.
Authorship
Study concept and design: CRP, HK, LdC, CH, SMP, JCK, SEB, DG, VXDY; Acquisition, analysis, or interpretation of data: CRP, HK, LdC, CH, SMP, JCK, SEB, DG, VXDY; Drafting the article: CRP, HK, LdC, DG, VXDY; Administrative, technical, or material support: CRP, HK, LdC, CH, VXDY; Statistical analysis: CRP, HK, DG, VXDY; Study supervision: VXDY.






