Introduction
Antipsychotic (AP) medications are effective in treating several psychiatric conditions in children and adolescents. Although not curative, they allow adequate control of clinical symptoms in lifelong psychiatric diseases (Caccia et al., Reference Caccia, Clavenna and Bonati2011). Yet, the use of APs is associated with a substantial number of adverse effects in this population and different agents present highly variable safety profiles (Pringsheim et al., Reference Pringsheim, Lam, Ching and Patten2011; Seida et al., Reference Seida, Schouten, Boylan, Newton, Mousavi, Beaith, Vandermeer, Dryden and Carrey2012). Therefore, prescribing of APs involves a difficult balance between the need to relieve mental disease symptoms and the risk of drug-induced toxicity.
Over the past three decades, studies have consistently demonstrated that the prevalence of the use of APs and duration of the AP therapy is increasing over time in the pediatric population (Vitiello et al., Reference Vitiello, Correll, van Zwieten-Boot, Zuddas, Parellada and Arango2009; Steinhausen and Bisgaard, Reference Steinhausen and Bisgaard2014; Halfdanarson et al., Reference Halfdanarson, Zoega, Aagaard, Bernardo, Brandt, Fuste, Furu, Garuoliene, Hoffmann, Huybrechts, Kalverdijk, Kawakami, Kieler, Kinoshita, Litchfield, Lopez, Machado-Alba, Machado-Duque, Mahesri, Nishtala, Pearson, Reutfors, Saastamoinen, Sato, Schuiling-Veninga, Shyu, Skurtveit, Verdoux, Wang, Yahni and Bachmann2017). Despite marketing authorisations for the use of some first-and second-generation antipsychotics (FGAs and SGAs) specifically in children and adolescents (Kaguelidou and Acquaviva, Reference Kaguelidou and Acquaviva2016), the vast majority of these medications are still prescribed ‘off-label’ in the pediatric population. The observed increase in the use of APs probably reflects the ‘off-label’ prescribing of APs in non-psychotic disorders, such as attention deficit hyperactivity disorder (ADHD) or disruptive behaviour, and in children younger than the approved age ranges (Penfold et al., Reference Penfold, Stewart, Hunkeler, Madden, Cummings, Owen-Smith, Rossom, Lu, Lynch, Waitzfelder, Coleman, Ahmedani, Beck, Zeber and Simon2013).
However, the majority of these drug utilisation studies are based on data from Northern American countries and information on population-based use of these medications in Europe is more limited (Zoega et al., Reference Zoega, Baldursson, Hrafnkelsson, Almarsdottir, Valdimarsdottir and Halldorsson2009; Verdoux et al., Reference Verdoux, Tournier and Begaud2010; Penfold et al., Reference Penfold, Stewart, Hunkeler, Madden, Cummings, Owen-Smith, Rossom, Lu, Lynch, Waitzfelder, Coleman, Ahmedani, Beck, Zeber and Simon2013; Steinhausen and Bisgaard, Reference Steinhausen and Bisgaard2014; Verdoux et al., Reference Verdoux, Pambrun, Cortaredona, Tournier and Verger2015; Waszak et al., Reference Waszak, Zagozdzon, Pierucka and Kubanek2018). Differences in the diagnosis and management of psychiatric conditions as well as in the attitude to prescribe APs may hinder extrapolation of results from one continent to the other. In fact, with regard to the use of SGAs, a wide inter-country variability was observed in a study from the year 2000, where dispensations of SGAs represented 66% of total AP use in children and adolescents in the US v. 48% in the Netherlands and only 5% in Germany (Zito et al., Reference Zito, Safer, de Jong-van den Berg, Janhsen, Fegert, Gardner, Glaeske and Valluri2008).
Therefore, the aim of this study was to describe the prevalence and incidence of AP use in children and adolescents in five European countries.
Methods
Data sources
Data were extracted from five population-based electronic healthcare databases in Europe. The Health Improvement Network (THIN) is a database of primary care medical records of about 5.9 million patients from 500 general practices (GPs) in the United Kingdom (UK). The PHARMO Database Network is a patient-centric data tracking system that captures medical information, including information on drug dispensing, for approximately 4 million inhabitants in 65 municipal areas in the Netherlands (NL). The Aarhus University Hospital Database comprises medical information including hospital and outpatient visits from 1.8 million inhabitants in Denmark (DN). The German Pharmacoepidemiological Research Database (GePaRD) consists of claims data from four German statutory health insurance providers, three of which accepted to contribute data for this study resulting in a source population of 8 million insurants. Finally, the Emilia Romagna Regional database (ERD) is a claims database that contains information on all reimbursable healthcare services, including drugs, for about 4.5 million inhabitants of the Emilia Romagna region in Northern Italy (IT). These databases contain information from the healthcare records of almost 27 million European citizens. THIN contain records from GP, while PHARMO, AARHUS, GePARD and ERD are comprehensive administrative/record-linkage systems in which drug dispensing data for a well-defined population are linked to a registry of hospital discharge diagnoses and various other registries.
All databases and their content have been extensively described in previous ARITMO publications and have already been used for the conduct of pharmacoepidemiological studies in compliance with European guidelines for the use of medical data for research (Holstiege et al., Reference Holstiege, Enders, Schink, Innocenti, Oteri, Bezemer, Kaguelidou, Molokhia, Poluzzi, Puccini, Ulrichsen, Sturkenboom, Trifiro and Garbe2015; Mor et al., Reference Mor, Froslev, Thomsen, Oteri, Rijnbeek, Schink, Garbe, Pecchioli, Innocenti, Bezemer, Poluzzi, Sturkenboom, Trifiro and Sogaard2015; Oteri et al., Reference Oteri, Mazzaglia, Pecchioli, Molokhia, Ulrichsen, Pedersen, Poluzzi, De Ponti, Garbe, Schink, Herings, Bezemer, Sturkenboom and Trifiro2016). Also, methodological aspects of multiple database studies carried out in ARITMO and other EU funded projects have been fully described in a previous publication (Trifiro et al., Reference Trifiro, Coloma, Rijnbeek, Romio, Mosseveld, Weibel, Bonhoeffer, Schuemie, van der Lei and Sturkenboom2014). Data were analysed using a distributed network approach, in which data holders maintain control over their original data and only anonymised and aggregated data are shared. This was done through the preparation of data according to a common data input model followed by local data aggregation using custom-built software, Jerboa© (Trifiro et al., Reference Trifiro, Coloma, Rijnbeek, Romio, Mosseveld, Weibel, Bonhoeffer, Schuemie, van der Lei and Sturkenboom2014). The respective scientific and ethics committees of each database approved the conduct of the study. With regard to GePaRD, the use of data was approved by the statutory health insurance providers and their authorities.
Study design and population
This was a dynamic retrospective cohort study. The study population comprised all children and adolescents (⩽18 years of age), registered with the databases during the study period with at least 1 year of valid data (except for newborns). The period for data collection differed between databases. It was longer in NL and UK (2000–2009) followed by DN (2001–2008), Germany (2005–2008) and IT (2006–2010). Children were followed from the start of the study period or, if later, the start of entry into the database until their 19th birthday, the end of the study period, exit from the database, death or latest data recorded, whichever came first.
Antipsychotic medications
All drugs under the ‘N05A’ pharmacological subgroup of the Anatomic Therapeutic Chemical classification system (except for lithium [N05AN]) were included. APs were sub-classified into second-generation (clozapine, olanzapine, quetiapine, asenapine, sulpiride, amisulpride, risperidone, aripiprazole, paliperidone, iloperidone, ziprasidone and sertindole) and first-generation agents (all the remaining). AP exposure was assessed using dispensing and/or prescription data from all databases.
Statistical analysis
Prevalence of AP use was defined as the number of children and adolescents that received at least one AP drug dispensing divided by the number of person-years (PYs) of follow-up in the study period and expressed as rate per 1000 PYs. Incidence of AP use was defined as the number of ‘new antipsychotic users’ per 1000 PYs. ‘New users’ were children and adolescents that had a first prescribing or dispensing of any AP drug after a drug-naïve period of 1 year. Because of the dynamic nature of the population we used PYs rather than the total number of individuals as denominators. Of note, PYs of exposure of prevalent users were not included in the denominator for calculation of the incidence of use. Both prevalence and incidence of use were estimated per calendar year and stratified by sex, age group (⩽4, 5–9, 10–14 and 15–17 years) and country. Prevalence and incidence estimates are provided with 95% confidence intervals (95% CI) calculated according to the asymptotic method based on a normal approximation. Relative changes (RC) in prevalence and incidence rates over the study period were expressed as percentage changes and calculated for each country as the difference in prevalence or incidence between the respective first and last year for which data was available, divided by the prevalence or incidence in the first data year.
Dispensing frequency by class of AP agents (FGAs and SGAs), age group and country was also measured. The number and type of APs covering 90% of all AP dispensing (DU 90%) were estimated by age group and country.
Data were described in narrative and tabular forms. Statistical analyses were performed using the SAS software version 9.3 software (SAS Inc, Cary, North Carolina).
Results
During the study period, average annual study population and population exposed to APs were respectively: 336.576 and 752 children and adolescents in DN, 1.340.163 and 1.949 in Germany (GE), 768.631 and 2.206 in UK, 798.431 and 2.149 in NL and 760.866 and 342 in IT.
Prevalence and incidence of AP use per country and calendar year with 95% CI are shown in Figs 1 and 2, respectively.
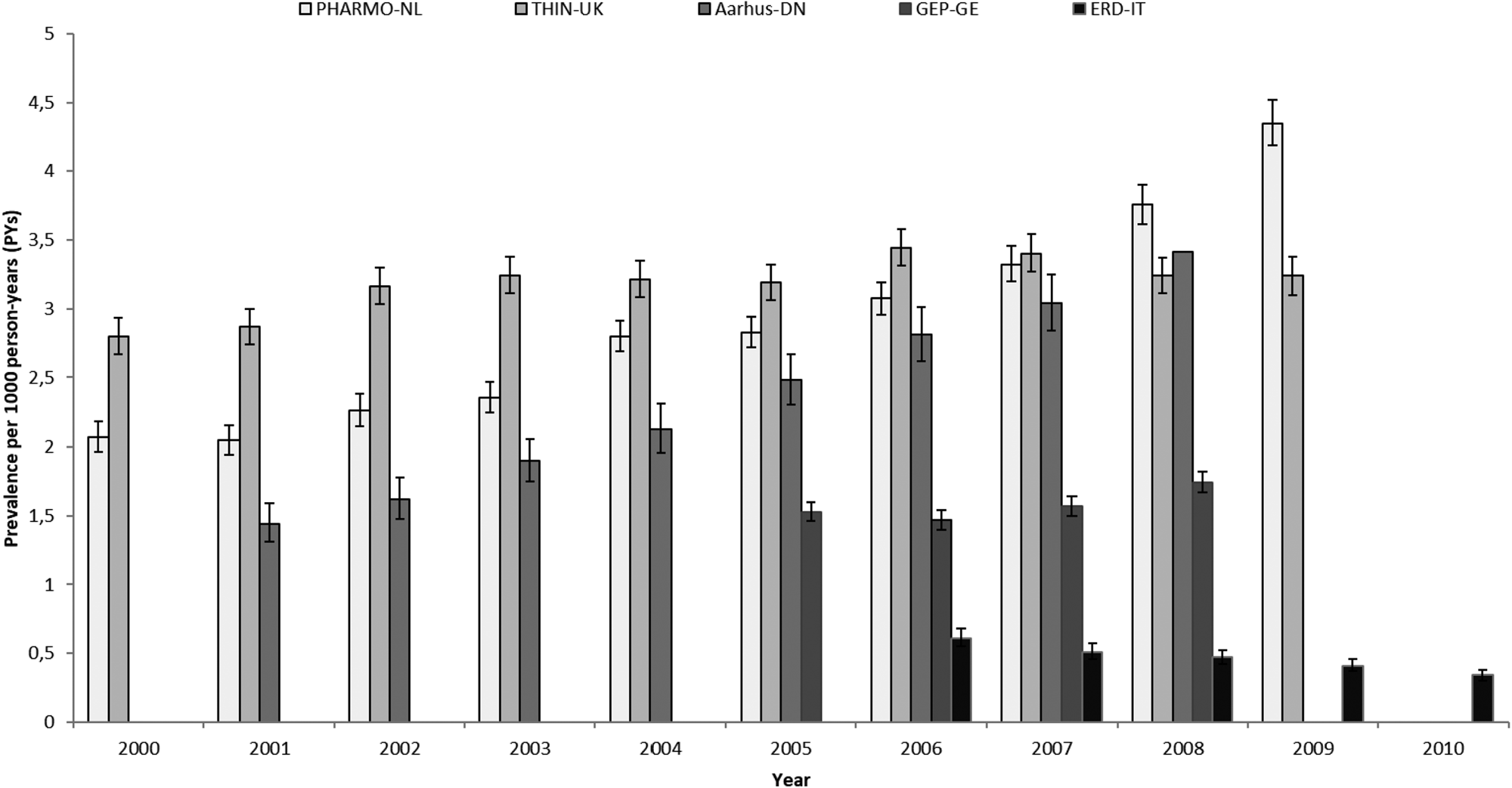
Fig. 1. Prevalence of AP use by country and calendar year. PHARMO-NL: PHARMO Database Network, the Netherlands. THIN-UK: The Health Improvement Network, United Kingdom. Aarhus-DN: Aarhus University Hospital Database, Denmark. GEP-GE: German Pharmacoepidemiological Research Database (GePaRD), Germany. ERD-IT: Emilia Romagna Regional database, Italy.
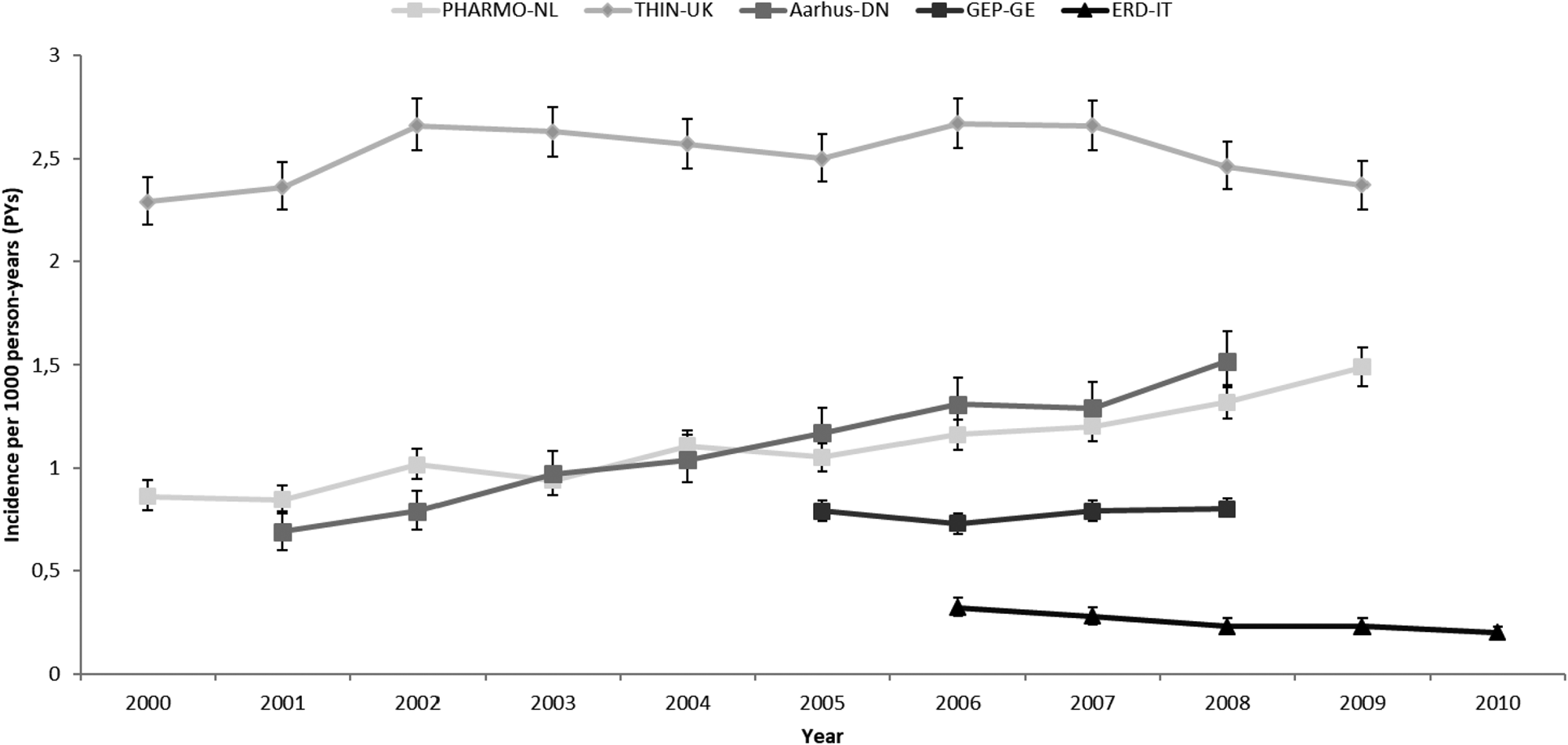
Fig. 2. Incidence of AP use by country and calendar year. PHARMO-NL: PHARMO Database Network, the Netherlands. THIN-UK: The Health Improvement Network, United Kingdom. Aarhus-DN: Aarhus University Hospital Database, Denmark. GEP-GE: German Pharmacoepidemiological Research Database (GePaRD), Germany. ERD-IT: Emilia Romagna Regional database, Italy.
Increases over time in prevalence and incidence rates were observed in DN and NL. In DN, prevalence increased from 1.44 (CI 95%: 1.31–1.58; 2001) to 3.41/1000 PYs (CI 95%: 3.21–3.62; 2008) (+137% RC) and in NL, from 2.07 (CI 95%: 1.96–2.18; 2000) to 4.35/1000 PY (CI 95%: 4.19–4.52; 2009) (+110% RC). Incidence rates increased from 0.69 (CI 95%: 0.6–0.79; 2001) to 1.52/1000 PY (CI 95%: 1.39–1.66; 2008) (+137% RC) in DN and from 0.86 (0.79–1.93; 2000) to 1.49/1000 PYs (1.39–1.58; 2009) in NL (+73% RC).
In UK and Germany, no increase in the use of APs was seen. In UK, prevalence and incidence changed respectively, from 2.8 (CI 95%: 2.67–2.93; 2000) to 3.24/1000 PYs (CI 95%: 3.1–3.38; 2009) (+16% RC) and from 2.29 (CI 95%: 2.18–2.41; 2000) to 2.37/1000 PYs (CI 95%: 2.25–2.49; 2009) (+3.5% RC). Of note, the UK had the highest incidence rates observed among all countries. In Germany, prevalence changed from 1.53 (CI 95%: 1.46–1.6; 2005) to 1.74/1000 PYs (CI 95%: 1.67–1.82; 2008) (+14% RC) and incidence remained stable from 0.79 (CI 95%: 0.74–0.84; 2005) to 0.8/1000 PYs (CI 95%: 0.75–0.85; 2008) (+1.2% RC).
The only country where both prevalence and incidence of AP use slightly decreased over the years was IT. Prevalence amounted to 0.61 (CI 95%: 0.55–0.68) in 2006 and 0.34/1000 PYs (CI 95%: 0.3–0.38) in 2010 (−44% RC) and incidence was 0.32 (CI 95%: 0.28–0.37) in 2006 and 0.2/1000 PYs (CI 95%: 0.17–0.23) in 2010 (−37.5% RC).
During the entire study period, the overall use (both prevalence and incidence) of APs was higher with increasing age, with a maximal use observed between 15 and 18 years of age, and more prevalent in boys than girls at all ages (Fig. 3). However, maximal prevalence and incidence of use was observed in boys between 10 and 14 years of age in NL and in girls between 15 and 18 years in the UK. Moreover, the use of APs in children ⩽4 years of age was null in Denmark, limited in IT and the highest in NL followed by UK and Germany.
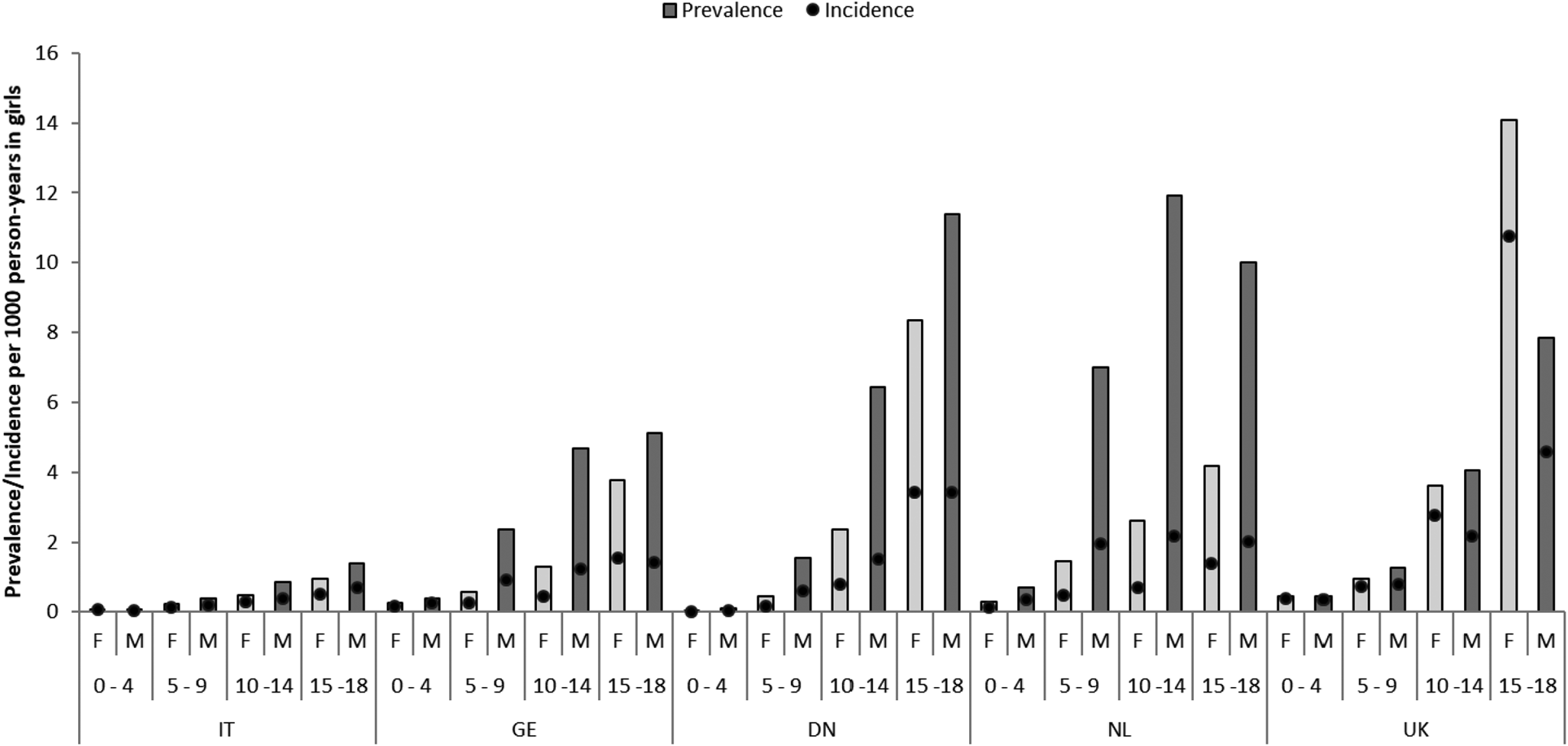
Fig. 3. Prevalence and incidence rates per sex, age group and country. NL: the Netherlands; UK: United Kingdom; DN: Denmark; GE: Germany; IT: Italy; F: female users; M: male users.
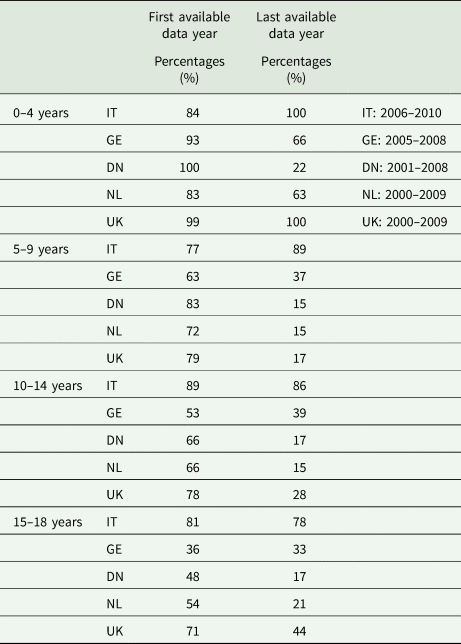
The frequency of AP dispensing by type of AP, age group and country for the year 2008 are illustrated in Fig. 4. Overall, SGAs were more frequently prescribed than FGAs and this is most obvious in the latest data years in all countries (Table 1). Yet, wide variation across different age groups was noted. In the youngest (⩽4 years), FGAs were clearly preferred with the exception of DN (22% of all prescriptions in 2008). The use of FGA decreased with age and years of study accounting for less than 50% of prescriptions in children above 4 years of age in all countries except IT in the most recent data-available year. In IT, FGAs remain frequently prescribed, 89% of all prescriptions in 5–9 years, 86% in 10–14 years and 78% in the 15–18 years age group. Of note, in the UK, FGA still accounted for 44% of all AP prescriptions in adolescents between 15 and 18 years of age, a percentage much higher than those observed in the other countries with the exception of IT. Variability in the use of FGA and SGA between countries and age groups is shown in Fig. 4 for the calendar year 2008 which was the only year with data available from all databases.
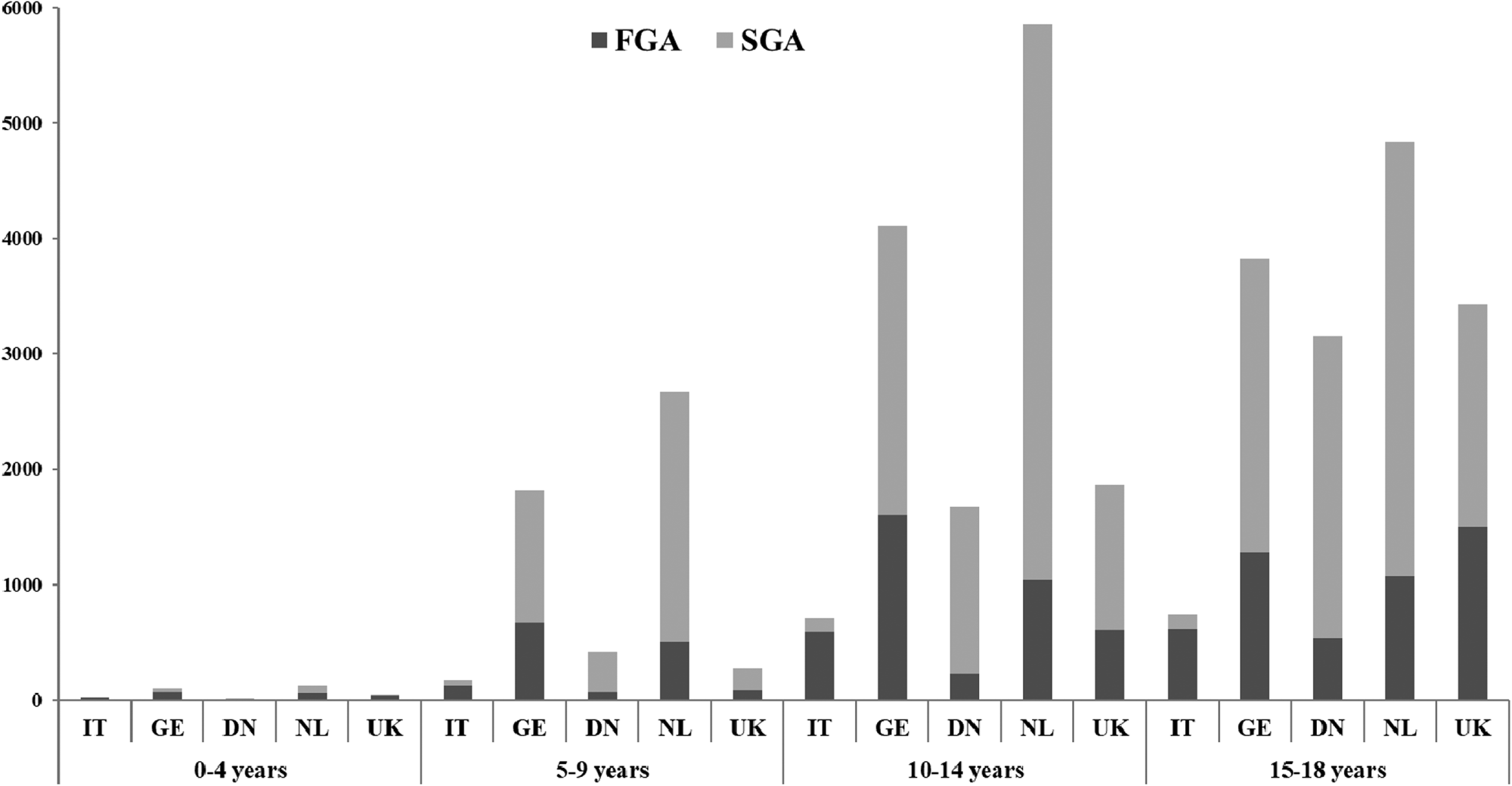
Fig. 4. Frequency of dispensing of AP drugs by type of AP, age group and country for year 2008. FGA: first generation antipsychotics, SGA: second generation antipsychotics. NL: the Netherlands; UK: United Kingdom; DN: Denmark; GE: Germany; IT: Italy
Table 1. Percentage of FGA prescriptions on total AP prescriptions in the first and the last available data years in the different countries
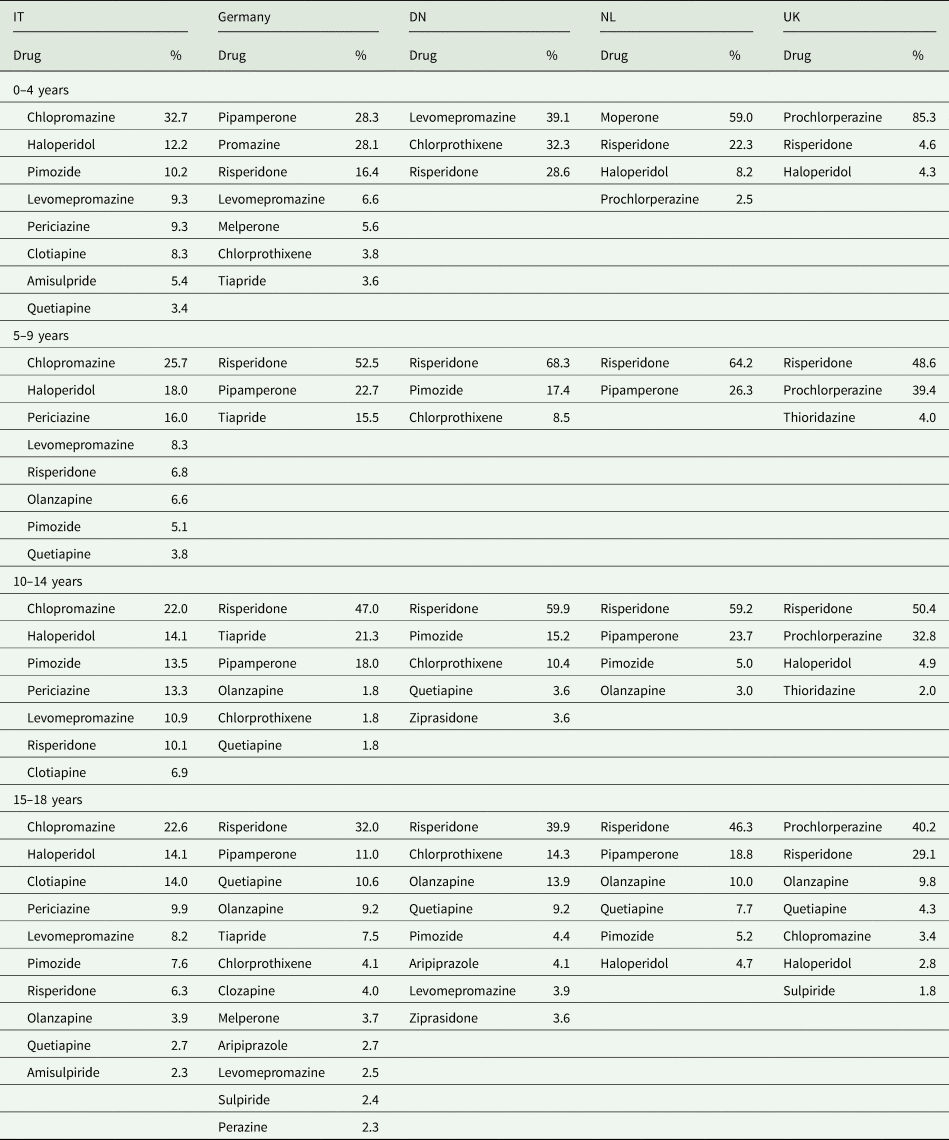
Ninety percent of all AP prescriptions (DU90%) in the population were covered by 9 drugs in IT, 8 in Germany, 6 in DN, 4 in NL and 5 in UK. Although, the number of drugs accounting for the DU90% increased with age, it is noteworthy that in the 0–4 years age group the number of prescribed drugs was higher than that in the 5–9 years group (Table 2). Risperidone is the most frequently prescribed AP in all countries with the exception of IT where chlorpromazine is generally prescribed at all ages. Also, prochlorperazine, an AP with mainly antiemetic properties, was the most commonly prescribed drug in the UK at all ages also prescribed in the NL in the ⩽4 years age group.
Table 2. APs that cover 90% of all prescriptions per country and age group during available data years
Discussion
This study provides a comprehensive overview of the use of APs in children and adolescents in five European countries: Denmark, Germany, Italy, the Netherlands and the United Kingdom. Prevalence and incidence of use varied widely between countries. The use was globally more important among adolescents however, that observed in younger age groups (5–9 years) was found to be comparatively high in the Netherlands. Prescriptions of SGAs were privileged over the years except for the youngest age groups where clinicians still favoured FGAs with the exception of one country.
In the recent years, several studies have alerted on the increasing rate of AP use worldwide in children and adolescents (Halfdanarson et al., Reference Halfdanarson, Zoega, Aagaard, Bernardo, Brandt, Fuste, Furu, Garuoliene, Hoffmann, Huybrechts, Kalverdijk, Kawakami, Kieler, Kinoshita, Litchfield, Lopez, Machado-Alba, Machado-Duque, Mahesri, Nishtala, Pearson, Reutfors, Saastamoinen, Sato, Schuiling-Veninga, Shyu, Skurtveit, Verdoux, Wang, Yahni and Bachmann2017; Kalverdijk et al., Reference Kalverdijk, Bachmann, Aagaard, Burcu, Glaeske, Hoffmann, Petersen, Schuiling-Veninga, Wijlaars and Zito2017) yet, there are few published AP utilisation studies in Europe. The present study showed that the point prevalence of AP use almost doubled over the years in countries like the NL and DN; however, the increase was moderate in the UK and Germany and the prevalence rate actually decreased in IT. These results are in accordance with some previously published studies. In the NL, estimations of prevalence in a previous study ranged from 3‰ in 1997 to 6.8‰ in 2005 (Kalverdijk et al., Reference Kalverdijk, Tobi, van den Berg, Buiskool, Wagenaar, Minderaa and de Jong-van den Berg2008). Accordingly, in DN where the prevalence of use was very low in 1996 (0.3‰), it increased over 6-fold in 2010 (Steinhausen and Bisgaard, Reference Steinhausen and Bisgaard2014). For these two countries, increasing rates were also reflected in the incidence rates and were confirmed in more recent international utilisation studies that covered larger study periods and different databases (Halfdanarson et al., Reference Halfdanarson, Zoega, Aagaard, Bernardo, Brandt, Fuste, Furu, Garuoliene, Hoffmann, Huybrechts, Kalverdijk, Kawakami, Kieler, Kinoshita, Litchfield, Lopez, Machado-Alba, Machado-Duque, Mahesri, Nishtala, Pearson, Reutfors, Saastamoinen, Sato, Schuiling-Veninga, Shyu, Skurtveit, Verdoux, Wang, Yahni and Bachmann2017; Kalverdijk et al., Reference Kalverdijk, Bachmann, Aagaard, Burcu, Glaeske, Hoffmann, Petersen, Schuiling-Veninga, Wijlaars and Zito2017). It is highly probable that this is related to frequent ‘off-label’ prescription of APs, particularly in the youngest, mainly to treat ADHD, conduct and behavioural disturbances (aggression, self-injury, disruptive behaviour, etc.) and mood disorders without solid efficacy and safety evidence for this practice (Penfold et al., Reference Penfold, Stewart, Hunkeler, Madden, Cummings, Owen-Smith, Rossom, Lu, Lynch, Waitzfelder, Coleman, Ahmedani, Beck, Zeber and Simon2013; Baribeau and Anagnostou, Reference Baribeau and Anagnostou2014; Hawton et al., Reference Hawton, Witt, Taylor Salisbury, Arensman, Gunnell, Hazell, Townsend and van Heeringen2015; Loy et al., Reference Loy, Merry, Hetrick and Stasiak2017). Nevertheless, increasing public awareness of pedopsychiatric disorders, greater acceptance of the use of psychotropic medications in children and a subsequently increasing demand for quickly effective therapies in these countries may stimulate AP prescribing. National pedopsychiatry therapeutic guidelines can also explain such practices and inter-country differences though most of the guidelines in Europe plead for non-pharmacological therapeutic approaches especially in AP ‘off-label’ indications such as ADHD or neurotic disorders (Taylor et al., Reference Taylor, Dopfner, Sergeant, Asherson, Banaschewski, Buitelaar, Coghill, Danckaerts, Rothenberger, Sonuga-Barke, Steinhausen and Zuddas2004; Hodgkins et al., Reference Hodgkins, Setyawan, Mitra, Davis, Quintero, Fridman, Shaw and Harpin2013). Regulatory approvals for APs are also harmonised across Europe and thus less susceptible to explain inter-country differences in use. Therefore, reasons for increasing AP use in NL and DN should be specifically explored.
In the UK, prevalence of AP use doubled between 1992 and 2005 (0.39 to 0.77‰) (Rani et al., Reference Rani, Murray, Byrne and Wong2008) but our estimations, although comparatively higher, showed that the increase between 2000 and 2009 was slight. Apart from the differences in study period and data sources used, another possible explanation for the higher prevalence estimation in the present study is that it also included the use of prochlorperazine, which is an AP frequently prescribed in the UK as an antiemetic agent. The latter may also explain why the number of new AP users was close to the number of prevalent users in the country and that incidence of use was the highest observed among all countries. In Germany, recent studies described prevalence ranging from 2.03‰ in 2006 to 2.61‰ in 2011 (Schroder et al., Reference Schroder, Dorks, Kollhorst, Blenk, Dittmann, Garbe and Riedel2017) and 3.3‰ in 2012 (Kalverdijk et al., Reference Kalverdijk, Bachmann, Aagaard, Burcu, Glaeske, Hoffmann, Petersen, Schuiling-Veninga, Wijlaars and Zito2017). Our prevalence estimations are lower which is probably related to the difference in study periods, study populations and/or methodological approaches. Nonetheless, they described the same discreet increment in use over the years. Both in the UK and in Germany, incidence rates were unchanged during the study period. Hence, the slight increases in prevalence observed were most probably due to an increase in the duration of the AP treatment rather than an increase in the number of prescriptions to newly diagnosed users.
Finally, a previous Italian study described a decrease in the prevalence of AP use from 0.63‰ in 1998 to 0.53‰ in 2004 (Clavenna et al., Reference Clavenna, Rossi, Derosa and Bonati2007) and this tendency was confirmed in our updated data. Globally, the increase in the use of APs observed worldwide has been largely attributed to their off-label use in pedopsychiatry. However, it is possible that in Italy other classes of psychotropic drugs such as antidepressants may be more largely prescribed especially in behaviour disorders or autism (Clavenna et al., Reference Clavenna, Rossi, Derosa and Bonati2007; Clavenna et al., Reference Clavenna, Andretta, Pilati, Dusi, Gangemi, Gattoni, Lombardo, Zoccante, Mezzalira and Bonati2011; Piovani et al., Reference Piovani, Clavenna, Cartabia and Bonati2016). Also, the low prevalence of AP use and the very low prescription rate of SGAs observed in the ERD might be explained by the fact that, in Italy, these drugs are partially dispensed through direct distribution from local psychiatric services and cannot be captured in the outpatient pharmaceutical dispensing flow. As a consequence, an underestimation of the use of APs especially SGA may have occurred.
Altogether, European prevalence rates were lower than those described in the US (39.4‰ per 2-year interval) (Cooper et al., Reference Cooper, Arbogast, Ding, Hickson, Fuchs and Ray2006) but in Canada, prevalence is comparable to that in the NL (1.66 in 1996 to 6.37% in 2001) (Ronsley et al., Reference Ronsley, Scott, Warburton, Hamdi, Louie, Davidson and Panagiotopoulos2013) although data were not available on the same study period.
Another interesting finding was the age distribution of AP prescriptions. Countries like NL have a very high use of APs in the youngest age groups (0–4 years and 5–9 years) followed by the UK, Germany and Denmark whereas in IT, use is very low in children of less than 9 years of age. Onset of psychotic disorders typically occurs in adolescence as opposed to conduct or autistic disorders associated with aggressive behaviour that can be detected in rather young ages. Therefore, high use in the youngest underlines the off-label use of AP agents for the treatment of a wide range of psychiatric illnesses without solid efficacy evidence and despite major concerns regarding their safety profile especially in very young children (Fraguas et al., Reference Fraguas, Correll, Merchan-Naranjo, Rapado-Castro, Parellada, Moreno and Arango2011; Ho et al., Reference Ho, Panagiotopoulos, McCrindle, Grisaru and Pringsheim2011; Kaguelidou and Acquaviva, Reference Kaguelidou and Acquaviva2016; Pisano et al., Reference Pisano, Catone, Veltri, Lanzara, Pozzi, Clementi, Iuliano, Riccio, Radice, Molteni, Capuano, Gritti, Coppola, Milone, Bravaccio and Masi2016). The extent of the off-label use highly varies between countries and it would be useful to monitor this use by assessing the ratio of prevalence rates as described in this study. On the other hand, gender differences with a higher use in boys compared to girls have been observed as expected in these pathologies (Ronsley et al., Reference Ronsley, Scott, Warburton, Hamdi, Louie, Davidson and Panagiotopoulos2013; Kalverdijk et al., Reference Kalverdijk, Bachmann, Aagaard, Burcu, Glaeske, Hoffmann, Petersen, Schuiling-Veninga, Wijlaars and Zito2017). The only exception was in the UK where girls in the 15–18 years of age had the higher prevalence and incidence of use mainly due to the prescription of prochlorperazine.
The nature and the number of prescribed AP agents also varied among countries and age groups. Altogether, there is a clear tendency in limiting FGAs prescribing over the years. Risperidone was the most frequently prescribed SGA in almost all countries. This is highly consistent with numerous national and international AP utilisation reports (Rani et al., Reference Rani, Murray, Byrne and Wong2008; Ronsley et al., Reference Ronsley, Scott, Warburton, Hamdi, Louie, Davidson and Panagiotopoulos2013; Halfdanarson et al., Reference Halfdanarson, Zoega, Aagaard, Bernardo, Brandt, Fuste, Furu, Garuoliene, Hoffmann, Huybrechts, Kalverdijk, Kawakami, Kieler, Kinoshita, Litchfield, Lopez, Machado-Alba, Machado-Duque, Mahesri, Nishtala, Pearson, Reutfors, Saastamoinen, Sato, Schuiling-Veninga, Shyu, Skurtveit, Verdoux, Wang, Yahni and Bachmann2017). Switch in the early 2000s was initially triggered by the fact that SGAs were marketed as being overall safer than FGAs. Since then, SGAs have indeed been associated with a lower risk of neurological adverse reactions than FGAs but they have been clearly associated with a higher risk of weight gain and metabolic abnormalities in both adults and children (Maher et al., Reference Maher, Maglione, Bagley, Suttorp, Hu, Ewing, Wang, Timmer, Sultzer and Shekelle2011; Seida et al., Reference Seida, Schouten, Boylan, Newton, Mousavi, Beaith, Vandermeer, Dryden and Carrey2012; Caccia, Reference Caccia2013). Also, despite initial expectations, variability in APs' safety profile appears to be greater among specific SGA agents than between FGA and SGA classes (Fraguas et al., Reference Fraguas, Correll, Merchan-Naranjo, Rapado-Castro, Parellada, Moreno and Arango2011; Masi and Liboni, Reference Masi and Liboni2011; Kaguelidou and Acquaviva, Reference Kaguelidou and Acquaviva2016). Yet, based on our findings, the switch from FGAs to SGAs concerned essentially the older paediatric age groups. In the youngest groups, FGAs were still commonly prescribed even in the most recent study years underlining the fact that in paediatric medical practice, new molecules without specific marketing authorisation are initially prescribed ‘off-label’ in adolescents and older children until more knowledge becomes available on their efficacy and safety. Certainly, inter-country differences observed in the choice of prescribed molecules are directly related to differences in market availability, variation in the diagnosis of paediatric psychiatric disorders, prescribers' habits and therapeutic approaches.
The results of the present study should be considered in view of some limitations. Firstly, we used outpatient prescription/dispensing data and had no information about the actual adherence of patients to their treatment and therefore, the real use of these medications. In addition, use in inpatient/institutionalised children and adolescents has not been assessed although it is unlikely that an AP therapy initiated in an hospital/institution would be discontinued in the outpatient setting especially since recommendations urge to minimise inpatient journeys in minors. Secondly, we do not directly compare national utilisation data given the differences in the nature of corresponding databases. Also, we acknowledge quantitative differences with some more recent studies published in the field. However, despite such differences, conclusions remain the same; some European countries have an already high and increasing rate of AP use (north of Europe) where in others, including UK and Germany, the rates are less important and increasing in a slower pace. In addition, we present data from Italy, a country that had not been previously included in multinational studies. Finally, we did not describe AP polypharmacy or therapeutic associations with other psychotropic drug classes as this was beyond the scope of the present study.
Conclusions
The use of APs varies widely among European countries, with some presenting an increase in use over the years and others, a stabilisation or slight decrease. While the use is overall higher in adolescents, in some countries there is a clear increase of AP prescribing in younger children. SGAs overall dominate prescribing preferences but FGAs are still prescribed in the youngest in varying proportions. The use of APs in children of ⩽9 years of age underlines their off-label use and should be carefully monitored as the risk/benefit ratio of these medications remains unclear especially in the youngest. Future studies should focus on exploring factors that drive AP use in children and adolescents in each country specifically. As the regulatory context regarding APs is quite homogenous in Europe, it is possible that societal and parental awareness and demands as well as prescribers' preferences play a determining role.
Acknowledgements
None.
Financial support
The current study is part of the EU-funded ARITMO Research and Development project funded by the Health Area of the European Commission under the VII Framework Programme (FP7/2007–2013) under grant agreement no. 241679-the ARITMO project.
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this study.








