INTRODUCTION
During the first 3 years (2003–2005) of enhanced surveillance for invasive Streptococcus pyogenes (iGAS) disease in Greece, we observed erythromycin resistance – primarily mef(A)-mediated – at a rate of 11·9%, and a high prevalence of emm-type 1 strains (fully susceptible to erythromycin) and emm-type 12 strains [Reference Stathi1]. This surveillance covered both adult and paediatric cases and presented for the first time data from Greece, as part of the Strep-EURO initiative [Reference Stathi1]. Preliminary observations regarding the epidemiology within each age group led us to further investigate possible epidemiological and clinical differences of iGAS infections between the two groups, during the extended period of 2003–2007, as similar comparable data are scarce in the literature and the majority of previous studies simply presented results on overall iGAS infections, or compared invasive and superficial infections [Reference O'Loughlin2–Reference Megged17]. In addition, typing of the isolates using emm-gene sequencing was performed, in order to assess strain variation.
MATERIALS AND METHODS
The study was conducted prospectively from 2003 to 2007; during 2003–2005, it was part of the Strep-EURO initiative [Reference Stathi1]. All enrolled cases were hospitalized in 17 general hospitals, 13 of which were in the Athens metropolitan area, two in northern, one in southern and one in western Greece. Reporting was on a voluntary basis. The study area covered about 40% of the total Greek population (2001 census: 10 964 020) (http://www.statistics.gr/portal/page/portal/ESYE/PAGE-themes?p_param=A1604).
Cases were identified as patients with S. pyogenes isolated from a normally sterile site, and diagnosed with streptococcal toxic shock syndrome (STSS), as defined previously by the Working Group on Severe Streptococcal Infections [Reference Breiman18], necrotizing fasciitis (NF), cellulitis/soft tissue infections (CSTI), osteomyelitis, arthritis, meningitis, primary bacteraemia with and without an apparent focus, empyema, pneumonia, and lymphadenitis. Only one isolate (the first) per patient was included in the study, except where putatively different strains [based on different minimum inhibitory concentrations (MICs)] were isolated from repeat specimens or different body sites.
Isolates were identified as S. pyogenes using standard methodology [Reference Stathi1]. Susceptibility testing to penicillin, erythromycin, clindamycin, tetracycline and vancomycin was performed using the disk diffusion method according to CLSI guidelines [19]. MICs to erythromycin, clindamycin and tetracycline were determined by a gradient strip method (Etest, bioMérieux, France) according to the manufacturer's instructions. All results were interpreted using CLSI breakpoints [20]. Erythromycin-resistant isolates were screened for macrolide-lincosamide-streptogramin B (MLSB) resistance phenotypes as described previously [Reference Seppala21].
Crude DNA extracts were prepared using a previously published protocol [Reference Stathi1]. Assignment of emm-types was performed with PCR and DNA sequencing according to the protocols and the Blast-emm search engine of the CDC website (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). Presence of speA, speB and speC genes was investigated as described previously [Reference Stathi1].
The following data were collected prospectively, using the same standardized questionnaire throughout the study period, from patients' charts and the attending physician: clinical presentation (as described above), treatment [surgical intervention, intensive-care unit (ICU) admission, ventilatory assistance], outcome within the first 7 days after onset of the symptoms (successful treatment, improvement, or death), and risk factors present at least 4 weeks prior to infection (trauma/skin lesion, prior surgical operation, pharyngotonsillitis, varicella infection, immunosuppression, malignancy, steroid use, intravenous drug abuse, diabetes mellitus, impetigo, alcohol abuse).
Statistical analysis was performed using SPSS v. 16.0 statistical software (SPSS Inc., USA). The patients were divided in two age groups for comparison: paediatric cases (<18 years) and adult cases (⩾18 years).
For qualitative variables, absolute and relative frequencies were computed; mean (standard deviation; s.d.) and median (interquartile range) for quantitative variables. Mann–Whitney tests were used for age comparisons between the two groups. For comparison of proportions, χ 2 and Fisher's exact tests were used, as appropriate. χ 2 tests for homogeneity were computed in order to evaluate differences in the monthly or yearly distribution of isolates. In case of a significant association in the total sample, adjustments were made for age group (children or adults) using logistic regression models. All P values reported are two-tailed and statistical significance was set at 0·05.
RESULTS
A total of 142 cases of invasive S. pyogenes infections were enrolled in the study. The basic microbiological data of the first 101 cases (period 2003–2005) have been presented previously [Reference Stathi1]. Isolates were derived from blood (48% of cases), deep soft tissue (sampling performed during surgery, 37%), synovial fluid (7%), pleural fluid (4%), cerebrospinal fluid (2%) and bronchoalveolar secretions (1%). Only the first isolate for each case was included.
Patients' age ranged from 6 months to 90 years (mean 20 years). A total of 96 (68%) cases were paediatric (mean age 5·4 years, s.d. = 3·27) and 46 (32%) cases were adults (mean age 50 years, s.d. = 19·65). Of the paediatric cases, 52 (54%) were boys, and 44 (46%) girls; of the adult cases, 32 (70%) were men and 14 (30%) women.
Clinical manifestations included bacteraemia with or without focus (96 cases, 67·6%) of which primary bacteraemia (without any apparent focus) was detected in 20 cases, CSTI (64, 45·1%), STSS (19, 13·4%), NF (17, 12·0%), lymphadenitis (16, 11·3%), arthritis (12, 8·5%), pneumonia (11, 7·7%), osteomyelitis (8, 5·6%), empyema (7, 4·9%), myositis (4, 2·8%) and meningitis (3, 2·1%). A total of 104 patients presented with two or more clinical conditions.
All isolates were fully susceptible to penicillin or vancomycin. A total of 22 (15·5%) isolates were resistant to erythromycin (EryR; MIC range 1 to >256 mg/l), of which six were additionally resistant to clindamycin (MIC >256 mg/l), expressing the constitutive resistant (cMLSB) phenotype. Of the remaining 17 EryR isolates, six belonged to the M phenotype, and 11 to the inducible resistant (iMLSB) phenotype. Most (7/11) of the iMLSB strains were isolated during 2006 and 2007, with a parallel disappearance of the M phenotype during the same period (data not shown). Forty-three (30·3%) isolates were non-susceptible to tetracycline (TetR), of which eight were intermediately resistant (MIC = 4 mg/l) and 35 fully resistant (MIC ⩾8 mg/l). No significant differences were recorded regarding antibiotic resistance between the adult and paediatric age groups.
Varicella and streptococcal pharyngotonsillitis, were the main predisposing factors in the paediatric group (Table 1), whereas in adults, malignancy, diabetes mellitus, and intravenous drug or alcohol abuse were detected more often.
Table 1. Significant differences in children and adults with respect to clinical and epidemiological characteristics
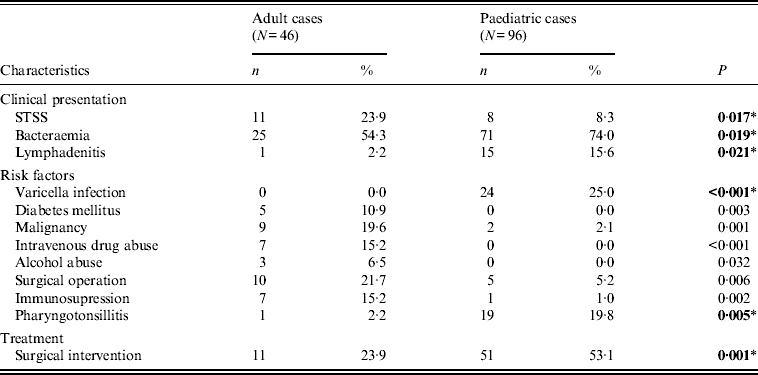
STSS, Streptococcal toxic shock syndrome.
* All P values were calculated using Fisher's exact test, except those indicated by an asterisk, which were calculated using χ 2.
Bold values indicate significant differences that were confirmed with logistic regression analysis.
While bacteraemia was the major clinical presentation in both age groups, it was significantly more frequent in children, as was lymphadenitis. On the other hand, STSS was more frequent in adults (Table 1); nevertheless, it proved more fatal in children (Table 2), as those that presented with STSS were more likely to die within 7 days, compared to non-STSS cases, a difference not detected in adults.
Table 2. Significant differences between paediatric and adult patients, regarding the correlation of specific clinical and microbiological characteristics with clinical severity, outcome and predisposing risk factors

ICU, Intensive care unit; n.s., not significant; STSS, streptococcal toxic shock syndrome; NF, necrotizing fasciitis.
* All P values were calculated using Fisher's exact test except that indicated by an asterisk, which was calculated using χ 2.
Bold values indicate significant differences that were confirmed with logistic regression analysis.
A total of 138 strains were emm-typed successfully and assigned to 31 different emm-types. Overall, the most common emm-types (with a predominance of ⩾5%) were emm-types 1 and 12 (28·2 and 8·5%, respectively), together comprising over a third of all isolates. As shown in Figure 1, the proportion of infections due to emm-type 12 remained relatively stable during the whole study period. In contrast, that of emm-type 1 fell sharply (P = 0·026) in 2005, rising gradually to reach its 2003 levels, by 2007.
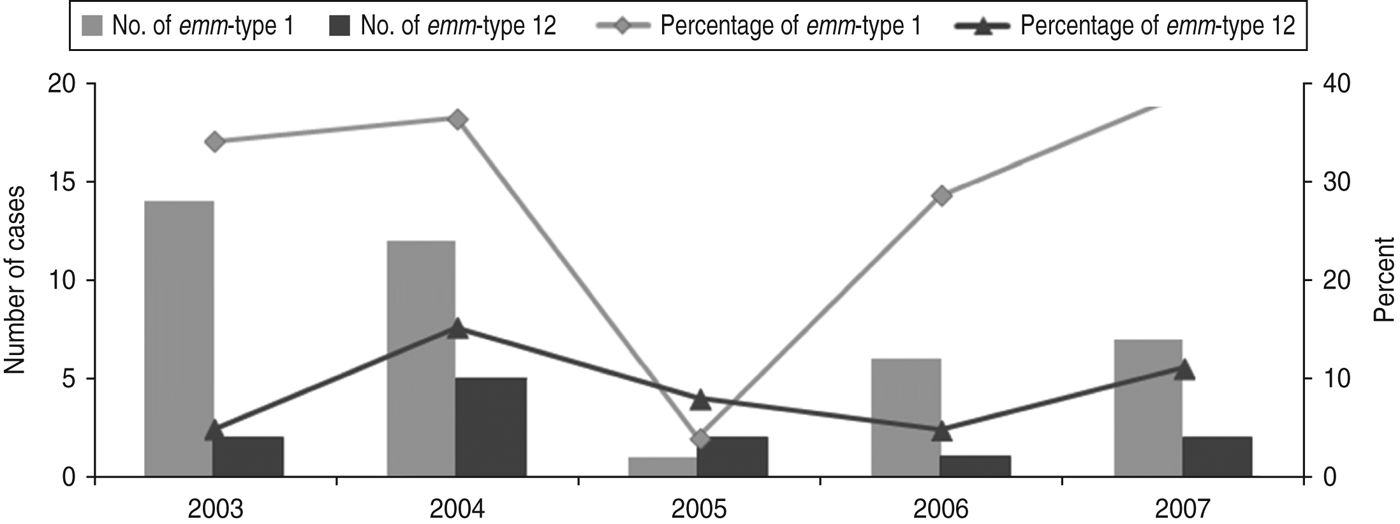
Fig. 1. Time fluctuation of invasive diseases due to emm-types 1 and 12. The percentage values refer to the proportion of all invasive disease attributable to emm-types 1 and 12. The number of emm-type 1 and emm-type 12 cases per year is plotted on the primary y-axis and the percentage on the secondary y-axis.
In contrast to adults, where emm-type 1 was the only predominant type (12/46, 26·1%), a great variety was observed in children: emm-types 1 (28/96, 29·2%), 12 (10/96, 10·4%), 22 (7/96, 7·3%), 4 (5/96, 5·2%), and 77 (5/96, 5·2%). Furthermore, emm-types 22, 28, 75, 78, 83, 84 and 115 were detected exclusively in paediatric isolates, whereas emm-types 19, 44, 80, 87, 101, 113, and 117 were detected exclusively in isolates from adults. Nevertheless, these emm-types were represented by only a few isolates each, thus rendering statistical evaluation impossible.
However, even for the two types common to both age groups, age-related differences could be observed, as shown in Tables 2–4, although these differences were not always confirmed by logistic regression analysis. For example, when comparing erythromycin or tetracycline susceptibility, STSS, empyema and lymphadenitis in relation to emm-type (the predominant emm-type 1, or other emm-types) within the two age groups, a statistical difference was detected within the paediatric group but not within the adult group (Table 3). In addition, a serious clinical condition, using ICU admission as an indicator, was more likely to be due to an emm-type 1 strain infection, and related to rash and respiratory distress syndrome (RDS) in children (Table 2). Finally, an association between the presence of the speA gene and emm-type 1 was detected within all age groups, as well as between the presence of speC gene and emm-type 1, but only within the paediatric group (Table 4).
Table 3. Significant differences between emm-type 1 and emm-type 12 cases compared to all other emm-type cases within each of the two age groups
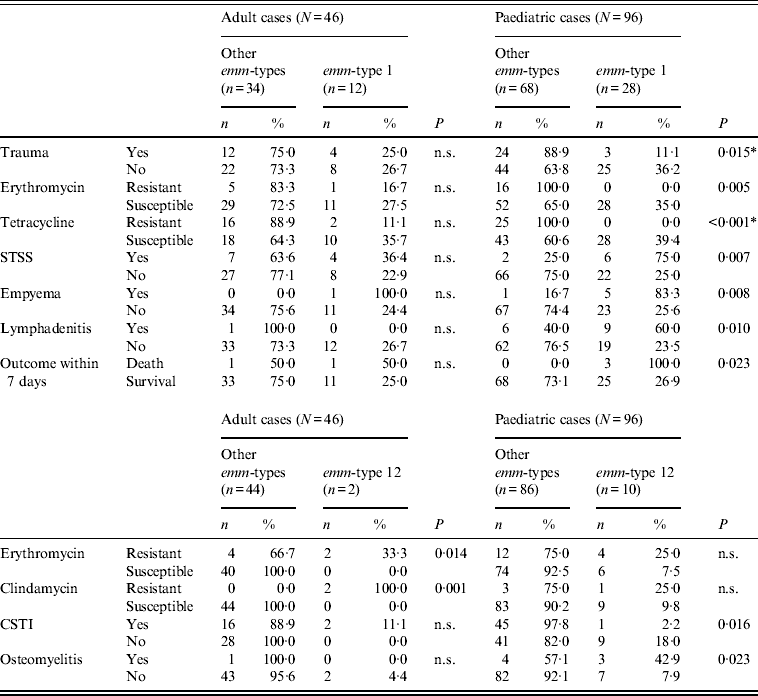
n.s., Not significant; STSS, streptococcal toxic shock syndrome; CSTI, cellulitis/soft tissue infections.
* All P values were calculated using Fisher's exact test except those indicated by an asterisk, which were calculated using χ 2.
Table 4. Significant differences between the presence and absence of speA and speC toxin genes, emm-types 1 and 12, STSS and outcome within the two age groups
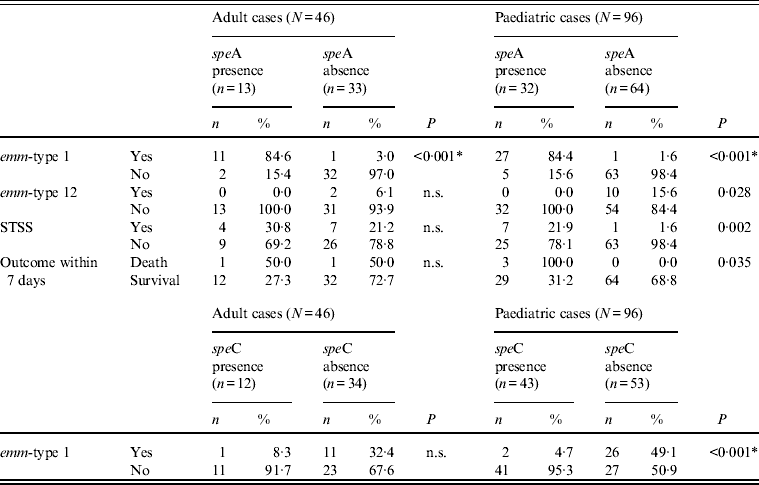
n.s., Not significant; STSS, streptococcal toxic shock syndrome.
* All P values were calculated using Fisher's exact test except those indicated by an asterisk, which were calculated using χ 2.
Regarding all other microbiological, clinical or epidemiological parameters, no significant differences were detected between the age groups.
DISCUSSION
Since the early 1990s, different studies [Reference O'Loughlin2–Reference Megged17], including those of the multi-centre European initiative Strep-EURO [Reference Darenberg6–Reference Luca-Harari9], have attempted to address the problem of increasing invasive S. pyogenes (iGAS) diseases. Nevertheless, only in a few of these studies was a direct and thorough comparison of putative epidemiological and clinical differences between adult and paediatric iGAS disease attempted [Reference Huang13, Reference Megged17]. Therefore, our study focused on investigating possible differences between adults and children, with respect to the emm-types, risk factors, clinical presentation, treatment, and outcome.
The study was prospective and on a voluntary basis for participating hospitals. Therefore, underreporting cannot be ruled out. In addition, the extrapolation of the data presented here to the total of the Greek territory should be made with caution, as no previous comparable study exists. Nevertheless, it should be noted that the areas mentioned above were simultaneously covered with respect to both their adult and paediatric populations, rendering patient samples comparable.
Some of the age-specific differences we observed, such as lower STSS rates and higher varicella zoster incidence in paediatric infections, have been described previously [Reference Laupland11], as has the fluctuation in the emm-type 1 isolation rate [Reference Wahl4–Reference Darenberg6, Reference Passaro10], which has been suggested to peak periodically every few years. Nevertheless, we were not able to confirm all the differences using logistic regression analysis, possibly due to the limited number of cases represented in each category. It is of interest that specific emm-types were detected only in paediatric or adult cases, to our knowledge an observation reported for the first time, although their limited number made statistical evaluation difficult.
Despite the importance of varicella infection and pharyngotonsillitis as risk factors, about half of paediatric cases occurred in previously healthy children, in contrast to adult cases where numerous risk factors were present in >90% of cases, which is similar to previous observations [Reference Bingöl-Koloğlu12, Reference Huang13]. Varicella infections in healthy children have been associated with increased risk of iGAS infections, including clinical presentations not directly related to skin. Possible immunosupression of the T-cell-mediated immunity [Reference Laupland11, Reference Zurawski14, Reference Fujimura22] has been suggested as an explanation.
Regarding clinical presentation, STSS in paediatric cases was associated with emm-type 1 (leading also to higher mortality, as indicated by the increased number of deaths within the first week), whereas, in adult cases, it was not associated with a specific emm-type – a difference, to our knowledge, not hitherto reported. The immaturity of the paediatric immunological system, coupled with the epidemiological differences in the emm-types detected between the age groups may explain the differences. This observation, if confirmed to be of clinical importance in further studies with higher patient enrolment, could be used to design age-focused prevention strategies. In contrast to STSS, we detected no clear association between other serious clinical conditions (e.g. NF or CSTI) and a risk factor within any specific age group. An exception was trauma as a risk factor for NF within the paediatric group and for CSTI in all cases, an observation also reported previously [Reference Laupland11, Reference Huang13, Reference Davies15].
The other dominant emm-type, 12, was associated with varicella as a risk factor. The literature on this is conflicting, as one study [Reference Tyrrell16] reported that varicella was associated with emm-types 1 and 3, whereas in another study [Reference Laupland11] no association was detected. Differences in the epidemiology of skin vs. throat isolates and the specific study population may offer a possible explanation.
Finally, the presence of the speA gene (its expression not having been assessed) was associated with emm-type 1 in both age groups, and with emm-type 12 isolates, STSS and death during the first week only within the paediatric population. As STSS is usually mediated by the SpeA pyrogenic exotoxin, this association between the speA gene, emm-type 1 and STSS may indicate that these strains actually expressed the gene. In addition, the association within the paediatric population may be explained by the fact that this population has been exposed to the pathogen to a lesser extent than adults, resulting in limited herd immunity and lower anti-SpeA antibody titres, which offer some protection against the toxin [Reference Musser23–Reference Schlievert, Assimacopoulos and Cleary25].
In conclusion, apart from a major fluctuation with time of the predominant emm-type 1, responsible for invasive disease of particularly high severity in children, we also observed several significant clinical and epidemiological differences in the two age groups with invasive S. pyogenes disease. These differences could prove useful in designing age-focused strategies for intervention and prevention, empirical antimicrobial chemotherapy, or vaccine candidates.
APPENDIX. The Hellenic Strep-EURO Study Group
Avlamis A., Foustoukou M., Gizaris V., Iordanidou M., Kanellopoulou M., Kondyli L., Kouppari G., Levidiotou-Stefanou S., Malamou-Ladas H., Makri A., Paniara O., Perogambros A., Petroxeilou V., Vogiatzi A., Tsagaraki A.
ACKNOWLEDGEMENTS
During 2003–2005, this study was part of the EU-funded Strep-EURO surveillance project (5th Framework Program, QLK2-CT-2002-01398). Helpful suggestions and guidance from Dr Aftab Jasir and Dr Androulla Efstratiou throughout that project are gratefully acknowledged.
DECLARATION OF INTEREST
None.







