In the UK there are over 55 000 new cases of breast cancer diagnosed every year(1). Worldwide the incidence varies geographically, with a higher incidence in Western populations compared to those in Africa and East Asia(2). Whilst a small number of cases can be attributed to a germline mutation in a high penetrance breast cancer susceptibility gene (e.g. BRCA1/BRCA2), a significant proportion is thought to be related to modifiable risk factors(Reference Loibl, Poortmans and Morrow3). These modifiable risk factors include high BMI in the postmenopausal state, physical inactivity and alcohol, as well as exogenous female hormone use and reproductive factors(Reference Britt, Cuzick and Phillips4).
Rates of obesity in the UK are rising with approximately 28 % of adults in England currently being classed as having obesity and over 36 % overweight(5). It is estimated that 23 % of breast cancer cases in the UK are preventable, with 8 % of cases being attributable to higher BMI(1,5) . These high rates of obesity are likely to be contributing to the rising incidence of breast cancer. In turn, the relationship between obesity and breast cancer is resulting in a higher prevalence of breast cancer patients with overweight or obesity(Reference Britt, Cuzick and Phillips4).
The management of breast cancer depends on patient fitness and comorbidities, and cancer stage and biological subtype. Modern oncological strategies are determined by the oestrogen receptor (ER) and HER2 receptor status of the cancer, with three main subtypes: ER positive/HER2 negative, ER positive or negative/HER2 positive and ER negative/HER2 negative (usually also progesterone negative and known as triple negative breast cancer)(Reference Waks and Winer6). Treatment of early breast cancer is multi-modal involving surgical resection, a range of systemic anti-cancer therapies (such as chemotherapy including chemotherapy, targeted therapy, immunotherapy), endocrine therapy for ER positive disease and radiotherapy(Reference Cardoso, Kyriakides and Ohno7).
Improvements in breast cancer treatment have led to an increase in the number of long-term survivors of breast cancer, in both those treated for early-stage disease as well as those living with metastatic breast cancer(8). This narrative review will describe the impact of lifestyle factors on breast cancer survivorship, the existing evidence on lifestyle interventions and the need for additional research and guidance in this expanding field.
Breast cancer and obesity
Obesity is becoming more prevalent in the UK and global population(9). Understanding the role of obesity in cancer and cancer outcomes is therefore becoming increasingly important. There is a known association between obesity and the development of thirteen different cancers including those of the endometrium, oesophagus, ovary and colon(Reference Lauby-Secretan, Scoccianti and Loomis10).
The link between elevated BMI and risk of developing breast cancer is dependent on menopausal status. A positive correlation is found in postmenopausal women, with an increased relative risk of developing breast cancer of 1⋅12 per 5 kg/m2 BMI increase(Reference Garcia-Estevez, Cortes and Perez11), however an inverse correlation with breast cancer risk and BMI in younger premenopausal women(Reference van den Brandt12,Reference Anderson, Renehan and Saxton13) . It has been postulated that this may be due to lower oestrogen levels in premenopausal women with a high BMI compared with those of normal BMI, caused by fewer ovulatory cycles and suppression of ovulatory function(Reference Garcia-Estevez, Cortes and Perez11). Postmenopausal women with a high BMI more frequently have hormone receptor positive disease than healthy-weight women(Reference Munsell, Sprague and Berry14,Reference Litton, Gonzalez-Angulo and Warneke15) . In premenopausal women obesity is associated with an increased risk of hormone receptor negative and triple negative breast cancer, which has a worse prognosis than other subtypes of breast cancer(Reference Picon-Ruiz, Morata-Tarifa and Valle-Goffin16).
Molecular mechanisms underlying obesity and breast cancer risk
Accumulation of adipose tissue is associated with the development of metabolic syndrome, characterised by insulin resistance, impaired glucose tolerance, reduced HDL cholesterol levels, raised TAG, abdominal obesity and hypertension(Reference Rochlani, Pothineni and Kovelamudi17). It is well documented that these factors increase the risk of CVD and diabetes, however the mechanisms by which obesity promotes tumourigenesis is less clear. A study of over 2000 women with breast cancer showed that the risk of distant metastases was more than double in those with metabolic syndrome(Reference Berrino, Villarini and Traina18).
Adipose tissue is comprised of multiple cell types, from adipocytes to endothelial cells and immune cells. It is an immunologically active tissue and is also involved in endocrine signalling, via adipokines(Reference Birts, Savva and Laversin19). Inflammatory cytokines, including TNFα and IL-6, are secreted, which can lead to a chronic inflammatory state and an increased risk of cancer. Higher levels of insulin and insulin-like growth factors are associated with an increased breast cancer risk(Reference Khandekar, Cohen and Spiegelman20). Higher circulating concentrations of insulin and insulin-like growth factors lead to reduced concentrations of sex-hormone-binding globulin, which result in an increased bioavailable fraction of oestradiol(Reference Ligibel21). Furthermore, in mouse models, a reduction in blood insulin and insulin-like growth factor 1 levels with a resulting inhibition of the PI3K–AKT–mTOR pathway, important for cell growth and survival, was associated with enhanced endocrine therapy activity(Reference Caffa, Spagnolo and Vernieri22). In postmenopausal women adipose tissue is the main site of oestrogen biosynthesis through aromatisation, therefore in those with a higher BMI there is a higher peripheral production of oestrogen which increases the risk of breast cancer(Reference Ligibel21). Aromatase inhibitors, a form of endocrine therapy, are not as effective in suppressing oestradiol levels in those with obesity compared to individuals with lower BMIs(Reference Pfeiler, Königsberg and Hadji23). These various mechanisms may not only increase the risk of developing breast cancer but also lead to worse outcomes in those with the disease(Reference Lorincz and Sukumar24).
Cancer survivorship
In the UK, cancer survivors are defined as any individual who has a personal history of cancer(Reference Jahan, Cathcart-Rake and Ruddy25). As outcomes from cancer treatment improve, there is a growing population of people surviving or living long-term with cancer. There are multiple components that contribute towards the care of cancer survivors (Fig. 1). However current cancer services are often poorly designed to optimally manage this population and provide adequate support for the unique issues cancer survivorship brings(Reference Jefford, Howell and Li26). Managing all aspects of survivorship requires coordination between specialist teams and primary care(Reference Jefford, Howell and Li26).
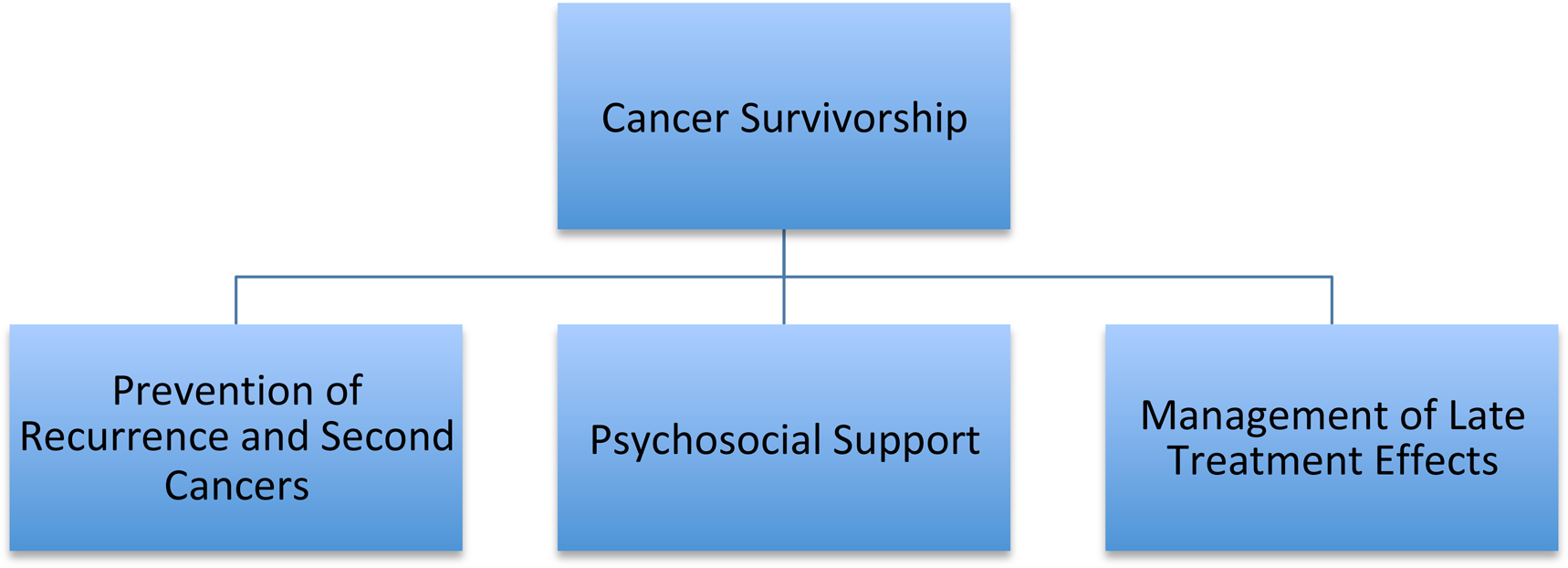
Fig. 1. Aspects of cancer survivorship.
Impact of lifestyle factors on recurrence following breast cancer diagnosis
Obesity predicts poorer outcomes in those with breast cancer regardless of menopausal status(Reference Protani, Coory and Martin27,Reference Copson, Cutress and Maishman28) . In a meta-analysis of eighty-two studies, including 213 075 patients with previously treated breast cancer, BMI prior to diagnosis was associated with a relative risk of total mortality of 1⋅41 for women with obesity when compared to women with a BMI in the healthy range(Reference Chan, Vieira and Aune29). When categorised by menopausal status the relative risk was 1⋅75 for pre-menopausal and 1⋅34 for post-menopausal breast cancer(Reference Chan, Vieira and Aune29). The reasons behind this observation are now considered to be multi-factorial, including biological factors, delays in diagnosis and treatment-related issues.
Several studies have reported that women with obesity have larger tumours and more metastatic axillary lymph nodes at diagnosis which may be due to difficulties in palpating small tumours due to body habitus(Reference Wang, Huang and Chagpar30). Therefore, although mammographic sensitivity is not affected by obesity, in those under the breast cancer screening age (currently 50 years in the UK), this may delay initial diagnosis(Reference Haakinson, Leeds and Dueck31). A study of over 2000 women found that 45 % of postmenopausal women with a BMI ≥25 kg/m2 had a tumour diameter of over 2 cm, compared to 33⋅4 % of those with a healthy-range BMI(Reference Biglia, Peano and Sgandurra32).
Another potential explanation is that patients with obesity may receive less effective treatment for breast cancer(Reference Lee, Kruper and Dieli-Conwright33). A study of 18 967 women treated for early-stage breast cancer found that chemotherapy and endocrine therapy seemed to be less effective after 10 years in patients with a BMI >30 kg/m2(Reference Ewertz, Jensen and Gunnarsdóttir34). There are known limitations to current methods of chemotherapy dosing, which are based on body surface area for the majority of chemotherapeutic agents(Reference Sparreboom, Wolff and Mathijssen35). An American study indicated that up to 40 % of patients with obesity receive capped chemotherapy doses not based on actual body weight due to concerns regarding toxicity; this may explain in part the poorer outcomes seen in this population(Reference Griggs, Mangu and Anderson36). There is limited evidence on the best way to dose chemotherapy in patients with overweight or obesity, with some studies reporting no increase in toxicity when chemotherapy is dosed according to actual body weight; however, this may not hold true for regimens containing anthracyclines and taxanes which are commonly used in early breast cancer(Reference Griggs, Mangu and Anderson36). The American Society of Clinical Oncology published guidance in 2012 regarding chemotherapy dosing in patients with obesity and concluded that full weight-based doses should be used predominantly, with a few notable exceptions for certain agents(Reference Griggs, Mangu and Anderson36). There is even less evidence on appropriate dosing of endocrine and targeted therapies(Reference Renehan, Harvie and Cutress37–Reference Furlanetto, Eiermann and Marmé39). A study of 297 patients treated with immune checkpoint inhibitors, which are used in triple negative breast cancer, found those patients with a high BMI had better outcomes with weight-based dosing rather than a fixed-dose strategy(Reference Ahmed, von Itzstein and Sheffield40).
Another vital component of early-stage breast cancer management is surgical resection. Obesity increases the incidence of wound complications, including infection(Reference El-Tamer, Ward and Schifftner41). This is important as delayed wound healing can lead to delays in other cancer treatments, including chemotherapy or radiotherapy, as well as potentially impacting future cosmetic outcomes and quality of life(Reference Sparreboom, Wolff and Mathijssen35). A study investigating outcomes following unilateral mastectomy found the incidence of minor and major complications was significantly increased in patients with obesity(Reference Garland, Hsu and Clark42). The complication rate is higher in those undergoing breast reconstruction, with wound dehiscence being 2⋅51 times more likely in women with obesity(Reference Garland, Hsu and Clark42,Reference Panayi, Agha and Sieber43) . A challenging complication of breast cancer and breast cancer treatment itself is lymphoedema which can occur due to axillary nodal disease, axillary dissection or radiotherapy and the risk of this is also increased in those with high BMI(Reference Iyigun, Duymaz and Ilgun44).
Post-operative radiotherapy is recommended routinely after breast-conserving surgery and to selected patients after mastectomy and increased toxicity has been noted in those with an increased BMI(Reference Rodriguez-Gil, Takita and Wright45). This may be due to dose inhomogeneity or increased doses to critical structures underlying the breast, such as the heart or lung(Reference Rodriguez-Gil, Takita and Wright45). Radiotherapy techniques are continuously improving and modifications can be made to reduce these complications, however technically this remains challenging in practice(Reference Krengli, Masini and Caltavuturo46).
Impact of lifestyle factors on treatment side effects
Late treatment toxicities associated with breast cancer treatments can include fatigue, premature ovarian failure, infertility, cardiotoxicity and increased risk of second malignancy(Reference Ewertz and Jensen47).
Cardiovascular health
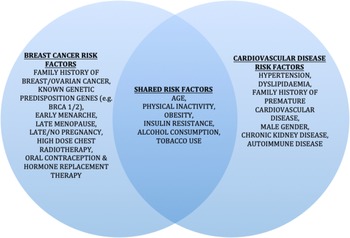
CVD and breast cancer are linked by shared risk factors (Fig. 2) and the cardiotoxic effects of certain breast cancer treatments(Reference Coughlin, Majeed and Ayyala48). CVD is the leading cause of death in breast cancer survivors over fifty, with an elevated risk of CVD in this population compared to age-matched controls (incidence rate ratio of 1⋅13)(Reference Blaes and Konety49).
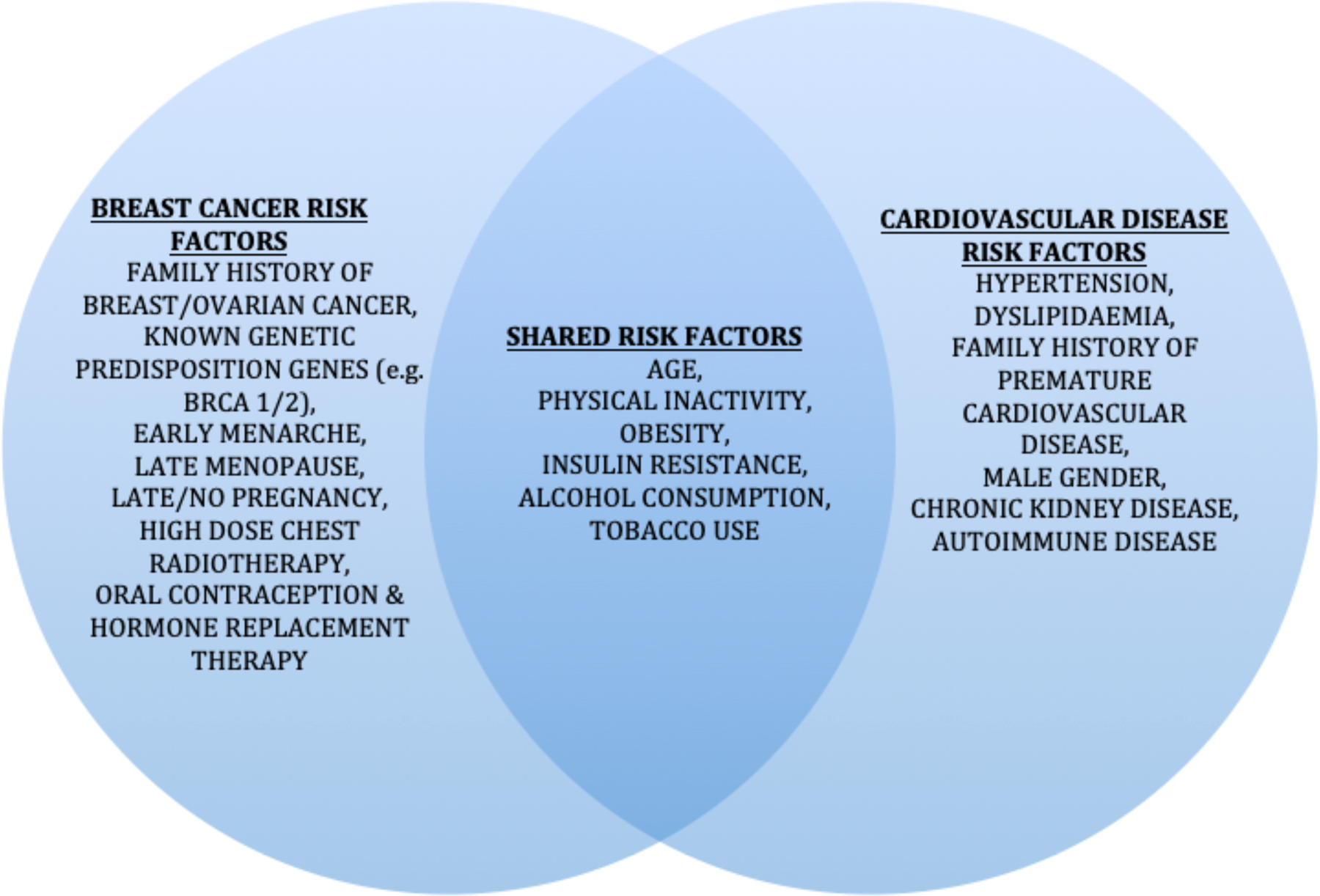
Fig. 2. Risk factors for breast cancer and CVD. BRCA1/2, breast cancer gene 1/2.
Anthracycline chemotherapy, trastuzumab and radiotherapy can increase the risk of cardiac disease and therefore focusing interventions in breast cancer patients and survivors to specifically improve cardiac health could be beneficial(Reference Greenlee, Iribarren and Rana50,Reference Chen, Yeh and Wei51) . There appears to be a protective role of physical activity in subclinical cardiac dysfunction, suggesting that physical activity programmes may help to reduce the impact of CVD in this group of patients(Reference Naaktgeboren, Groen and Jacobse52).
Bone health
Bone health is another key survivorship issue for patients with breast cancer as many breast cancer therapies affect bone health and increase the risk of osteoporosis. Chemotherapy can induce an early menopause and ovarian suppression may be used in premenopausal women as part of their treatment(Reference Ramaswamy and Shapiro53). Endocrine therapies also have an impact on bone health, with drugs such as tamoxifen mimicking menopause and aromatase inhibitors reducing peripheral oestrogen production in postmenopausal women(Reference Ramaswamy and Shapiro53,Reference Reid, Doughty and Eastell54) . There is extensive guidance on the medical management of bone health, including monitoring and medical interventions, however the beneficial effect of weight-bearing exercise on bone health is another positive reason why clinicians should promote physical activity in these patients(Reference Reid, Doughty and Eastell54,Reference Coleman, Body and Aapro55) .
Psychosocial issues
Psychosocial issues are also frequently experienced, from the disruption of body image and self-identity to increased rates of depression and anxiety(Reference Burney56). Breast surgery and premature menopause can impact body confidence and sexual function as well as libido(Reference Burney56). Understandably there is often a fear of recurrence and it can be difficult for patients to adjust to the reduced support received from oncological services after treatment for early breast cancer is completed(Reference Vachon, Krueger and Champion57). The regular clinic visits become less frequent, and whilst this can be a welcome relief for some patients, it can lead to a sense of abandonment in others(Reference Burney56,Reference Vachon, Krueger and Champion57) . All patients are unique and the effect these issues have on their quality of life will vary, however healthcare professionals need to ensure these issues are discussed openly with patients, who may feel reluctant to disclose their concerns(Reference Vaz-Luis, Masiero and Cavaletti58).
Effect of weight gain during and after treatment
Weight gain during and following treatment for breast cancer is common, with women gaining on average between 2⋅5 and 5 kg with gain of fat mass and loss of lean body mass(Reference Vance, Mourtzakis and McCargar59,Reference van den Berg, Winkels and de Kruif60) . A sub-study of the Prospective study of Outcomes in Sporadic versus Hereditary breast cancer (POSH), a prospective observational study of almost 3000 women aged ≤41 years at first breast cancer diagnosis showed 30 % of premenopausal women 293 with early breast cancer gained more than 5 % body weight, compared to their weight at diagnosis, and this was more common in those with a lower BMI at base-line(Reference Gandhi, Copson and Eccles61). There are many factors that may contribute to this phenomenon including reduced activity due to fatigue and other treatment effects, a change in menopausal status, steroid use in treatment, psychological impact of diagnosis and treatment leading to altered eating habits and metabolic changes(Reference Sedjo, Byers and Ganz62). There is a link with chemotherapy use, but no proven link to the use of endocrine therapy or radiation therapy(Reference Makari-Judson, Judson and Mertens63). Importantly, this may impact breast cancer outcomes. Follow-up of participants from the nurses' health study showed that those whose BMI increased between 0⋅5 and 2 kg/m2 or more than 2 kg/m2 had an increased risk of breast cancer-related death(Reference Kroenke, Chen and Rosner64). A meta-analysis of twenty-three trials (n 23 832) reported that weight gain of ≥10 % was associated with increased all-cause mortality (hazard ratio = 1⋅23, 95 % CI = 1⋅09, 1⋅39, P < 0⋅001) but there was heterogeneity of effect for more moderate weight gain(Reference Playdon, Bracken and Sanft65).
There is also an increase of lymphoedema and a rise of other obesity related diseases, as well as the impact weight gain may have on people's quality of life(Reference Makari-Judson66). Weight gain in the absence of fluid retention is currently not listed as a side effect on the systemic anti-cancer treatment consent forms used in the UK for standard early breast cancer chemotherapy regimens and as a result patients are frequently not advised regarding this risk. This clearly demonstrates an area that oncological services must recognise to ensure that patients are appropriately educated when they start their treatment.
Evidence for impact of lifestyle interventions on breast cancer survivors
Lifestyle interventions
It is challenging to study complex interventions such as lifestyle modifications independently, for example often increased physical activity alongside healthy dietary changes result in weight loss, making it difficult to assess the impact of each component. Whilst there is growing evidence of the positive impact of healthy lifestyle changes, there are limitations to the existing available evidence(67,Reference Ligibel, Basen-Engquist and Bea68) . The 2022 American Society of Clinical Oncology Guidance on exercise, diet and weight management during cancer treatment, concluded that regular exercise should be recommended but that there was limited evidence for specific dietary interventions or weight loss(Reference Ligibel, Bohlke and May69). A number of trials have investigated the effect of lifestyle interventions on outcomes of those with breast cancer, which we will discuss next. Whilst there are data to support the premise that lifestyle interventions can lead to weight loss in patients with breast cancer, there are little data on the effect of this on long-term cancer-related outcomes.
Weight loss
The women's intervention nutrition study and women's healthy eating and living studies looked at the impact of dietary interventions, including reducing fat intake and increasing vegetable and fruit intake, on breast cancer events and mortality(Reference Pierce70,Reference Chlebowski, Blackburn and Thomson71) . The women's healthy eating and living trial did not show a reduction in breast cancer events or mortality whilst participants in the women's intervention nutrition study trial assigned to the dietary intervention group did lose weight and there was a trend towards a reduction in breast cancer recurrence, although no statistically significant difference in overall survival(Reference Pierce70,Reference Chlebowski, Blackburn and Thomson71) .
The LISA trial investigated a telephone-based weight loss intervention in women with early breast cancer with a BMI >24 and aimed to look at disease-free survival and overall survival(Reference Goodwin, Segal and Vallis72). Unfortunately they were unable to meet their initial recruitment target, and this study was therefore underpowered for the primary endpoint of disease-free survival. Weight loss was significantly improved in those in the interventional arm of the study in the first 24 months, however this effect was not maintained in the longer term(Reference Goodwin, Segal and Vallis72).
The ENERGY study of 692 women with a BMI of 25–45 kg/m2 with a history of early breast cancer, demonstrated a mean weight loss of 6⋅0 % associated with a group-based behavioural intervention to support weight loss compared to 1⋅5 % in the control group(Reference Rock, Flatt and Byers73). The SUCCESS-C trial compared two chemotherapy regimens with a secondary intervention to compare lifestyle changes for weight loss via nutritional changes and increased physical activity(Reference Hauner, Rack and Friedl74). Among those who completed the 2-year programme, those in the lifestyle intervention arm had a significantly better disease-free survival than those in the control group(Reference Hauner, Rack and Friedl74).
Overall prospective studies have therefore demonstrated that it is possible to successfully implement weight loss interventions in breast cancer survivors. Direct evidence for translation of these interventions into longer term healthy weight maintenance and oncological benefits remain less clear.
Physical activity
There is evidence that physical activity may reduce the risk of premenopausal breast cancer. Importantly for cancer survivorship physical activity in patients with a diagnosis of breast cancer has been found to improve emotional health, anxiety as well as stamina and reduce body fat(Reference Lahart, Metsios and Nevill75). It is proposed that lower endogenous hormone levels, reduced inflammation and reversal of insulin resistance may in part explain the benefits of exercise(Reference Hamer and Warner76).
An observational study of 2987 women found that the risk of death from breast cancer reduced in women doing more than three metabolic equivalent of task hours per week of physical activity following a breast cancer diagnosis, with more benefit seen in those with hormone receptor positive disease(Reference Holmes, Chen and Feskanich77). A meta-analysis of twenty-two studies found reduced breast cancer mortality in those engaging in physical activity, with most benefit seen in postmenopausal women and those with a BMI >25 kg/m2(Reference Lahart, Metsios and Nevill78). Higher intensity exercise appears to be more beneficial than lower intensity exercise(Reference Lahart, Metsios and Nevill78).
Physical activity during chemotherapy has been found to improve fitness, reduce fatigue and potentially improve cognitive function(Reference Furmaniak, Menig and Markes79). Aerobic exercise is safe during chemotherapy-based treatments and may help to improve quality of life and reduce some side effects, however there remains limited evidence regarding the benefit of this on cancer-related outcomes and survival(Reference Furmaniak, Menig and Markes79,Reference Cave, Paschalis and Huang80) .
Recently published guidelines by the American Society of Clinical Oncology recommend that oncology providers should recommend aerobic and resistance exercise during active treatment of adults with curative intent to mitigate side effects of treatment(Reference Ligibel, Bohike and Alfano81).
Metastatic breast cancer
Although treatment of early breast cancer has improved significantly over the past two decades, approximately 20 % of early breast cancer patients subsequently develop metastatic disease, which is incurable(Reference Kennecke, Yerushalmi and Woods82). In this setting sequential systemic anti-cancer treatments are used with the aims of reducing disease burden, and improving life expectancy and quality of life(83).
In contrast to patients with early breast cancer who rarely exhibit systemic cancer symptoms, patients with progressive metastatic breast cancer are likely to experience weight loss and muscle wasting due to cancer cachexia: a syndrome related to the chronic inflammatory state in cancer(Reference Aoyagi, Terracina and Raza84). Patients with metastatic breast cancer may also be sarcopaenic, unrelated to the cancer itself. Sarcopaenia is characterised by the generalised and progressive loss of skeletal muscle mass and function related to age, diet and other comorbidities(Reference Cruz-Jentoft, Bahat and Bauer85). In patients with metastatic breast cancer, sarcopaenia is associated with an increased risk of severe chemotherapy toxicity(Reference Aleixo, Williams and Nyrop86). Studies suggest that physical activity may have a role in preventing cancer cachexia and sarcopaenia(Reference Yoo, No and Heo87,Reference Hardee, Counts and Carson88) which may improve survival in patients with metastatic breast cancer.
Studies investigating the effect of BMI on survival in women with metastatic breast cancer have found that BMI is not associated with survival(Reference Alfari, Salamoon and Kadri89,Reference Gennari, Nanni and Puntoni90) . However, more detailed body composition analysis is required to understand these results more completely. As patients with progressive metastatic breast cancer are likely to have a degree of skeletal muscle loss, BMI is a particularly poor measure of body composition in these patients as it is unable to identify patients with a low skeletal muscle mass.
The majority of previous lifestyle and physical activity interventions in breast cancer have been aimed at patients with early (curable) disease. However, a study by Yee et al. found that women with metastatic breast cancer were significantly less aerobically fit, weaker and less active compared to the healthy controls, highlighting that physical activity interventions may have an important role in maintaining a patient's independence and quality of life(Reference Yee, Davis and Beith91). NICE guidance on the management of advanced breast cancer recommends the use of an exercise programme in patients experiencing cancer-related fatigue(92). However, these guidelines do not refer to alcohol consumption, smoking cessation and general physical activity, meaning that clinicians are currently unable to routinely refer patients with metastatic breast cancer for lifestyle interventions(92).
There have been few reported physical activity intervention studies in women with metastatic breast cancer. Slower decline in physical well-being and lower increases in fatigue scores over time were seen in a seated exercise programme, which included thirty-two women who were receiving chemotherapy for their metastatic disease(Reference Headley, Ownby and John93).
A randomised trial of women with metastatic breast cancer investigated the effect of moderate-intensity exercise on physical functioning, cardiorespiratory fitness (using the modified Bruce ramp treadmill test) and quality of life(Reference Ligibel, Giobbie-Hurder and Shockro94). Participants were randomised to follow a 16-week moderate-intensity aerobic exercise intervention or to receive routine care. Although no adverse events were recorded in the intervention group, illustrating that moderate-intensity physical activity was safe in the study population, significant differences in the change of physical functioning, cardiorespiratory fitness or quality of life from baseline between the intervention and control groups were not identified(Reference Ligibel, Giobbie-Hurder and Shockro94). This may be because of a small population and heterogeneity in treatments received, as data from only seventy-six participants were analysed: 53 % of patients receiving endocrine therapy only and 2 % of patients receiving no treatment(Reference Ligibel, Giobbie-Hurder and Shockro94).
The results from these trials suggest there may be a role for physical activity interventions in the setting of metastatic breast cancer to improve patients' quality of life, although further trials with larger sample sizes are required to determine the optimum type of exercise for these patients.
The role of dietary intervention in patients with a high BMI at the time of metastatic breast cancer diagnosis is currently not known, but results are awaited from the B-AHEAD 3 trial, which involved patients who were receiving first-line chemotherapy for metastatic breast cancer(95). In this study, participants were randomly allocated to either follow a diet and exercise programme and resistance training three times weekly or to follow resistance training three times weekly in order to investigate the effect of intermittent energy restriction and resistance exercise on endpoints including time to disease progression and chemotherapy toxicity(95).
Limitations of evidence base
Many studies of lifestyle factors in breast cancer patients have omitted to collect important confounding factors; biological subtype, menopausal status, cancer stage and treatment received all impact oncological outcomes(Reference Anderson, Martin and Renehan96). In particular, early and metastatic breast cancer populations should be investigated separately as the interventions and outcomes that are appropriate in these groups differ.
Lifestyle factors are intertwined, physical inactivity and weight gain are clearly linked and it is difficult to examine the impact of these separately. Various methods to measure excess body fatness have been used in research studies, BMI is by far the most common method, due to its simplicity and ease of use. However, BMI is a surrogate marker of body fatness with significant limitations. Furthermore, the relationship between BMI and body fat varies by sex, ethnicity and age(Reference Romero-Corral, Somers and Sierra-Johnson97). Anthropometric methods to assess fat distribution such as waist circumference and waist-to-hip ratio can add additional information to BMI alone but are limited in their sophistication(Reference Sommer, Teufer and Szelag98). Imaging techniques such as computerised tomography and dual energy X-ray absorptiometry or bioelectrical impedance assays can provide more precise measurements of body composition, but can be difficult to implement into clinical practice due to cost and time constraints(Reference James, Wootton and Jackson99).
Obesity is more prevalent in low socio-economic groups and is higher in those who identify as Black or White British(5). There is growing evidence that Black women with breast cancer have worse outcomes than those from other ethnic backgrounds(Reference Gathani, Chaudhry and Chagla100). This is in part due to a higher frequency of more aggressive and larger tumours(Reference Copson, Maishman and Gerty101). However even after adjusting for BMI, and tumour pathology, Black ethnicity remains a marker of poor prognosis(Reference Copson, Maishman and Gerty101) and this requires further investigation as under-representation of ethnic minorities in clinical trials currently limits the evidence base in this area(Reference Gathani, Chaudhry and Chagla100).
Current UK guidelines for breast cancer survivors
NICE guidance on the management of early breast cancer, published in 2018, advises that people with breast cancer should be informed that having a healthy lifestyle is associated with a lower risk of recurrence and that they should limit alcohol consumption to below 5 units/week, undertake regular physical activity and be offered smoking cessation support(102). This guidance signposts the reader to general advice on preventing excess weight gain and obesity for the general population, rather than specific advice for those being treated for cancer(102).
Barriers to improving lifestyle factors
Both patients and clinicians in the UK report unsatisfactory experiences of nutritional care for those receiving cancer treatment and highlight a need for better evidence to be available so that more consistent advice is provided(103).
Oncology follow-up for those who have received treatment for breast cancer has changed over the past couple of decades with most patients now being discharged back to the care of their general practitioner a few years after completion of their primary treatment(102). This provides the oncology team with limited opportunities to give advice or provide support for lifestyle changes.
Barriers that can make it difficult for cancer patients to improve lifestyle include patient factors, such as fatigue, lack of confidence, pain, surgical scars and weight gain from treatment(Reference Webb, Ardill and Smemerald104). Results from a patient survey regarding this have demonstrated that nutritional advice given by healthcare professionals to cancer patients is often vague(103). In addition to this clinicians and healthcare professionals are often not taught how to discuss these sometimes sensitive issues with patients and may lack awareness of the existing evidence to support lifestyle changes in cancer survivors. The recently updated medical oncology curriculum discusses acting as an advocate for health promotion and advises taking into account lifestyle factors when giving advice to patients but trainees currently receive little if any formal teaching on this topic(105). Current NHS oncology services are under significant pressure, meaning detailed discussions about lifestyle in clinics are not easily feasible. There is currently a lack of commissioned services for oncologists and surgeons to refer patients to limiting the support available for patients regarding nutritional information, weight loss advice or access to exercise programmes(Reference Jefford, Howell and Li26). High-quality research is needed to guide practice and to provide the evidence to allow the commissioning of support services and lifestyle interventions for our patients.
Where should we go from here
Other specialities have demonstrated a benefit from rehabilitation and exercise training programmes, with inclusion of this is the routine care of patients. For example, following a myocardial infarction, whilst there is conflicting evidence as to whether cardiac rehabilitation is beneficial in reducing mortality and re-infarction, current NICE guidance advises patients are offered a cardiac rehabilitation programme(Reference Lawler, Filion and Eisenberg106,Reference West, Jones and Henderson107) . Similarly NICE guidance recommends the use of pulmonary rehabilitation for patients with chronic obstructive pulmonary disease(108,Reference Fastenau, van Schayck and Winkens109) . In the cancer care setting prehabilitation prior to surgery can improve postoperative functional capacity and is becoming more commonplace prior to extensive cancer surgery(Reference Michael, Lehrer and Schmitz110). However given breast surgery outside of reconstruction, is typically a day case procedure prehabilitation is unlikely to be particularly relevant in this group(111).
Current evidence is sufficient to show that maintaining a healthy weight and being physically active can improve outcomes; however there are limited data on important oncological outcomes such as disease-free survival and overall survival. All healthcare professionals therefore have a responsibility to encourage a healthy lifestyle during and following breast cancer treatment. This can represent a teachable moment where we can educate our patients on simple measures to improve their health and potentially quality of life(Reference Lawson and Flocke112). The importance and impact of lifestyle factors need to be integrated into oncology training to provide clinicians with the appropriate skills and knowledge to advise and support patients(Reference Vaz-Luis, Masiero and Cavaletti58). In addition, access to more formal support and lifestyle intervention programmes are required for our patients. To facilitate this, more evidence from high-quality research is needed to help guide practice and commission services.
There is an under-representation of patients from racial and ethnic minority backgrounds in clinical trials, with recent American Society of Clinical Oncology recommendations published to help encourage more diverse and representative populations participating in clinical trials(Reference Oyer, Hurley and Boehmer113). The inclusion of lifestyle research in the recent strategic priorities of the National Cancer Research Institute's Breast Group highlights the growing importance of this issue(114).
Gold standard clinical trial evidence comes from randomised controlled trials rather than observational studies, but long-term follow-up is needed especially if we are going to establish the effect an intervention has on survival. Surrogate markers for such long-term outcomes may, however, be needed to enable us to gather the evidence required more quickly for our patients. Careful thought and planning are required for the design of lifestyle interventions for breast cancer patients, to ensure these are both effective as well as manageable for patients. The involvement of patient groups in the study design process is therefore crucial.
Conclusions
Many cancer organisations have identified the need to better understand the effect lifestyle has on patients with cancer. As obesity and physical inactivity become more prevalent in our society, these issues are affecting more of our patients. Services to support lifestyle and health interventions are more developed for CVD however breast cancer patients may equally see benefit including from cardiovascular, bone health and psychological perspectives, all of which are potentially impacted by breast cancer and its treatments, in addition to possible oncological benefits. Patients with both early and metastatic breast cancer should be included although it may be more appropriate to consider them separately both in terms of intervention delivery and evaluation. Further research is needed to define the optimal lifestyle recommendations and support for our patients to achieve healthy survivorship.
Acknowledgements
We acknowledge all the patients who participated in the clinical trials described in this review.
Financial Support
No funding has been received for this piece of work.
Conflict of Interest
E. R. C. and R. I. C. report research support funding from Astra-Zeneca and research equipment provision from SECA. E. R. C. additionally reports: consultancy fees and honoraria from Astra-Zeneca, Eli-Lilly, Roche, Novartis and Pfizer, and accommodation and travel expenses from Roche. N. J. C. and C. B. report no conflicts of interest.
Authorship
N. J. C. conducted literature reviews and drafted the text for all sections on early breast cancer. C. B. conducted literature reviews and drafted the text for all sections on metastatic breast cancer. R. I. C. provided breast surgical expertise, and reviewed and revised the manuscript. E. R. C. provided oncological expertise, designed the overall structure of the review and reviewed and revised the manuscript.