INTRODUCTION
In children aged <5 years, Haemophilus influenzae type b (Hib) had been a major cause of serious infections (e.g. meningitis, pneumonia, epiglottitis) before conjugate vaccine was licensed in 1987 [1, 2]. In 2006, less than 200 possible Hib cases (<5 deaths estimated) were reported in the USA, a >99% decline from the pre-vaccine era (20 000 cases and 1000 deaths annually) [Reference Roush and Murphy3].
In December 2007, Merck & Co., Inc. (West Point, USA) voluntarily recalled specific lots of its Hib conjugate vaccine polyribosylribitol phosphate outer membrane protein (PRP-OMP) (trade names PedvaxHIB® and Comvax®), resulting in shortages in the US Hib vaccine supply [4]. PRP-tetanus toxoid (PRP-TT) vaccines (trade names ActHIB®, TriHIBit®, and Pentacel®; Sanofi-Pasteur, USA) were unaffected by the recall. PRP-OMP vaccine requires a two-dose primary series, with doses recommended in the USA at ages 2 months and 4 months, and a booster at age 12–15 months, while PRP-TT vaccine requires a three-dose primary series, with doses recommended in the USA at ages 2, 4, and 6 months and a booster at age 12–15 months. As a result of the vaccine shortage, deferral of the 12–15 months booster dose for children other than those in specific high-risk groups was recommended to ensure sufficient vaccine for all children to complete the primary series [4].
During the vaccine shortage, in 2008, the Minnesota Department of Health (MDH) detected the highest incidence of invasive Hib cases (1·41/100 000 children aged <5 years) in Minnesota since 1992 [5]. Of the five affected patients (aged ⩽3 years), four had incomplete/no Hib vaccination [5]. In addition, a January 2009 assessment of Minnesota immunization registry data indicated that primary Hib vaccine coverage during the Hib vaccine shortage (96%, 82%, and 47% for the first, second, and third doses, respectively) was lower than other three-dose primary series vaccines – 17% lower than diphtheria, tetanus, and acellular pertussis (DTaP) vaccination (97%, 86%, and 63% by dose, respectively), and pneumococcal vaccination (PCV-7) (97%, 86%, and 63% by dose, respectively) [5].
The increase in invasive Hib cases in young children in Minnesota, mainly in children whose parents refused Hib vaccination, and the overall decreased Hib vaccine coverage prompted concern of increased community-wide Hib transmission in young children. Therefore, MDH conducted a survey to ascertain whether Hib carriage or vaccine refusal was widespread. In this report, we present survey results for Hib carriage, Hib vaccination in children, and their parents' overall vaccination beliefs in the context of surveillance for invasive Hib cases during 2008–2009.
METHODS
Surveillance for invasive cases
Since 1995, Minnesota has conducted active, population-based surveillance for invasive H. influenzae infection [Reference Triden6], thereby monitoring statewide disease incidence in a defined population [Reference Schuchat7]. A case is defined as isolation of H. influenzae from a normally sterile site, and isolates are sent to the MDH Public Health Laboratory for species and serotype confirmation [Reference Triden6]. Surveillance officers actively contact infection control practitioners and microbiology laboratories to identify cases and regularly perform audits to detect additional unreported cases [Reference Triden6]. Hib isolates detected during 2008–2009 were characterized by pulsed-field gel electrophoresis (PFGE) as previously described [Reference Popovic8], and grouped by similarity of PFGE subtype [Reference Boxrud9, Reference Tenover10]. Although previously summarized as an alert to public health communities in the Morbidity and Mortality Weekly Report [5], the details of the invasive Hib cases detected during 2008 are described here for ease of comparison with 2009 invasive Hib cases.
H. influenzae carriage survey
Children were enrolled from a convenience sample of 18 outpatient clinic sites throughout Minnesota, targeting counties near the residences of the Hib cases reported in 2008. The Centers for Disease Control and Prevention, MDH, and participating sites' institutional review boards approved the survey as a non-research public health response. Eligible children were Minnesota residents aged 4 weeks–60 months who had not been administered antibiotics during the previous 4 weeks. A sample size of 1500 children was estimated to be sufficient to detect 1% prevalence (50% error, α=0·05). After informed consent was obtained from parents/guardians, trained survey staff administered a questionnaire, collecting information on demographics, potential H. influenzae colonization risk factors, and immunization practices and beliefs. Consent documents and questionnaires were translated into Spanish, Hmong, and Somali, and were reviewed for accuracy by native speakers. Vaccination histories were collected from Minnesota's immunization registry [Minnesota Immunization Information Connection (MIIC)] or from primary-care providers.
An oropharyngeal swab specimen was obtained from each child and placed in a tube containing 1 ml skim milk, tryptone, glucose, and glycerine transport medium (STGG) [Reference Kaijalainen11]. Inoculated STGG media were refrigerated or kept in coolers for ⩽4 h before being frozen at −20°C or plated onto selective growth medium for H. influenzae isolation. Thawed specimens were vortexed for 10 s to resuspend and distribute contents. One drop (~0·05 ml) was transferred to Chocolate II agar with 16 500 U/l Bacitracin® (Becton, Dickinson and Company, USA), then streaked for isolation. Cultures were incubated (37°C with 5% CO2) for 24–48 h. Suspected colonies were transferred to Chocolate II agar supplemented with haemoglobin and IsoVitalex® (Becton, Dickinson and Company) for additional screening, including Kovac's oxidase test to determine presence of cytochrome oxidase, and porphyrin test to exclude haemin-independent Haemophilus strains. Selected colonies were identified using API NH strips (bioMérieux, USA) and BBL Hemo ID QUAD® plates (Becton, Dickinson and Company). Serotyping of H. influenzae isolates was performed by slide agglutination by screening with commercial polyvalent antisera-containing serotypes a–f (Difco™; Becton, Dickinson and Company). Isolates that did not react with the polyvalent antisera were considered non-typable H. influenzae. The serotype of isolates that tested positive for the polyvalent antisera was determined by using specific antisera for serotypes a–f (Remel, USA).
Hib primary series completion was defined as receipt of two doses of PRP-OMP vaccine (recommended at ages 2 and 4 months) or three doses of PRP-TT vaccine (recommended at ages 2, 4, and 6 months). Completion of DTaP and PCV-7 primary series was defined as receipt of three doses. Primary series completion was evaluated by year of birth for children aged >7 months. Completion of Hib primary series was compared with completion of DTaP and PCV-7 primary series (McNemar test). Odds ratios and 95% confidence intervals evaluated completion of children's vaccination series by parents' reported vaccination-related beliefs. Odds ratios and 95% confidence intervals were calculated for factors associated with H. influenzae carriage; simultaneous adjustment was made by multivariable logistic regression. Missing data/non-response to questions on risk factors for H. influenzae carriage were evaluated as separate strata in logistic regression analyses. Data were managed with Microsoft Access® (Microsoft Corporation, USA). Statistical analyses were conducted using SAS® version 9.1 (SAS Institute Inc., USA) and Stata® v. 10 (StataCorp, USA).
RESULTS
Surveillance for invasive cases
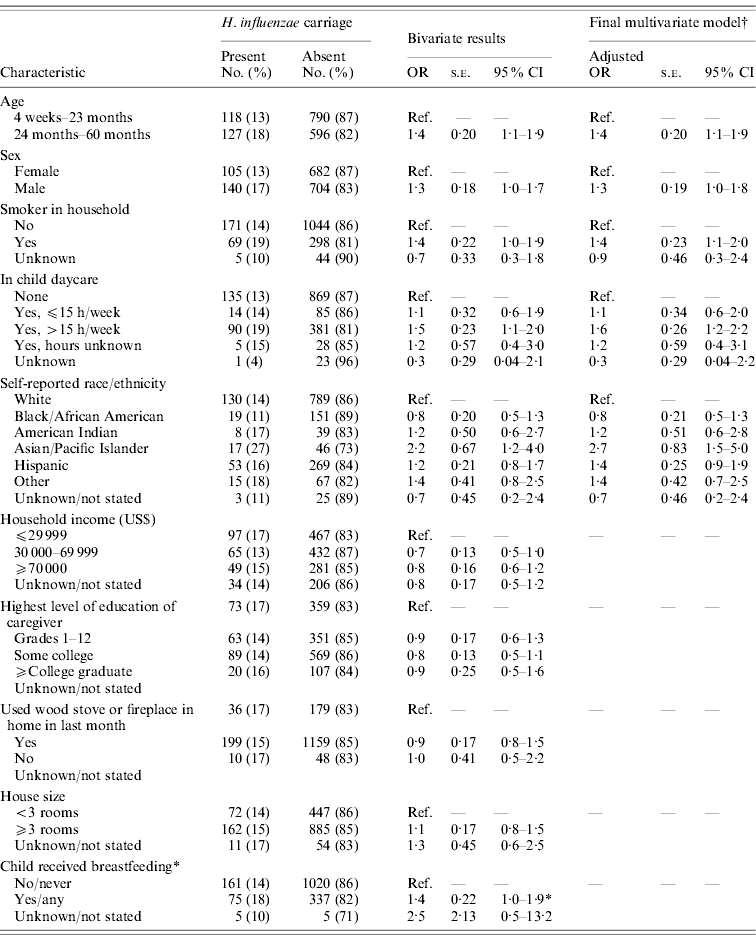
Active surveillance identified five cases of invasive Hib disease in 2008, prior to the survey, and two cases in 2009, following the survey (Fig. 1 a). Of the five affected patients in 2008 (aged ⩽3 years), one died. Three were unvaccinated because of parental refusal; one had age-appropriate but incomplete primary vaccination with PRP-TT; and another had completed the two-dose primary series with PRP-OMP. However, this patient was diagnosed with hypogammaglobulinaemia [5]. The 2008 patients had no known relationship with each other, but all resided in south-central Minnesota (Fig. 1 b). None attended group childcare. Isolates 1 and 2 were indistinguishable from each other by PFGE analysis (Fig. 1 a); isolate 5 had a closely related band pattern to isolates 1 and 2. Isolates 6 and 7 differed from each other and were possibly related to other isolates. The 2009 cases of invasive Hib disease, an 8-year-old with unknown vaccination status and a fully vaccinated 5-year-old, resided in the same county in northwestern Minnesota (Fig. 1 b); their isolates (3 and 4) were indistinguishable (Fig. 1 a) from each other and from isolates 1 and 2. Of all seven patients with invasive Hib during 2008–2009, five were male; five were white/non-Hispanic, one was Hispanic, and one had race/ethnicity not reported.
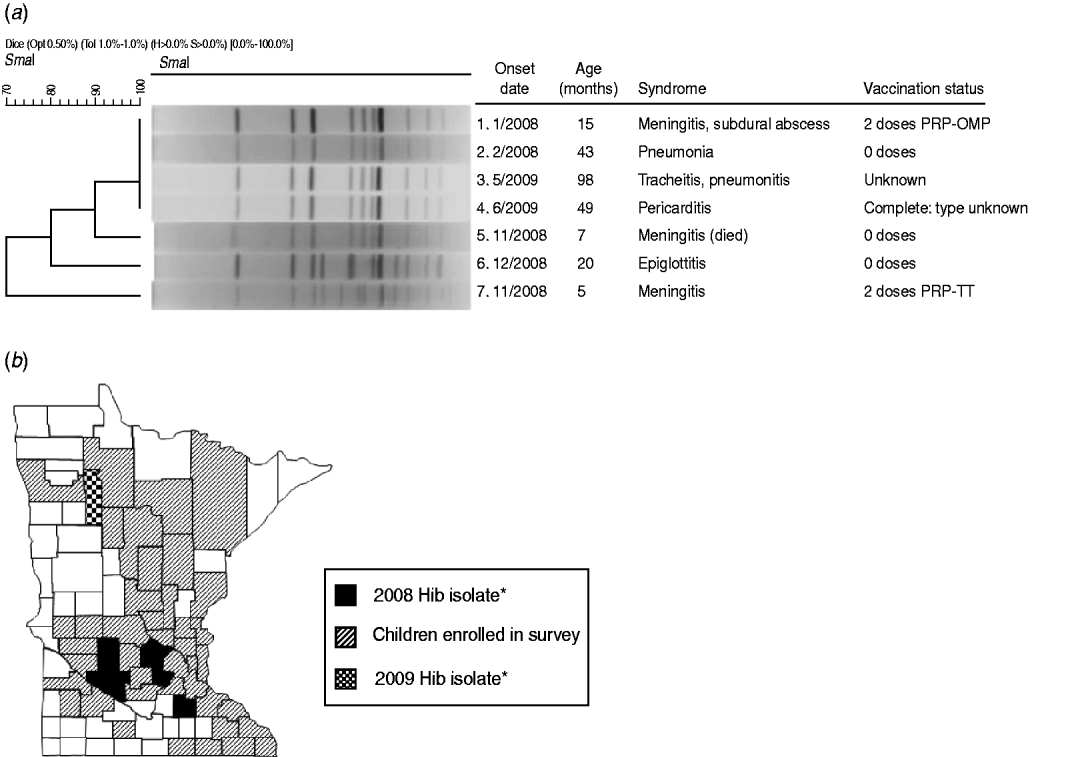
Fig. 1. (a) Results of pulsed-field gel electrophoresis testing of isolates of Haemophilus influenzae type b isolates detected in Minnesota residents, 2008–2009. (b) Counties of residence of patients with invasive Hib disease during 2008–2009 and of children enrolled in 2009 carriage survey. * Indicates these counties also had children enrolled in the survey.
Carriage survey
During 2 February–20 March 2009, we enrolled 1631 children in the carriage survey. Of the participants, 884 (54%) were male, 908 (56%) were aged <24 months, 919 (56%) were white, 322 (20%) reported Hispanic ethnicity, 170 (10%) were black, 63 (4%) were Asian/Pacific Islander, 47 (3%) were American Indian, 82 (5%) reported another race/ethnicity, and 28 (2%) did not report race/ethnicity. H. influenzae was isolated from 245 (15%) children. Of these, no Hib was isolated; 235 were non-typable, five were serotype e, and five were serotype f.
Non-type b H. influenzae carriage was more likely in children aged 24–60 months, in males, in children who lived in a household with a smoker, and in children attending daycare >15 h/week (Table 1). Children with non-type b H. influenzae carriage were more likely to have been reported by parents as Asian/Pacific Islander (Table 1). Children reported as having any breastfeeding were more likely to have carried H. influenzae in bivariate analysis, although this association did not remain statistically significant in multivariate analysis (data not shown). In a multivariate model, male sex, age 24–60 months daycare attendance >15 h/week, having a smoker in the household, and being reported as Asian/Pacific Islander race/ethnicity remained associated with carriage (Table 1).
Table 1. Risk factors for H. influenzae carriage in Minnesota children, 2009
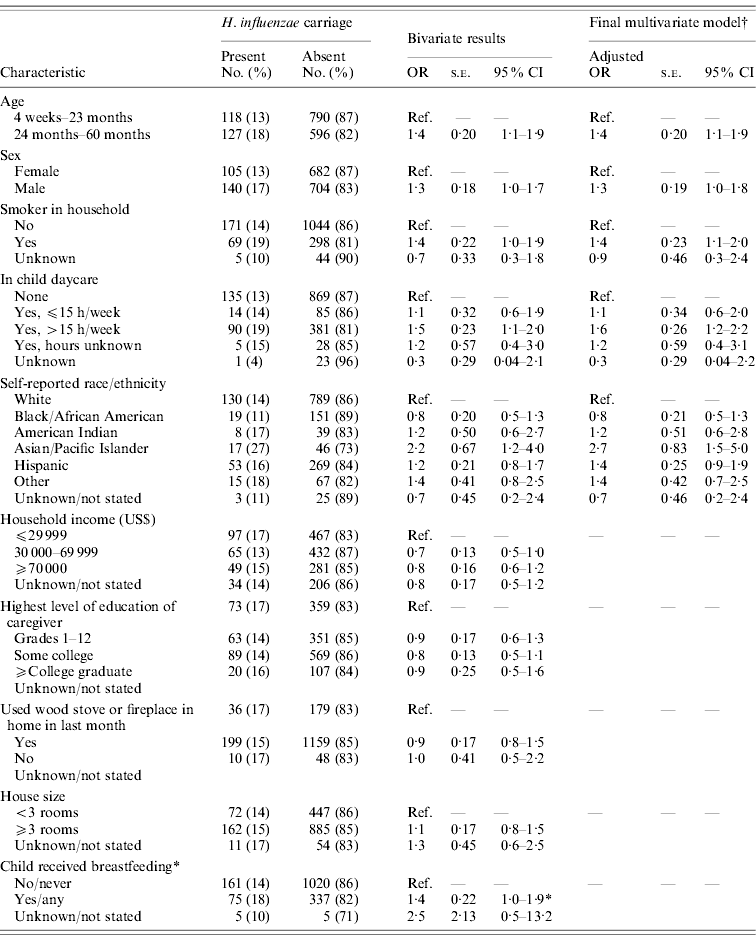
OR, Odds ratio; s.e., standard error; CI, confidence interval; Ref., reference.
* Breastfeeding was not found to be statistically associated with H. influenzae carriage in an interim model that contained all variables identified as statistically significant (confidence interval did not overlap 1·0) in bivariate analysis (data not shown).
† The final results represent a reduced, parsimonious model that includes all variables found statistically significant upon bivariate analysis (confidence interval did not overlap 1·0) and that remained statistically significant when combined in a multivariate logistic model.
Vaccination records were obtained from the MIIC for 1568 children, from primary providers for 51 children, and were not obtained for 12 children. For 1298 children aged >7 months, those born before 2008 (prior to the Hib vaccine shortage) more often completed their Hib primary series with two doses of PRP-OMP, compared to those born in 2008, who more often completed their Hib primary series with three doses of PRP-TT. The percentage of children with incomplete or no Hib vaccination increased during 2004–2008 (P<0·001) (Fig. 2 a); the percentage of children receiving a booster dose declined (Fig. 2 b). For 1268 children aged >7 months with complete data for all three vaccines, the Hib vaccination series was less frequently completed (80%) than DTaP (89%, P<0·001) and PCV-7 (87%, P<0·001) series.
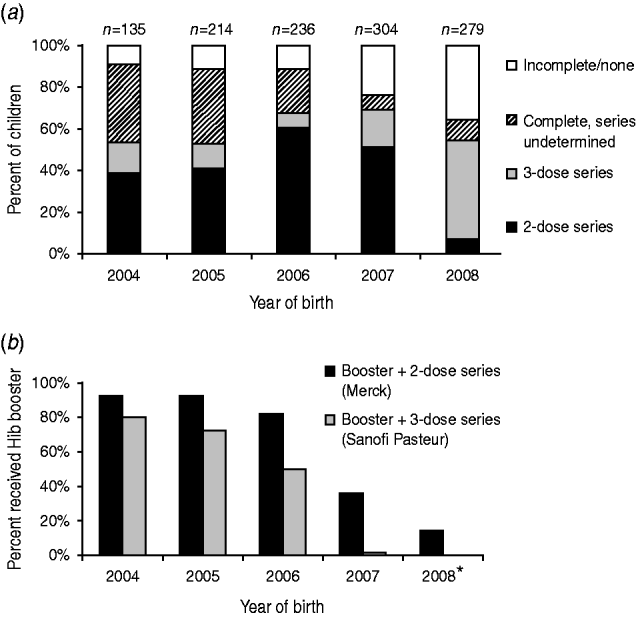
Fig. 2. (a) Completion of Haemophilus influenzae vaccination in children aged >7 months by primary vaccine series and year of birth. (b) Proportion of children who received H. influenzae type b booster dose by primary vaccine series (2 doses vs. 3 doses) and year of birth. * Children aged ⩾12 months.
Of the 80 parents (5%) who reported having reasons for not having their children vaccinated, 16 cited medical exemptions; 17 cited philosophical exemptions; 18 cited other reasons (e.g. personal choice); and 29 provided no reason. Of the 132 (8%) parents who reported not using the routine schedule for vaccines, 44 followed a schedule based on their own beliefs; eight reported vaccine unavailability; 26 gave other reasons (e.g. missed appointment); and 53 gave no reason for not adhering to the routine schedule. Compared to children whose parents reported no reasons for avoiding childhood vaccination, children whose parents reported reasons for not having their children vaccinated were less likely to have complete vaccination for the Hib primary series [43 (72%) vs. 855 (81%)], the DTaP series [50 (77%) vs. 1056 (90%)], and the PCV-7 series [46 (71%) vs. 1038 (88%)], for those aged >7 months. Similarly, compared to children whose parents reported following the routine childhood vaccination, children whose parents reported following an alternative childhood vaccination schedule were less likely to have complete vaccination for the Hib primary series [65 (68%) vs. 828 (82%)], the DTaP series [81 (78%) vs. 1017 (91%)], and the PCV-7 series [75 (72%) vs. 1002 (89%)], for those aged >7 months.
DISCUSSION
The 2008 increase in invasive Hib cases during a Hib vaccine shortage and decreased primary vaccination rates were a matter of concern. Matching isolates during 2008–2009 provided evidence of ongoing circulation of at least one strain [Reference Tenover10]. Despite a national Hib vaccine shortage and decreased vaccination coverage in young children that probably decreased population immunity in young children, no Hib carriage was detected in young children. Therefore, young unvaccinated or vulnerable children appear to continue to be at risk of invasive Hib disease even though child carriage levels remained low.
Vaccine distribution in Minnesota was substantially altered by Merck Hib vaccine availability. Hib booster dose coverage declined corresponding with the recommendation for deferral of booster dose; Minnesota providers shifted from predominantly using PRP-OMP to mainly using PRP-TT for primary series. Hib primary series completion decreased in 2008 in children enrolled in the survey, similar to decreases observed in the immunization registry in Minnesota [5] and in other sentinel locations [Reference White, Pabst and Cullen12]. Decreased coverage might have been a result of parental or provider confusion regarding the change from a two-dose to a three-dose primary series or to mismatches between vaccine supply and demand. Confusion in appropriate Hib primary vaccination was also noted in Alaska after community-wide changes in Hib vaccine type [Reference Singleton13]. Booster dose recommendations were reinstated in June 2009 after PRP-TT vaccine production increased sufficiently provide the infant booster dose [14].
Three families with children diagnosed with invasive Hib disease in 2008 either declined Hib vaccination or deferred Hib vaccination past the recommended age. We also identified children in the 2009 survey who lacked complete vaccination because of parental preference. Vaccination refusal or delay increases risk of infection in children and, because of the potential impact on herd immunity, increases risk of colonization and disease in others in the community [Reference Glanz15–Reference Fry17].
Our findings were consistent with findings elsewhere that detected <1% Hib carriage in young children [Reference McVernon18, Reference Jacups, Morris and Leach19], a change from the pre-vaccine era when the prevalence of Hib carriage was 1–4% in young children [Reference Howard, Dunkin and Millar20–Reference Mohle-Boetani23]. Several reasons might explain the lack of Hib carriage. Hib carriage might have been less than 0·5%, outside the detectable range for our survey. Persons aged >5 years, not included in our survey, might serve as a reservoir for Hib, and it has been shown elsewhere that Hib carriage is more prevalent in persons aged >5 years than in children aged <5 years [Reference Singleton13, Reference Adam24–Reference Singleton28]. Although enrolment was targeted to communities of greatest concern for transmission, Hib transmission may be limited to direct contacts of cases, as has been documented in past Hib outbreaks in selected communities that have identified carriage in family contacts [Reference Fry17]. In our setting with Hib cases unrelated to each other and with declining vaccination coverage, an investigation focused on family contacts of Hib cases or in children whose parents declined vaccination might have detected Hib. However, such an investigation would not have determined if Hib carriage had re-emerged in the broader community. Documenting these changes in the epidemiology of H. influenzae demonstrates the importance of continued surveillance for carriage and invasive cases.
Understanding these risk factors for non-b serotypes and non-encapsulated H. influenzae carriage is of growing importance, because they represent an increased proportion of H. influenzae invasive infections in vaccinated populations [Reference Adam24, Reference Shuel27, Reference McConnell29–Reference Laupland32], and because of the potential for their prevention by the new conjugate pneumococcal non-typable H. influenzae protein D conjugate vaccine [Reference Prymula33, Reference Prymula34]. The risk factors for non-b serotypes and non-encapsulated H. influenzae carriage in this survey (age ⩾24 months, increased daycare attendance, having a smoker in the household) were consistent with factors historically associated with Hib disease and carriage [Reference Howard, Dunkin and Millar20, Reference Galil35–Reference Lee37]. The prevalence of H. influenzae carriage in our survey was lower than in past studies [Reference Howard, Dunkin and Millar20, Reference Mohle-Boetani23]. This might indicate less than optimal specimens in some instances, a culture method less sensitive than antiserum agar used in other studies but unavailable at the time of our survey, or might characterize changes in the epidemiology of H. influenzae.
Several of the risk factors for H. influenzae carriage identified in this survey are consistent with circumstances amenable to epidemiological factors associated with transmission (e.g. hours spent in daycare) and biological plausibility (e.g. second-hand smoke). The finding of increased H. influenzae carriage in Asian/Pacific Islander children warrants further investigation to exclude the possibility of a spurious association. Rates of H. influenzae carriage and disease have varied by race/ethnic group, typically being higher in native or aboriginal populations [Reference Singleton13, Reference Jacups, Morris and Leach19, Reference Bruce38]. In our survey, American Indian children had elevated odds of H. influenzae carriage, but this was not statistically significant. Asia and the Pacific Islands include a broad region of origin, and further investigation might determine if H. influenzae carriage circulates within racially and ethnically specified communities in Minnesota.
As a convenience sample, our findings might not be generalizable and selection bias is possible. Parents of participants less frequently reported being white compared with Minnesota census data (86% in 2005), and more frequently reported being non-white minorities (black, American Indian/Alaska Native, two or more races, or Hispanic ethnicity) compared with the general Minnesota population (4%, 1%, 1%, 4% in 2005, respectively) [39]. However, overall vaccination rates in this survey were similar to the larger Minnesota population. Given that non-white racial/ethnic minority children historically have lower vaccination coverage than white children in Minnesota, one might have expected the level of Hib carriage in survey children to be higher in this survey than in a representative sample of the Minnesota child population.
CONCLUSION
Although childhood vaccination programmes have dramatically reduced incidence of diseases such as invasive Hib, success of these programmes depends on continued vaccine supply and on vaccine uptake in children. Even a minor shortage can cause difficulties in vaccine supply (e.g. requiring a change from a two-dose to a three-dose Hib primary series). Parental refusal of childhood vaccines and use of alternative vaccination schedules can decrease vaccine coverage, even when vaccine supply is optimal. The vaccine shortage has ended, but vaccine supply remains volatile. Maintaining vaccine supply and effectively communicating to parents the safety and importance of vaccination are critical to continued success of the childhood vaccination programme.
Although we did not detect any Hib carriage in the children surveyed, the five cases of invasive Hib disease in 2008 and two cases in 2009 of a similar strain based on PFGE patterns demonstrate continued circulation of Hib. The increased number of invasive Hib cases with the absence of detectable Hib carriage in young children, indicates that young unvaccinated and vulnerable children remain at risk of severe Hib disease. Decreases in primary Hib vaccine series completion, parental vaccination refusal, and use of alternative vaccination schedules are critical concerns that place young children at risk for invasive Hib disease and should be addressed by healthcare providers, parents, and advocates for children. Continued surveillance for typable and non-typable H. influenzae disease and carriage across all ages will enable ongoing characterization of H. influenzae infection risk and implementation of appropriate prevention and control measures.
ACKNOWLEDGEMENTS
This survey was funded in part, by the Emerging Infections Program – CDC Research Project Cooperative Agreement Grant no. 5U01CI000313-05, CFDA: 93.283. N. Shinoda was supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by CDC Cooperative Agreement U60/CCU007277. The authors thank the staff at all participating Hib survey sites, and thank Tom Hennessy and Jay Wenger for their input and review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
APPENDIX. The HIB Survey Team
E. Anderson, J. Besser, T. Clark, K. Como-Sabetti, A. DeVries, P. Gahr, K. Gall, J. Griffith, J. Harper, C. Hickman, K. Jeppesen, B. Krier, C. Lees, P. LeMaster, L. Lesher, C. Lexau, J. Mariotti, L. Mayer, J. MacNeil, N. Messonnier, C. Morin, J. Rainbow, T. Ristinen, E. Shade, C. Thomas, I. Triebold, A. Westbrook, and K. White.
DECLARATION OF INTEREST
None.