Fibre is known to have positive effects on gastrointestinal health and has been determined to have a link to decreased disease risk (CHD, colorectal cancer, breast cancer). Dietary fibre consumption in the USA is 12–18 g/d, which is well below the recommended adequate intake of 25 g/d for women and 38 g/d for men(1). Little research currently exists regarding select functional soluble fibres such as polydextrose (PDX) and soluble maize fibre (SCF). Furthermore, there is debate as to whether these functional soluble fibres deliver known health benefits associated with ‘traditional’ dietary fibres. Additional fibre consumption can lead to increased gas production and overall gastrointestinal discomfort. Therefore, finding functional fibres that can be added to food products that minimally disturb gastrointestinal tolerance and acceptance of the product may result in increased fibre consumption by humans.
PDX is a randomly bonded polysaccharide of glucose, with an average degree of polymerisation of 12. Soluble maize fibre is made from maize starch and contains oligosaccharides with random glycosyl bonds and may contain minor amounts of monosaccharides. These substrates are poorly digested ( < 30 % digested) in the small intestine, but are partially fermented by bacteria in the large bowel(Reference Flood, Auerbach and Craig2, Reference Stewart, Nikhanj and Timm3). Therefore, PDX and SCF can be considered dietary fibres; however, more information is needed on the health effects of these substrates. These functional carbohydrates are known to be water-soluble and have many properties similar to dietary fibres. Specifically, PDX has been reported to increase faecal bulk, soften stools and lower faecal pH due to its partial fermentability in the large bowel(Reference Nakagawa, Okamatsu and Fujii4–Reference Achour, Flourie and Briet6). Soluble maize fibre has been found to increase SCFA production and beneficial bacteria concentrations, while decreasing endproducts of protein fermentation in vitro (Reference Maathuis, Hoffman and Evans7); however, limited data are available in vivo. Determining in vivo characteristics of these fibres and their impact on gastrointestinal tolerance are important to determine their efficacy as supplemental fibres.
Beyond the expected effects of dietary fibres, soluble fibres such as PDX and SCF also may act as prebiotics. A prebiotic is a non-digestible food ingredient that beneficially affects the host by stimulating the growth or activity of specific species of bacteria in the hindgut, which are thought to improve gut health(Reference Gibson and Roberfroid8). Prebiotics increase the numbers of potentially beneficial bacterial species (for example, bifidobacteria and lactobacilli)(Reference Buddington9), while decreasing potentially pathogenic bacteria (for example, Clostridium perfringens and Escherichia coli). Pathogenic bacteria are controlled due to decreasing pH resulting from the increased production of SCFA and increased competition for nutrients(Reference Fuller and Gibson10).
The objective of the present study was to determine utilisation of two functional soluble fibres, PDX and SCF, in comparison with no supplemental fibre (no fibre control; NFC). This was determined by measuring: total faecal weights and laxation; faecal pH; faecal fermentative endproducts including ammonia, phenol, indole, SCFA and branched-chain fatty acids (BCFA); and quantification of specific bacterial species. Additionally, subjective scoring of gastrointestinal tolerance (burping, cramping, distension, flatulence, nausea, reflux and vomiting), stool characteristics (faecal score) and ease of stool passage were determined.
Materials and methods
Subjects
Healthy adult men (n 25) with an average daily intake of dietary fibre of approximately 13–15 g were recruited for this experiment. Subjects were recruited via an email list server from the College of Agricultural, Consumer and Environmental Sciences at the University of Illinois. Entrance demographics and vital signs were collected for all subjects to ensure general health. Of the twenty-one subjects who completed the study, thirteen were self-described as Latino, eight as Caucasian, two as Asian, one as African-American and one as East Indian. All subjects were free of antibiotics for at least 3 months before study initiation. Before initiation of the experiment, the protocol and informed consent form were approved by the University of Illinois Institutional Review Board.
To be eligible for the experiment, all subjects had to meet the following criteria: (1) be between 20 and 40 years of age; (2) be free of known metabolic and gastrointestinal diseases, with no history of metabolic or gastrointestinal diseases; (3) avoid taking medications that would have an impact on bowel function; (4) refrain from consuming pre- or probiotic supplements during the entire duration of the study; (5) limit their alcohol consumption to two servings per d; (6) agree to avoid any changes in chronic medications until the end of the study; (7) agree to maintain the same dosage of any mineral and vitamin supplements consumed until completion of the study; (8) maintain their current level of exercise and physical activity; (9) be willing to complete all necessary study questionnaires and to donate stool specimens as required; (10) consume a moderate-fibre diet (12–13 g fibre per d); and (11) voluntarily sign a written informed consent form before participation in the study.
Experimental design and treatments
A tolerance trial was conducted before the start of the present study evaluating PDX and SCF (7, 14 or 21 g/d) in the study population. Each fibre source was tested independently in a completely randomised design. In each group, seven subjects were asked to consume the varying fibre doses and record daily gastrointestinal tolerance and faecal description scores. Due to lack of differences in gastrointestinal tolerance subjective scores, it was decided to test the substrates at 21 g/d for the following study due to only limited increases in negative gastrointestinal tolerance scores among the subjects. Therefore, during the utilisation portion of the study, subjects were asked to consume three treatment bars per d corresponding with each meal (breakfast, lunch and dinner) for a total of 0 g (NFC) or 21 g PDX and 21 g SCF of supplemental fibre per d.
Subjects were enrolled in a randomised, double-blind, placebo-controlled cross-over study. Subjects were randomly assigned one of two fibre sources or no supplemental fibre during each period in a Latin-square design. There was no washout time between periods. The present study consisted of three periods lasting 21 d, with 16 d adaptation followed by 5 d of total faecal collections. Treatments included NFC (no supplemental fibre control), PDX (Litesse II®; Danisco, Copenhagen, Denmark) and SCF (PROMITOR™; Tate & Lyle Ingredients, Decatur, IL, USA). Litesse II® PDX is a low-glycaemic functional fibre that contains 1 kcal/g (about 4 kJ/g) of substrate. Promitor® SCF contains 70 % total dietary fibre and contains 2 kcal/g (about 8 kJ/g) of substrate. The fibre was provided in a snack bar made of rice crisps and manufactured by General Mills, Inc. (Minneapolis, MN, USA). Each snack bar contained approximately 7 g supplemental fibre or 0 g fibre (NFC).
The test bars were analysed for moisture, total fat, protein and water activity(11, 12). Total dietary fibre was determined and insoluble and soluble fractions determined. Resistant oligosaccharides and resistant oligosaccharides from fructans were determined according to Association of Official Analytical Chemists (AOAC) methods. Carbohydrates were then determined by difference.
Diet and stool records
Subjects were required to maintain daily diet and stool records throughout the entire study. The amount and type of all foods or liquids consumed in each 24 h period were recorded. Diet records were processed using the ESHA Food Processor SQL computer software program version 10.7.0 (ESHA Research, Salem, OR, USA). Dietary energy, protein, fat, fibre and carbohydrate intakes were calculated from the dietary records.
Subjects recorded the date, time, consistency and ease of passage of each bowel movement. Stool consistency was scored as follows: 1 = hard, dry pellets – small, hard mass; 2 = hard, formed, dry stool – remains firm and soft; 3 = soft, formed, moist – softer stool that retains shape; 4 = soft, unformed – stool assumes shape of container; and 5 = watery – liquid that can be poured. Ease of stool passage also was ranked on a five-point scale (1 = very easy, 2 = easy, 3 = neither easy nor difficult, 4 = difficult, 5 = very difficult). Subjects also ranked on a four-point scale (1 = none, 2 = mild, 3 = moderate, 4 = severe) the following subjective tolerance variables daily: burping, cramping, distension/bloating, flatulence, nausea, reflux (heartburn) and vomiting.
Stool collection and analysis
Total faeces were collected over the last 5 d of each period. An attempt was made to collect three fresh stool specimens during each collection period to account for daily variation. Samples were collected using Commode Specimen Collection Systems (Sage Products, Crystal Lake, IL, USA) and fresh samples were brought to the laboratory within 15 min of defecation, while all other samples were brought to the laboratory within 1 h or early the next morning; participants were asked to refrigerate these samples. Total faecal weight was measured using both the 5 d total weight and the last 4 d of faecal collection. Both values were reported due to some subjects forgetting to collect a faecal sample during the first day of the first period.
All stool samples were weighed upon arrival at the laboratory. A sample for DM analysis was obtained. Fresh samples were further processed by taking a pH measurement and a sample was removed for bacterial DNA extraction. The remainder of the fresh sample was then frozen at − 20°C and stored until the three fresh samples were obtained. Upon collection of all three fresh faecal samples from each individual, the samples were thawed and composited using manual stirring. Samples then were taken for SCFA, BCFA, ammonia, phenol and indole concentrations, and stored at − 20°C until analysed. The ammonia, SCFA and BCFA sample (5 g) was acidified with 5 ml of 2 m-HCl before refreezing.
Faecal DM was measured according to the Association of Official Analytical Chemists(13). Faecal pH was obtained using a Denver Instrument AP10 pH meter (Denver Instrument, Bohemia, NY, USA) equipped with a Beckman electrode (Beckman Instruments, Inc., Fullerton, CA, USA). Ammonia concentrations were measured according to the method of Chaney & Marbach(Reference Chaney and Marbach14). GC was used to measure SCFA according to Erwin et al. (Reference Erwin, Marco and Emery15). Briefly, acetate, propionate, butyrate, isobutyrate, isovalerate and valerate concentrations were determined on the supernatant fraction of acidified faecal samples using a Hewlett-Packard 5890A Series II gas chromatograph (Palo Alto, CA, USA) and a glass column (180 cm × 4 mm internal diameter) packed with 10 % SP-1200–1 % H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco, Bellefonte, PA, USA). N2 was the carrier gas with a flow rate of 75 ml/min. Oven, detector and injector temperatures were 125, 180 and 175°C, respectively.
Additionally, subsamples of stools collected for microbiota analysis were stored at − 80°C. Bacterial DNA was purified using QIAamp DNA stool mini kits (Qiagen, Valencia, CA, USA) using the repeated bead beating plus column (RBB+C) method described by Yu & Morrison(Reference Yu and Morrison16). Faecal DNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Quantitative PCR analysis
E. coli, Bifidobacterium genus and Lactobacillus genus were quantified via quantitative PCR using specific primers, as described in Hernot et al. (Reference Hernot, Boileau and Bauer17). Amplification was performed on a set of triplicate reactions for each bacterial group within each sample according to the procedures of Deplancke et al. (Reference Deplancke, Vidal and Ganessunker18). For amplification, 10 μl final volume containing 2X SYBR Green PCR Master Mix (Applied BioSystems, Foster City, CA, USA), 15 pmol of each primer and 5 ng of template DNA was used. Pure cultures of each bacterium were utilised to create a five-fold dilution series (10 × 100 to 10 × 105) in triplicate from target species(Reference Middelbos, Godoy and Fastinger19). DNA from each serial dilution was amplified along with faecal DNA samples using a Taqman ABI PRISM 7900HT Sequence Detection System (Applied BioSystems). The colony-forming units of each standard curve serial dilution was previously determined by plating the E. coli grown on Luria–Bertani (LB) medium (tryptose (10 g/l), yeast extract (5 g/l), NaCl (5 g/l); pH = 7), Lactobacillus genus on Difco Lactobacilli de Man–Rogosa–Sharpe (MRS) broth (Becton, Dickenson and Co., Sparks, MD, USA) and the Bifidobacterium genus on Difco Reinforced Clostridial Medium (Becton, Dickenson and Co.). Cycle threshold values were plotted against standard curves for quantification (colony-forming units per g faeces) of the target bacterial DNA from faecal samples.
Calculations and statistical analyses
Data were analysed using the Mixed Models procedure of SAS (SAS Institute, Inc., Cary, NC, USA). The fixed effect of treatment was tested. Period and subject were considered random effects. Total dietary fibre consumed, as determined through diet records, was used as a covariate. Differences among treatments were determined using a Fisher-protected least significant difference with a Tukey adjustment to control for experiment-wise error. Non-continuous survey data were compared using the GLIMMIX procedure of SAS. These data were averaged by period. The fixed effect of treatment was tested. A probability of P < 0·05 was accepted as statistically significant and a probability of P < 0·10 was considered a trend. Reported pooled standard errors of the mean were determined according to the Mixed Models procedure of SAS.
Results
Of the twenty-five subjects enrolled, three were removed from the study before study initiation and one did not complete the study. Two (one Latino and one Asian) were dropped due to moving away, one (Caucasian) was dropped due to starting medication restricted by the study, and one (Latino) was dropped due to aberrant faecal patterns (greater than three watery stools per d throughout the study) compared with the remainder of the study population (as determined during the initial tolerance study and before initiation of the present study). The descriptions of the twenty-one subjects are listed in Table 1. Macronutrient intake for each treatment group is presented in Table 2. Macronutrient intake did not differ among treatment groups (P>0·05).
Table 1 Physical characteristics of the experimental subjects
(Mean values, standard deviations and ranges)
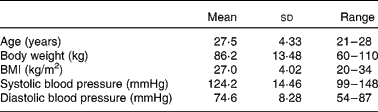
Table 2 Macronutrient intake of healthy adult men consuming no supplemental fibre (no fibre control; NFC), polydextrose (PDX; 21 g/d) or soluble maize fibre (SCF; 21 g/d)
(Mean values with their pooled standard errors for twenty-one subjects)
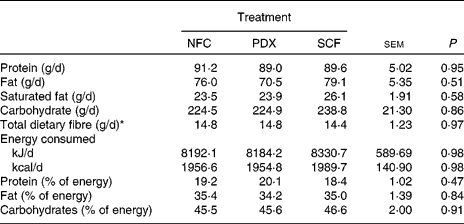
* Total dietary fibre values reflect dietary fibre consumed in the normal diet of the subjects only; supplemental fibre is not included.
Analysis of the test bars indicated that they were very similar in chemical composition (Table 3). Resistant oligosaccharides and total dietary fibre concentrations indicated that the treatment bars with functional fibres contained approximately 8 g fibre per bar (PDX = 8·06 g/bar; SCF = 7·60 g/bar). After removal of the intrinsic fibre of the bar (1·0 %), the bars contained approximately 7·5 g/bar (PDX = 7·64 g/bar; SCF = 7·18 g/bar), which is close to the formulated value for these bars (7 g/bar).
Table 3 Composition of experimental fibre snack bars
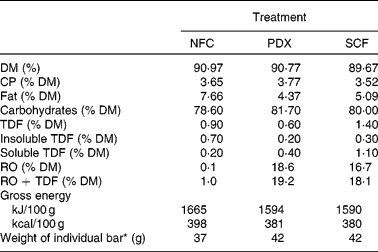
NFC, no supplemental fibre control; PDX, polydextrose; SCF, soluble maize fibre; CP, crude protein; TDF, total dietary fibre; RO, resistant oligosaccharides.
* Bars were manufactured to weigh as designated above for each treatment, ± 2 g.
The main effects of treatment for gastrointestinal tolerance, ease of stool passage and faecal scoring (stool consistency) are presented in Table 4. Flatulence (P = 0·001) and distention (P = 0·07) were greatest when subjects consumed PDX or SCF. Reflux was greater (P = 0·04) when subjects consumed SCF compared with NFC. Ease of stool passage and stool consistency did not differ due to treatment.
Table 4 Gastrointestinal tolerance scores and stool characteristic subjective scores during the entire treatment period for healthy adult men consuming no supplemental fibre (no fibre control; NFC), polydextrose (PDX; 21 g/d) or soluble maize fibre (SCF; 21 g/d)
(Mean values with their pooled standard errors for twenty-one subjects)
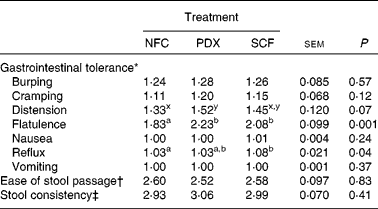
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
x,y Mean values within a row with unlike superscript letters tended to differ (P < 0·10).
* Gastrointestinal tolerance was scored using the following scale: 1 = none, 2 = mild, 3 = moderate, 4 = severe.
† Ease of stool passage was scored using the following scale: 1 = very easy, 2 = easy, 3 = neither easy nor difficult, 4 = difficult, 5 = very difficult.
‡ Stool consistency was scored using the following scale: 1 = hard, dry pellets – small, hard mass; 2 = hard, formed, dry stool – remains firm and soft; 3 = soft, formed, moist – softer stool that retains shape; 4 = soft, unformed – stool assumes shape of container; 5 = watery – liquid that can be poured.
Faecal fermentative endproducts are presented in Table 5. Faecal ammonia concentration was decreased (P < 0·0001) when subjects consumed either of the functional fibres, but was lowest with PDX. Faecal 4-methylphenol, indole, and isobutyrate, isovalerate and total BCFA concentrations were decreased (P < 0·01) when subjects consumed the functional fibre sources compared with NFC. Faecal valerate concentrations were lower (P < 0·005) when subjects consumed PDX compared with NFC, with SCF intermediate. Faecal 2,3-methyl indole did not differ (P = 0·72) among treatments.
Table 5 Faecal fermentative endproducts of healthy adult men consuming no supplemental fibre (no fibre control; NFC), polydextrose (PDX; 21 g/d) or soluble maize fibre (SCF; 21 g/d)
(Mean values with their pooled standard errors for twenty-one subjects)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
x,y Mean values within a row with unlike superscript letters tended to differ (P < 0·10).
Faecal acetate, propionate and butyrate concentrations were lowest (P < 0·05) when subjects consumed PDX compared with SCF and NFC. Faecal SCFA molar ratios of acetate were higher (P < 0·0001) and ratios of butyrate lower (P = 0·005) when subjects consumed the PDX or SCF compared with NFC. Faecal propionate molar ratio tended to be lower (P = 0·09) when subjects consumed PDX compared with NFC.
Faecal pH was lower (P = 0·01) when subjects consumed SCF compared with NFC, while PDX was intermediate (Table 6). Faecal 5 d wet weight tended to be greater (P = 0·06) when subjects consumed SCF compared with NFC, while faecal 5 d dry weight was greater (P = 0·02) when subjects consumed PDX or SCF compared with NFC. Faecal 4 d wet weight was greatest (P = 0·03) when subjects consumed SCF compared with NFC, with PDX intermediate. Faecal 4 d dry weight tended to be greater (P = 0·07) when subjects consumed PDX compared with NFC. Average (g/d) faecal wet weight tended to be greater (P = 0·06) when subjects consumed SCF compared with NFC, while average faecal dry weight was greater (P = 0·02) when subjects consumed either supplemental fibre. The functional fibres led to 1·4 and 0·9 g (PDX and SCF, respectively) increases in faecal dry mass per g supplemental fibre intake. Number of defecations per period did not differ (P = 0·56) among treatments.
Table 6 Faecal pH, weight and mass increase per g for fibre intake of healthy adult men consuming no supplemental fibre (no fibre control; NFC), polydextrose (PDX; 21 g/d) or soluble maize fibre (SCF; 21 g/d)
(Mean values with their pooled standard errors for twenty-one subjects)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
x,y Mean values within a row with unlike superscript letters tended to differ (P < 0·10).
Faecal microbiota data are presented in Table 7. Bifidobacterium spp. were present in higher (P < 0·05) concentrations when subjects consumed SCF compared with NFC, while PDX was intermediate. Faecal Lactobacillus spp. and E. coli populations did not differ among treatments.
Table 7 Faecal microbiota (log colony-forming units/g DM faeces) of healthy adult men consuming no supplemental fibre (no fibre control; NFC), polydextrose (PDX; 21 g/d) or soluble maize fibre (SCF; 21 g/d)
(Mean values with their pooled standard errors for twenty-one subjects)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
The average dietary fibre intake in the USA is well below the recommended amount, 38 g/d for an adult man(1). Therefore, finding ways of increasing fibre consumption in the USA is of importance to aid colonic health. Adding non-digestible, fermentable carbohydrate sources that act as dietary fibres and that can be added to new or existing food products is one way to potentially increase fibre consumption. Therefore, the objective of the present study was to evaluate digestive physiological outcomes elicited by functional fibres fed to healthy adult men. PDX and SCF were evaluated for effects on gastrointestinal tolerance, faecal BCFA and putrefactive compound concentrations, faecal SCFA concentrations, laxation and faecal microbiota populations.
Dietary fibre ingestion, even in relatively small amounts, can lead to gastrointestinal discomfort but varies widely among individuals. Therefore, before determining efficiency of PDX and SCF, a dose–response experiment with the same individuals that were enrolled in the main experiment was conducted. It was determined through subjective scoring that the subject population was able to consume as much as 21 g PDX or SCF per d with little gastrointestinal discomfort (Supplemental Tables 1–4, available online at http://www.journals.cambridge.org/bjn). Furthermore, this amount of supplemental fibre bridged the gap to the fibre requirement of adult men (average intake 15 g/d, recommended intake 38 g/d, fibre gap 23 g/d).
Overall, compliance was excellent throughout the study. All diet records were analysed and it was determined that the subjects consumed a moderate-fibre diet (average fibre intake, 14·7 g/d). Dietary macronutrient composition also remained steady over time, as it did not differ due to treatment. The final composition of the test bars provided close to the 7 g/bar that was formulated. There was 1 % intrinsic fibre in the test bars (NFC), with the PDX bar containing approximately 7·6 g fibre/bar and the SCF bar containing 7·2 g fibre/bar after accounting for the intrinsic fibre present.
Excessive consumption of some fermentable carbohydrates has been reported to lead to gastrointestinal side effects such as bloating, abdominal cramps, flatulence and borborygmi, especially in sensitive individuals(Reference Sandler, Stewart and Liberman20). These symptoms are transient and cease or lessen when the fibre intake is reduced or stopped completely(Reference Flood, Auerbach and Craig2). Supplemental PDX and SCF were well tolerated by all subjects, with only mild increases in distension, flatulence and reflux. Interestingly, although only moderately, the greatest amount of flatulence was noted by subjects consuming PDX. Jie et al. (Reference Jie, Bang-yao and Ming-jie21) noted no changes in gastrointestinal discomfort when subjects consumed as much as 12 g PDX/d. Furthermore, total gas production was lowest for PDX compared with other substrates when evaluated in vitro (Reference Hernot, Boileau and Bauer17, Reference Vester Boler, Hernot and Boileau22); however, SCF was not tested in these experiments. Supplemental SCF consumed at 12 g/d was noted to increase self-reported flatulence and stomach noise scores(Reference Stewart, Nikhanj and Timm3). This increase in flatulence is similar to that noted in the present study. Stewart et al. (Reference Stewart, Nikhanj and Timm3) noted, on a ten-point scale, flatulence scores of 4·2 in subjects consuming SCF v. 2·8 in subjects consuming a maltodextrin control treatment. It was noted, however, that all subjects tolerated the supplemental fibre sources well. Similarly, in the present study, average tolerance scores were low ( < 2·5), indicating only mild to moderate discomfort.
We saw no changes in subjective scoring of stool consistency. These results are in contrast with previous literature indicating that PDX leads to a softening of the stool(Reference Flood, Auerbach and Craig2, Reference Jie, Bang-yao and Ming-jie21) and easier passage(Reference Middelbos, Godoy and Fastinger19). A softening of stools was not noted by Stewart et al. (Reference Stewart, Nikhanj and Timm3), however, when subjects consumed 12 g SCF/d. It should be noted that results from the present study were self-reported scores by the subjects; differences in objective measures of faecal weight and moisture content were noted.
Ammonia, phenol, indoles and BCFA (isobutyrate, isovalerate and valerate) are products of protein fermentation by gut microbiota. These putrefactive compounds are linked to bowel cancer and can damage the colonic epithelium and become tumour growth promoters(Reference Bone, Tamm and Hill23–Reference Bingham25). Decreases in faecal BCFA concentrations and ammonia are considered beneficial. Also, with increased stool production, there is a dilution effect, thereby potentially allowing less of the putrefactive compounds to come in contact with the intestinal epithelium. In the present study, supplemental fibre decreased all of the putrefactive compounds measured.
Lower concentrations of putrefactive compounds as a result of fibre ingestion have been reported previously. Pigs fed 30 g PDX/d had decreased BCFA concentrations in the distal colon(Reference Fava, Mäkivuokko and Siljander-Rasi26). Given that, in the present study, faecal BCFA were measured, distal colon values should be comparable with faecal values. Furthermore, it is the distal colon where carbohydrate is limiting and where protein is used as a fuel source for the microbiota. No effects were noted in ammonia concentrations when supplemental PDX was fed to pigs(Reference Fava, Mäkivuokko and Siljander-Rasi26). Indole, p-cresol, isovalerate and isobutyrate also were noted to decrease in human subjects fed a high-cholesterol diet with added PDX(Reference Endo, Kumemura and Nakamura5), and isobutyrate and isovalerate decreased in healthy adults consuming 8 g PDX/d(Reference Hengst, Ptok and Roessler27). PDX also decreased total BCFA in vitro (Reference Mäkivuokko, Kettunen and Saarinen28). Jie et al. (Reference Jie, Bang-yao and Ming-jie21) reported increased faecal isobutyrate concentrations when adult subjects consumed 8 or 12 g PDX/d. It is unclear why an increase was noted in that study as compared with the other literature. Soluble maize fibre decreased isobutyrate, isovalerate and ammonia production in vitro (Reference Maathuis, Hoffman and Evans7), which is consistent with the results of the present study.
Providing fermentable, non-digestible carbohydrates in a diet provides carbohydrate for the colonic microbiota. In the present study, we did not observe an increase in faecal SCFA, as would be expected, but rather a decrease when PDX was consumed, and no change between the NFC and SCF treatments. In vitro fermentation of fibres with the addition of PDX also reduced SCFA production compared with the substrates alone(Reference Vester Boler, Hernot and Boileau22). Furthermore, PDX fermented alone had the lowest SCFA production when compared with other fibre sources tested(Reference Hernot, Boileau and Bauer17, Reference Vester Boler, Hernot and Boileau22). Similar to the results of the present study, proximal, middle and distal colonic digesta samples from pigs fed 30 g PDX/d had numerically lower SCFA concentrations (acetate, propionate and butyrate)(Reference Fava, Mäkivuokko and Siljander-Rasi26). In the distal colon, as to faecal concentrations, both propionate and butyrate concentrations were statistically lower in pigs fed PDX. Again, in contrast to the reported literature, Jie et al. (Reference Jie, Bang-yao and Ming-jie21) noted greater faecal acetate and butyrate production when subjects consumed 8 or 12 g PDX/d as compared with subject baseline values.
The lack of SCFA differences between the SCF and NFC groups may be due to the increase in stool volume when subjects consumed the SCF, thereby leading to a dilution of SCFA in the faeces. A similar response was noted by Weaver et al. (Reference Weaver, Martin and Story29) in rats. When comparing faecal SCFA concentrations, only propionate had a greater concentration compared with the control group; however, caecal content weight was greater when rats consumed SCF compared with the control diet. Therefore, when SCFA were expressed on a total caecal contents basis, acetate, propionate and total SCFA amounts were greater in rats fed SCF compared with control(Reference Weaver, Martin and Story29). A study in human subjects noted similar results to those of the present study, with no difference in faecal total SCFA concentrations of human subjects fed 12 g SCF/d compared with the control(Reference Stewart, Nikhanj and Timm3). Neither of these functional fibres appeared to be butyrogenic, and limited fermentation was noted compared with that of the NFC group. Other literature supports the lack of an increase in faecal butyrate with PDX or SCF supplementation(Reference Stewart, Nikhanj and Timm3, Reference Fava, Mäkivuokko and Siljander-Rasi26, Reference Weaver, Martin and Story29). Faecal SCFA concentrations were similar to those reported in previous research evaluating human subjects consuming a resistant maltodextrin(Reference Fastinger, Karr-Lilienthal and Spears30).
Faecal SCFA molar ratios were similar to those reported by Fastinger et al. (Reference Fastinger, Karr-Lilienthal and Spears30), but the molar ratio of acetate was greater in the present study compared with that reported in previous research evaluating SCF(Reference Stewart, Nikhanj and Timm3). In that study, the SCFA molar ratios (0·43 acetate, 0·26 propionate and 0·31 butyrate) did not differ between control (maltodextrin) and SCF treatments. This indicates that the difference between the present results and those of Stewart et al. (Reference Stewart, Nikhanj and Timm3) is probably due to the subject population, dietary patterns of the subjects or the analytical procedures used.
The decrease in pH was minimal, but consistent with previous research with human subjects consuming 12 g PDX/d(Reference Jie, Bang-yao and Ming-jie21). While the supplemental fibres did not increase the number of defecations per d, they led to an increase in stool volume, indicating a laxation effect. This is similar to results from other reports of PDX ingestion by human subjects(Reference Endo, Kumemura and Nakamura5, Reference Achour, Flourie and Briet6), and greater caecal content weights with PDX and SCF supplementation of rats(Reference Mäkivuokko, Kettunen and Saarinen28). No change in stool weight was noted when subjects were supplemented with 8 g PDX/d, but the authors noted an increase in orofaecal transit time when subjects consumed PDX(Reference Hengst, Ptok and Roessler27). Although both total 5 d and 4 d total faecal collection values are provided, the 4 d total faecal weights are a more accurate measure of faecal mass due to subject error during the initial collection day of the first period.
The present results indicate that both PDX and SCF consumed at 21 g/d may have a prebiotic effect in healthy adult men, as both led to an approximate 1 log increase in Bifidobacterium spp., with the greatest effect being noted for SCF. Data regarding the prebiotic effect of PDX are mixed. Bifidobacteria populations were not changed with PDX supplementation of pigs(Reference Fava, Mäkivuokko and Siljander-Rasi26) or human subjects(Reference Endo, Kumemura and Nakamura5). Interestingly, in pigs, bifidobacteria populations decreased numerically compared with the control treatment(Reference Fava, Mäkivuokko and Siljander-Rasi26). Bifidobacteria populations were high in comparison with baseline data in the present study, 9·7–10·1 log10 per g wet faeces(Reference Endo, Kumemura and Nakamura5), which may have masked any effects of PDX on these populations. Similar to results of the present study, PDX at 4, 8 or 12 g/d led to increases in faecal bifidobacteria(Reference Jie, Bang-yao and Ming-jie21). A 1 log unit change was noted after supplementation with 12 g PDX/d(Reference Jie, Bang-yao and Ming-jie21); we noted a similar response with 21 g PDX/d supplementation. PDX also is reported to lead to increases in bifidobacteria in vitro (Reference Hernot, Boileau and Bauer17, Reference Vester Boler, Hernot and Boileau22, Reference Probert, Apajalahti and Rautonen31). There is limited literature regarding the utilisation of SCF, and only one study in the literature to date evaluating microbiota. Total bifidobacteria species populations were greater for SCF over time when analysed semi-quantitatively in vitro, with B. adolescentis and B. bifidum being increased the greatest(Reference Maathuis, Hoffman and Evans7). Given the magnitude of change in Bifidobacterium spp. (1·3 log units) when subjects consumed SCF in the present study, further investigation into its prebiotic potential is warranted.
Overall, these data are consistent with previous reports that PDX and SCF are fermentable fibres and may be beneficial to colonic health. The effects noted in the present study, including reduction in faecal putrefactive compounds, stool bulking and bifidogenic potential, indicate that these functional fibres exert positive health effects in humans. Furthermore, these supplemental fibres, even at relatively large dosages (21 g/d), minimally increased gastrointestinal tolerance scores, indicating only slight discomfort in the present study population, while still providing a highly acceptable snack bar product. Therefore, PDX and SCF may be good candidates as supplemental fibre sources in food products for humans.
Acknowledgements
The present study was supported by General Mills, Inc.
B. M. V. B., T. W. B., K. S. S. and G. C. F. designed the research; B. M. V. B., M. C. R. S. and L. L. B. conducted the research; B. M. V. B. analysed the data; M. S. and T. W. B. provided essential materials for this research; B. M. V. B. wrote the manuscript; and G. C. F. had primary responsibility for the final content. All authors read and approved the final manuscript.
M. S. and T. W. B. work for General Mills, Inc. who provided funding for the study. B. M. V. B., M. C. R. S., L. L. B., K. S. S. and G. C. F. have no conflicts of interest.









