Stunting, or low height-for-age(Reference de Onis1), also referred to as growth retardation, is the most common form of undernutrition among children under 5 years(2). With an estimated 149 million (22 %) of children affected(3), stunting has been identified as a global public health priority. Stunting is associated with an increased risk of morbidity and mortality, poor cognitive and physical development, with significant educational and economic consequences(Reference Victora, Adair and Fall4) as well as an increased risk of non-communicable diseases in adulthood(Reference Victora, Adair and Fall4,Reference Gluckman, Hanson and Beedle5) . The World Health Assembly has set targets to reduce stunting by 40 % between 2010 and 2025(6); however, at the current rate of reduction, several countries, especially low- and middle-income countries, are unlikely to achieve the targets(2).
For many years, policies to reduce child undernutrition in low- and middle-income countries, particularly in sub-Saharan Africa, have often focused on improving household income and food security(Reference Amugsi, Mittelmark and Lartey7,Reference Kennedy and Haddad8) . However, evidence shows that increasing household income and food security, though necessary, is not sufficient for improved child nutritional status(Reference Engle, Bentley and Pelto9). Research also shows that interventions to improve child nutritional status through improved household income and/or food security depend largely on child care practices within the household(Reference Engle, Lhostika and Armstrong10,Reference Kennedy and Peters11) . Several studies showed that better care practices related to feeding and preventive health practices can significantly improve child nutritional status, even in households with limited economic and food resources(Reference Brown, Zeitlin and Peterson12–Reference Zeitlin14).
In Rwanda, 35 % of children younger than 5 years were stunted in 2018(15). Data on prevalence of stunting in Rwanda show a national downward trend (48·3 % in 2000 to 35 % in 2018); however, the reduction has not been consistent across the country. For example, in fourteen out of thirty districts of Rwanda, 40–54 % of children under 5 years are stunted(15). Based on the WHO thresholds(Reference de Onis, Borghi and Arimond16), these rates of stunting are of very high public health significance and require urgent actions. Thus, research is needed to understand the factors underlying the persistence of child stunting in Rwanda. Given the significant disparity in stunting prevalence, a greater understanding of subnational level factors is needed to inform the design of more targeted programmes and policies to effectively address child stunting in Rwanda.
Studies(17,Reference Lee, Dusingizimana and Umutoni18) have documented gaps in care practices related to child feeding practices in Rwanda. For example, the number of children aged 6–23 months who met the minimum acceptable diet remained unchanged (17 %) between 2010 and 2018(15). Increasing evidence also suggests that poor care practices, including sub-optimal child feeding practices, are potentially contributing to the high rates of child undernutrition in Rwanda(15,Reference Lung’aho, Birachi and Butare19,20) . In addition, studies indicate inadequate health practices such as low utilisation of child growth monitoring for screening of child nutritional status(Reference Ngirabega, Leonard and Munyanshongore21). However, research is limited on the influence of child feeding patterns on child nutrition status. A few studies examining the relationship between infant and young child feeding (IYCF) practices and child nutrition status in Rwanda have assessed single indicators of feeding practices and produced mixed results. Uwiringiyimana et al. (Reference Uwiringiyimana, Ocké and Amer22) found that exclusive breast-feeding was significantly positively associated with height-for-age z-scores (HAZ) in children aged 5–30 months (n 138). Conversely, Matsiko(Reference Matsiko23) found that achieving minimum dietary diversity, minimum meal frequency and acceptable diet were not significantly associated with HAZ in children aged 6–12 months (n 192). Given that child feeding has several dimensions (e.g. the type, quality and variety of foods, the quantity of foods, responsive feeding, etc.), studies examining the association between single feeding practices and child nutrition status may not provide a comprehensive picture of the association between overall child feeding patterns and nutritional outcomes(Reference Arimond and Ruel24). Ruel & Menon(Reference Ruel and Menon25) proposed a novel approach of assessing the relationship between child feeding patterns and nutritional status using an index that combined various dimensions of child feeding. Drawing from Ruel and Menon’s approach, Condo et al. (Reference Condo, Gage and Mock26) examined the association between a feeding index and nutritional status in Rwanda. The authors found no association between the feeding index and child nutritional status; however, their findings may not apply to all children because the study focused on HIV-infected children whose caregivers’ feeding practices may differ from those of caregivers with healthy children.
The objectives of the present study were to identify the factors associated with child stunting in Rwanda, including the relationship between indices of child feeding and health practices and child stunting. Our assumption was that better feeding and health practices are positively associated with better child HAZ and that mothers of non-stunted children have better child feeding and health practices than those of stunted children. This study contributes to the knowledge on the determinants of child stunting in Rwanda and extends the literature on the usefulness of indices of child feeding and health practices in relation to child nutritional status in the Rwandan context.
Methods
Settings
The study was conducted in Rutsiro district, northwest of Rwanda, 150 km from the capital city, Kigali. The inhabitants of the district are predominantly rural and engaged in subsistence agriculture(15). About 50 % of the population in Rutsiro are food insecure, and the prevalence of stunting increased from 46 % in 2015 to 54 % in 2018(15). The public health system of the district consists of a network of seventeen health centres and one district hospital(27).
Design and sample size
This was a cross-sectional study involving mother–child pairs. The sample size was calculated using the Cochrane formula for estimation of a single proportion(Reference Cochran28). Although our focus was not to estimate the proportion of stunted children, this would allow us to analyse the determinants of child nutritional status with a 95 % confidence of having an anticipated prevalence of stunting.
where n is the sample size, Z is 1·96, p is the proportion of stunting (46 % at the time of the survey(29)), q is 1−p and d is the relative desired precision of 5 %. The calculated sample size (n 382) was increased by 5 % to account for contingencies such as recording error, resulting in a final sample of 400. Because of the variability in geographical landscape, it was assumed that child feeding and/or health practices might differ across the district. Therefore, the district was divided into three zones based on the main roads connecting Rutsiro district to its neighbouring districts. Health centres were used as an entry point to facilitate access to the community, and community health workers who work under supervisions of the health centres assisted in identifying potential participants. Three health centres (nine in total across the district) were purposively selected in each zone to maximise geographic distribution. Within each health centre’s catchment area, two administrative entities (i.e. cells) were selected. The cell containing the health centre was automatically selected, and the next was randomly selected among distant cells. In each cell, one village was randomly selected, a list of all potentially eligible mothers with children aged 6–23 months were compiled using growth monitoring records obtained from community health workers and children were randomly selected from the lists. Eligibility criteria were: (1) child aged 6–23 months; (2) child was apparently healthy and (3) being in the lowest socio-economic categories. All participants gave oral informed consent prior to data collection. The survey was undertaken from September 2018 to January 2019.
Ethical approval
All study guidelines were in accordance with the guidelines laid down in the Declaration of Helsinki. The study procedures were also approved by the Massey University Human Ethics Committee (reference: SOA 17/67) and the Institutional Review Board of the University of Rwanda’s College of Medicine and Health Sciences (reference: 003/CMHS IRB/2017). Permission to collect data was also obtained from the District public health office. Oral informed consent was obtained from all participants, after the purpose of the study was explained. Each participant received five soap bars (~$US 1) as a recognition for their participation.
Face-to-face interviews were conducted with mothers, and data were collected using a structured questionnaire digitally preprogrammed on the CommCare platform(30). The data were captured on a handheld tablet. Information on socio-demographic characteristics, maternal and child characteristics were collected. Mothers also reported infant birth weight, child morbidities in previous 4 weeks: diarrhoea (defined as ≥3 watery or loose stools per day), upper respiratory infections (coughing, runny nose or wheezing), fever and other illnesses. In addition, household socio-economic characteristics such as size, number of children under 5 years, ownership of agricultural land and other household assets, and source of drinking water were reported. Altitude and location of the household premises were recorded using a handheld Global Positioning System (Tremble Juno SB Handheld).
Measures
Anthropometry
Anthropometric measurements were taken following standard procedures(Reference Cogill31). Child height was measured to the nearest 1 mm in a recumbent position using a UNICEF designed length board. The height of mothers was measured using a wall-mounted portable stadiometer to the nearest 0·1 cm. The weight of both mother and child was measured to the nearest 100 g using an electronic scale (SECA model 874).
Feeding and health practices
Breast-feeding and complementary feeding practices, and health practices (antenatal care visits during the last pregnancy, attendance at growth monitoring, use of deworming tablets, child immunisation status, use of vitamin A supplements) were reported by the mother. Birth certificates and immunisation records were used to verify information on birth weight and immunisation status, respectively.
Infant and child feeding index
We adapted the methodology used by Ruel & Menon(Reference Ruel and Menon25) and others(Reference Jones32–Reference Sawadogo, Martin-Prevel and Savy34) to construct an infant and child feeding index (ICFI). The ICFI consisted of five child feeding practice components: current breast-feeding (yes/no), duration of exclusive breast-feeding (i.e. whether the mother exclusively breastfed for the first 6 months), dietary diversity indicator, feeding frequency and responsiveness during feeding. Dietary diversity and meal frequency were calculated using 24-h dietary recall data(35). The feeding practice components and the scoring system used to create the ICFI for the different age groups are described in the online Supplementary Table S1. Briefly, a score of 1 or 2 was assigned for a positive practice, and a score of 0 was assigned for a potentially negative practice. The practices were judged positive or negative based on child feeding recommendations(Reference Dewey36) as well as evidence about their potential benefits or risks(Reference Brown, Dewey and Allen37).
For children aged 6–8 months and 9–11 months, a score of 2 was assigned for mothers whose children were still breast-feeding. For children aged 12–23 months, a score of 1 was assigned for mothers whose children were still breast-feeding. For all children, a score of 0 was assigned for mothers whose children were not breast-feeding. A score of 1 was also assigned for mothers who reported exclusive breast-feeding for the first 6 months, and a score of 0 was given to those who introduced complementary foods to their children prior to 6 months.
A dietary diversity indicator was created using information collected on the number of food groups consumed by the child in the previous 24 h. Following the guidelines for young child feeding(35), seven food groups, adapted from the Rwanda’s Demographic and Health Survey(29), were considered: grains/roots/tubers, legumes/nuts, milk or dairy products, flesh foods (meat/fish/poultry), eggs, vitamin A fruits and vegetables, and other fruits and vegetables. A score of 1 was given for each food group consumed, resulting in a dietary diversity score ranging from 0 to 7 which was divided into three categories: low, medium and high. For all age groups, low category included children who had not received any foods. For both 6–8 months and 9–11 months age groups, a child was classified into medium category if a child received 1–2 food groups, or into high category if a child received three or more food groups. For children 12–23 months, a medium category composed of children who had received 2–3 food groups, whereas a high category comprised those receiving ≥4 food groups.
Feeding frequency scoring criteria used information collected on the number of meals received by the child in the past 24 h. This indicator reflects the WHO recommended minimum meal frequency for breastfed and non-breastfed children (i.e. breastfed children aged 6–8 months and 9–23 months should receive solid or semi-solid or soft foods at least two times/d and two times/d, respectively). Thin/watery porridges and trivial snacks were not counted(35).
Indicator of responsive feeding was based on current young child feeding recommendation(Reference Dewey36), and it was informed by a previous study(Reference Dusingizimana, Weber and Ramilan38) conducted in the same population. It was defined as caregiver’s self-report of encouraging food intake of children who refuse to eat due to loss of appetite or during diarrhoea(Reference Engle, Lhostika and Armstrong10). A score of 1 was assigned for an action to encourage food intake, and a score of 0 if the action potentially restricted/discouraged child’s food intake.
The ICFI was created by adding up the scores obtained from all the component practices. For each age group, the minimum ICFI score was 0 and maximum possible ICFI score was 9. The scores were ranked, and tertiles were created to form three categories: low, medium and high. Grouping the ICFI score into tertiles, although arbitrary, was done to allow comparison with other studies that have examined the relationship between ICFI and child HAZ.
Health practices index
A methodology used by Ruel et al. (Reference Ruel, Levin and Armar-Klemesu13) was adapted to develop a health practices index (HPI). Five health practices were considered: attendance at growth monitoring in the past month, the number of antenatal care visits when pregnant with the study child, child diarrhoea treatment methods, vitamin A supplementation (whether the child received vitamin A supplements in the previous 6 months) and immunisation status (whether the child received all age-specific vaccines). The scoring system for the different health practices is described in the online Supplementary Table S2.
Household hunger level
Household hunger level was assessed and categorised using a validated cross-cultural household hunger scale(Reference Ballard, Coates and Swindale39). Following the household hunger scale measurement guide, a six-point household hunger score was generated and categorised into three levels of household hunger: little or no hunger (score: 0–1 score); moderate hunger (score: 2–3) and severe hunger (score: 4–6). The moderate and severe hunger level categories were combined to form one category, that is, moderate/severe, as there were few households (4·2 %, n 16) in the severe hunger category.
Household wealth index
A household wealth index – a proxy measure of household socio-economic status – was constructed using a principal component analysis(Reference Vyas and Kumaranayake40), with the following twelve variables: access to agricultural land, purchase of commercial fertilizer in the previous cropping season, the quality of housing (type of floor, wall), degree of household crowding, that is, the number of persons sleeping in a room (1 if ≤ 3 persons/room and 0 if ≥ 4 persons/room), source of lighting (none, petrol lamp or solar/electricity), ownership of durable assets (radio, thermos, mattress, umbrella), ownership of health insurance for all household members and a measure of animal ownership expressed in tropical livestock units. The tropical livestock unit is a metric combining multiple species of livestock into a weighted measure representing total body weight and potential market value(Reference Mosites, Rabinowitz and Thumbi41). The tropical livestock unit variable was skewed and, therefore, three categories were created. All variables were thus categorical and were ranked in ascending order, that is, from the worst to the best. The scores of the first two extracted components of the principal component analysis were used as a measure of household wealth index and included as covariates in multivariable regression analyses.
Statistical analysis
The WHO Anthro software version 3.2.2 was used to calculate HAZ(42). A child was classified as stunted if his/her HAZ was 2 sd below the median of the WHO reference population(43). A binary indicator of stunting (stunted/non-stunted) was used to allow comparison between care practices and other characteristics of mothers of non-stunted and those of stunted children. Continuous variables were summarised using means and standard deviations or medians and interquartile ranges for non-normally distributed variables. Categorical variables were summarised using frequencies and percentages. The Mann–Whitney U test or independent t test (continuous variables) and χ 2 test (categorical variables) were used to test differences in care practices and other characteristics between non-stunted and stunted children. Multiple linear regression analyses were used to examine the relationship between various factors and child HAZ (dependent variable). To identify variables to include in the regression models, we first represented hypothetical assumptions about the causal relationships between various factors and child HAZ using DAGitty software(Reference Textor, Hardt and Knüppel44). The software was used to construct directed acyclic graphs which specify the relationships among factors based on a priori evidence, theoretical knowledge and researcher’s subject matter expertise(Reference Greenland, Pearl and Robins45), while enabling identifying covariates to adjust for in multiple regression models(Reference Greenland, Pearl and Robins45). Although we aimed to examine association between various factors and child HAZ, ICFI and HPI were particularly of interest because feeding and health practices are modifiable factors often targeted by interventions to prevent stunting. Therefore, two different regression models containing ICFI and HPI as main independent variables were fitted. The first model (model 1) examined association between ICFI and child HAZ, adjusting for all other covariates at child, maternal and household level included in the model. The second model (model 2) examined association between HPI and child HAZ, adjusting for the same covariates as in model 1. Since feeding practices are linked to child food intake, which is a direct determinant of child HAZ (besides illnesses), we also adjusted for ICFI in model 2. Because of age-related variability in child feeding practices(Reference Dewey36), we ran separate regression analyses: for all children, and for those aged 6–11 months and 12–23 months. All assumptions were assessed for all models. Multicollinearity was checked using the variance inflation factor of ten for all explanatory variables(Reference James, Witten and Hastie46). Homoscedasticity was assessed by visual inspection of a plot of standardised residuals v. standardised predicted values. Variables with P < 0·05 were considered statistically significant. All analyses were performed using SPSS version 25 (IBM).
Results
Of the 400 child–mother pairs recruited, twenty-one children (5 %) were excluded from the analysis due to preterm birth or lack of health cards to verify birth weight information. Thus, the descriptive analysis was performed on 379 children. Of these, thirty-five mothers (9 %) had no height measurements, whereas 10 (2·5 %) had no data on education. The final sample considered for regression analyses was thus 334 (~84 %) mother–child pairs. The median age was significantly lower (P < 0·001) for non-stunted children than for stunted children (Table 1). Mothers of non-stunted children were older and taller than those of stunted children. Approximately 60 % of the mothers had either never attended school or did not complete primary education; 16 % had some secondary education, and 65 % did not achieve the recommended number of ≥ 4 antenatal care visits. Of the households, 43 % had experienced moderate to severe hunger in the past 4 weeks.
Table 1. Child-, maternal- and household-level characteristics, by child nutritional status*
(Frequencies and percentages; means and standard deviations; medians and interquartile ranges (IQR))

HAZ, height-for-age z-score; ANC, antenatal care; masl, metres above sea level.
* Data are missing for maternal education (n 10) and maternal height (n 36).
† Source of drinking water defined according to UNICEF/WHO(47): improved = public taps, standpipes, tube wells, boreholes, protected well and springs, rainwater; unimproved = unprotected well and spring, surface water.
Nutritional status
The mean HAZ was −1·69 (sd 1·3). Of the children, 38 % were stunted (Table 3), and the stunting prevalence in older children was more than twice that in younger children (47 v. 21 %, P < 0·001). There was no significant difference in the prevalence of stunting between males and females or between household wealth index categories.
Table 2. Distribution of the infant and child feeding index (ICFI) and its components by child nutritional status
(Frequencies and percentages; ranges; means and standard deviations)
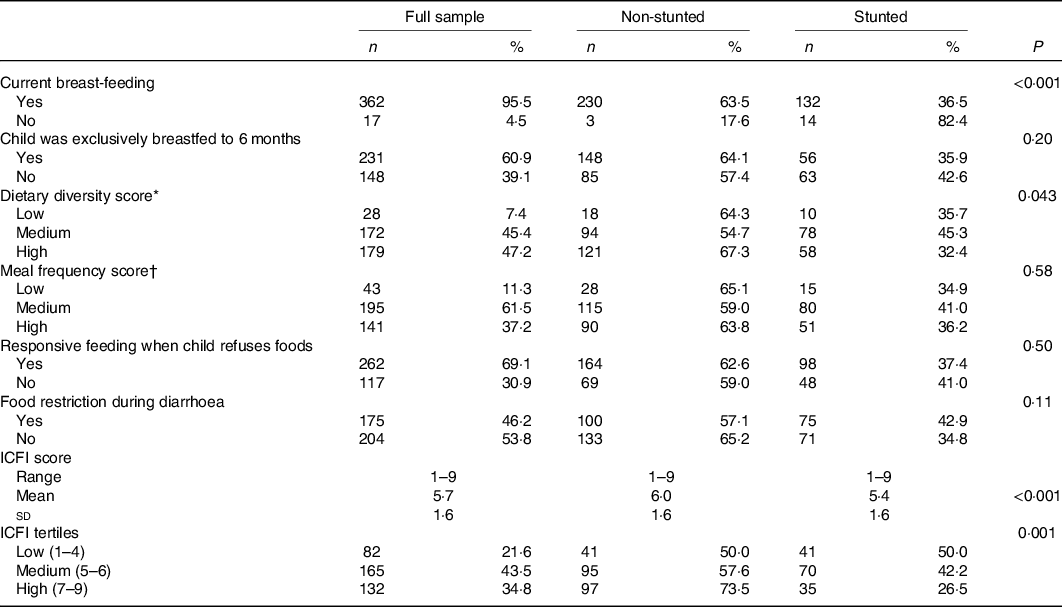
* For age 12–23 months, medium = 2–3 food groups/d and high = 4 or more food groups/d.
† For age 12–23 months, medium = 1–2 meals/d and high = 4+ meals/d.
Table 3. Distribution of the health practices index (HPI) and its components by nutritional status of children aged 6–23 months, Rutsiro district, Rwanda, September 2018 to January 2019
(Frequencies and percentages; ranges; means and standard deviations)

Child feeding practices
Almost all children (96 %) were still breastfed at the time of the survey. However, over one-third of the mothers (39 %) introduced complementary foods to their children before the recommended age (i.e. 6 months) (Table 2). Approximately half (54 %) of the children were fed at the recommended minimum frequency; however, the diversity of foods consumed was low. On average, children consumed three food groups and only 36 % met the WHO recommended minimum dietary diversity (≥4 food groups) (online Supplementary Table S3). As compared with children aged 12–23 months, children in the age group of 6–11 months were less likely to achieve the recommended minimum dietary diversity (23 v. 42 %, P = 0·001) (data not shown). Nearly all children (93 %) consumed staples (grains, roots or tubers), whereas the consumption of animal source foods was low (27 %) (online Supplementary Table S3). The mean ICFI score for all children was 5·8 (sd 1·6.) Non-stunted children had significantly higher ICFI score than stunted children (6·0 v. 5·4, P = 0·001) (Table 2).
Child morbidity and health practices
Morbidity among children during the previous 4 weeks was high: 47 % had experienced diarrhoea, 78 % had symptoms of respiratory infections, 43 % had fever and 11 % had other illness (online Supplementary Table S3). No significant difference was observed in the prevalence of morbidity between non-stunted and stunted children. However, results from sub-analyses (data not shown) showed that, in younger children (6–11 months), stunted children were more likely to have diarrhoea than non-stunted (64 v. 42 %, P = 0·05). About half (46 %) of the mothers whose children had suffered from diarrhoea reported either never seeking treatment or using home remedies to treat their child diarrhoea. The prevalence of stunting was higher among children whose mothers did not seek treatment or used home remedies for child diarrhoea than among children whose mothers sought diarrhoea treatment from a health centre or health worker (58 v. 48 %, P = 0·057). The mean HPI was 4·5 (sd 1·0), with no significant difference between non-stunted and stunted children (P = 0·58) (Table 3).
Association between infant and child feeding index, health practices index and child nutritional status
Results from bivariate analyses showed that ICFI was associated with child nutritional status. We found a significant difference of 0·34 in mean HAZ between children in the highest compared with the lowest ICFI tertiles (P < 0·001) (data not shown). Additionally, the prevalence of stunting was significantly lower among children whose mothers were in the high ICFI tertile compared with those in the lowest tertile (27 v. 50 %, P = 0·001) (Table 2). In bivariate analysis, the association between HPI and HAZ was not statistically significant (P = 0·10) (data not shown). The prevalence of stunting was also not as expected; it was significantly higher among children whose mothers were in the medium HPI tertile as compared with low and high tertiles (Table 3). However, when the two lowest tertiles (low and medium) were combined and compared with the high tertile, the prevalence of stunting was significantly lower among children in the highest tertile compared with the lowest tertile (33·5 v. 43·8 %, P = 0·04).
Results from multiple linear regression analyses are shown in Table 4. There was a significant and positive association between ICFI and HAZ, after controlling for potential confounders at child, maternal and household level. We found a mean difference of 0·14 HAZ between children of mothers in the high ICFI tertile compared with those in the low ICFI tertile (P = 0·039) (Table 4, model 1). When analysis was stratified by age, the association between ICFI and HAZ remains significant (β = 0·16, P = 0·025) only in older children (12–23 months) (online Supplementary Table S4). The association between HPI and HAZ was not statistically significant, neither when all age groups were combined (Table 4, model 2) nor in stratified analysis (online Supplementary Table S5). We also analysed individual components of ICFI and HPI, while controlling for variables as in ICFI or HPI models (data not shown). Among the components of ICFI, a significant and positive association was observed between breast-feeding and child HAZ (β = 0·13, P = 0·011). The association between dietary diversity indicator and child HAZ also approached statistical significance (β = 0·17, P = 0·087). None of the components of HPI was significantly associated with HAZ.
Table 4. Factors associated with height-for-age z-score (HAZ) of children aged 6–23 months in Rutsiro district, Rwanda, September 2018–January 2019*
(Standardised coefficients and 95 % confidence intervals)

β, Standardised coefficient; ICFI, infant and child feeding index; Ref., reference category; HPI, health practices index.
* Model 1 included ICFI as the main independent variable. Model 2 included HPI as the main independent variable. Both models included the same covariates, except that model 2 also included ICFI. (−) means that HPI was not included in model 1.
In both model 1 and model 2, child’s birth weight and maternal height were positively associated with HAZ. By contrast, child’s age, sex (male) and altitude were negatively associated with HAZ. In models including all children (6–23 months), respiratory infections tended to be significant and negatively associated with HAZ, but no association was observed between diarrhoea and HAZ. When analysed by age group, we found a statistically significant and negative association between both diarrhoea and respiratory infections and HAZ only in younger children (6–11 months) (online Supplementary Tables S4 and S5).
Discussion
Findings from the present study indicate that the burden of stunting among children aged 6–23 months in Rutsiro district is high (39 %). National estimates show that the prevalence of stunting increases from 18 % among children 6–8 months to 21 % among those aged 9–11 months to 49 % among those aged 18–23 months(29). Our results showed a similar trend and are comparable to those observed in other studies showing that the odds of stunting increases with age(Reference Binagwaho, Rukundo and Powers48).
In the present study, we found a feeding pattern that is similar to what has been observed in other studies conducted in Rwanda. For example, 39 % of the mothers reported introducing complementary foods to their children before 6 months. In a cross-sectional study(Reference Uwiringiyimana, Ocké and Amer22) and a longitudinal study(Reference Matsiko23) conducted in northern and southern provinces of Rwanda, respectively, researchers found that only 50 % of children were exclusively breastfed for the first 6 months. These results suggest that national estimates of exclusive breast-feeding prevalence (87 %)(29) may be an overestimation in rural Rwanda. In addition, the diet of children was less diverse, with majority of children (64 %) consuming less than the recommended four food groups, and the consumption of animal sources foods was extremely low. Other studies conducted in rural Rwanda have reported that 65–70 % of children younger than 24 months do not meet the WHO recommended dietary diversity(Reference Uwiringiyimana, Ocké and Amer22,29) . Although monotonous diets are correlated with household poverty in low- and middle-income countries(Reference Onyango49), several studies conducted in Rwanda revealed other important factors underlying poor child feeding practices, including maternal beliefs and food restrictions, especially during child illness(Reference Lee, Dusingizimana and Umutoni18,Reference Dusingizimana, Weber and Ramilan38) .
Our finding of a positive association between ICFI and child HAZ is consistent with other studies that have investigated the relationship between a summary of child feeding practices and child HAZ in rural(Reference Jones32,Reference Sawadogo, Martin-Prevel and Savy34,Reference Qu, Mi and Wang50) and urban populations(Reference Ruel, Levin and Armar-Klemesu13,Reference Moursi, Trèche and Martin-Prével51) . However, our result contrasts those of Ntab et al. (Reference Ntab, Simondon and Milet52) who found no association between ICFI and child HAZ in rural Senegalese children (n 500, age 12–42 months). These inconsistent results are probably due to differences in feeding practices. For example, contrary to our observation, breastfed children in the Senegalese study had lower mean HAZ than non-breastfed children because mothers prolonged breast-feeding for stunted children. Our findings of an association between ICFI and child HAZ only in older children are consistent with those of Ruel & Menon(Reference Ruel and Menon25) who found a positive association between ICFI and HAZ in older children (12–36 months). These authors suggested that the cumulative effect of improved care practices on child health may increase over time, and therefore, the effect of better child feeding practices may become more apparent in older children than in younger children(Reference Ruel and Menon25).
In the present study, two components of ICFI, that is, breast-feeding and dietary diversity indicators, were associated with child HAZ. Other studies in Rwanda(Reference Uwiringiyimana, Ocké and Amer22) and Kenya(Reference Onyango, Esrey and Kramer53) have reported positive associations between breast-feeding and linear growth among children < 24 months. However, our results regarding breast-feeding should be interpreted with caution because only a small number (5 %) of children were not breast-feeding. Our finding of a positive association between dietary diversity and child HAZ is also supported by findings from several countries in Africa (including Rwanda), Asia and Latin America(Reference Arimond and Ruel54,Reference Menon, Bamezai and Subandoro55) . This result underscores the use of the dietary diversity as a better proxy of feeding practices and suggests that interventions to diversify children’s diet have potential to improve child nutritional status of young children in Rwanda. However, studies found that complementary foods in Rwanda may be source of mycotoxins(Reference Grosshagauer, Milani and Kraemer56,Reference Matsiko, Kanyange and Ingabire57) . Consumption of mycotoxins, particularly aflatoxins, through complementary foods was found to be associated with child stunting in children (9–60 months)(Reference Gong, Hounsa and Egal58). This could be one of the reasons why the relationship of ICFI with child HAZ was not as strong as might be expected, but further investigation is needed in this area. Thus, to maximise the benefits of dietary diversification on children’s nutritional status, interventions should focus not only on improving the diversity of complementary foods, but also the safety of these foods.
The lack of association between HPI and child HAZ in the present study contrasts findings of Liu et al. (Reference Liu, Behrman and Stein59) who reported a significant and positive association between a health practices index (measured by access to prenatal care, timing and frequency of prenatal care visits) and child HAZ in a multi-country/pooled analysis of longitudinal data from four low- and middle-income countries. The discrepancy between our results and those of Liu et al. may be due to methodological differences. Our health index included five aspects of health practices, whereas that of Liu et al. included only indicators related to antenatal care. Additionally, some components (e.g. growth monitoring and antenatal care) used to construct the HPI serve as an entry point to nutrition services (e.g. counselling on IYCF practices and micronutrient supplementation); therefore, the effect of HPI in the present study on child nutritional status may be much more dependent on the quality of services provided to mothers. It was found in India and Bangladesh that growth monitoring programmes had little or no effect on child nutritional status because of failure to identify growth faltering among children or ineffective nutritional counselling to mothers(Reference Ashworth, Shrimpton and Jamil60).
Research shows that health promoting practices tend to cluster. That is, a mother who, for example, exclusively breastfeeds is more likely to engage in other positive practices(Reference Arimond and Ruel24). So, it has been suggested that summary indices may be useful to comprehensively assess the cumulative effect of various dimensions of child feeding and/or health practices on child nutritional status, while accounting for the interrelationships between these practices(Reference Moursi, Trèche and Martin-Prével51). Thus, conceptually, one would expect the effect size (i.e. adjusted mean HAZ difference between high and low categories) of the index on child HAZ to be higher than that of single practices. However, in the present analysis, only a few components (i.e. breast-feeding and dietary diversity) were associated with child HAZ and the effect size of each of these components on child HAZ was comparable to that of the ICFI. Moreover, the addition or removal of the ICFI from the model did not significantly change the adjusted R 2 (data not shown). These results suggest that, at least in this sample, these indices did not perform better than individual practices. Although indices have some advantages, such as summarising information on child feeding or health practices, they can also mask the individual practices they include(Reference Sawadogo, Martin-Prevel and Savy34). In addition, while composite indices have previously been used to examine associations between feeding/health practices and child nutritional status in various settings, there still no consensus on methodology to construct these indices. Therefore, further research is needed to validate these indices and to address their limitations. Such research could, for example, examine the performance of ICFI constructed by including other aspects of child feeding such as hygiene practices during food preparations, the types or amounts of foods as opposed to food groups.
Among covariates, child age, sex (male) and altitude were negatively associated with HAZ. These factors have been found to be associated with child stunting in Rwanda(Reference Habyarimana, Zewotir and Ramroop61,Reference Uwiringiyimana, Veldkamp and Amer62) and other sub-Saharan African countries(Reference Wamani, Åstrøm and Peterson63). In addition, maternal height and infant birth weight were positively associated with child HAZ. This relationship has been described in previous studies showing that both maternal height and child birth weight reflect the prenatal environment and strong determinants of subsequent child nutritional status(Reference Young, Nguyen and Gonzalez Casanova64,Reference Schmidt, Muslimatun and West65) . The association between maternal height and child HAZ may be explained by two mechanisms: shorter mothers have small uterine volume which constrains fetal growth(Reference Zhang, Cnattingius and Platt66), and/or placental transport mechanisms that limit provision of nutrients to the fetus and growth(Reference Price and Coe67). It is also suggested that this association could reflect shared genetic factors and/or common environmental factors that affect a mother during her early childhood and subsequently the growth of her offspring(Reference Hernandez-Diaz, Peterson and Dixit68). However, studies indicated that, in early childhood, the effect of environmental factors on child linear growth is stronger than that of genetic factors, especially in poorer regions where adverse environmental conditions, such as poor quality of diet and diseases, may not permit full expression of height potential(Reference Hernandez-Diaz, Peterson and Dixit68–Reference Liu, Yu and Gao70). A significant negative association was observed between child morbidities (diarrhoea and respiratory infection) and child HAZ, but only in younger children. This finding corroborates the previous aforementioned longitudinal study(Reference Matsiko23) that also found a significant negative association between days with diarrhoea or respiratory infection with decreased child linear growth among children aged < 12 months.
Strengths and limitations
One of the strengths of the present study is the use of summary indices to comprehensively examine the relationship between a variety of feeding and health practices and child HAZ. There are some limitations to the present study. First, the cross-sectional nature of the present study means that our analysis cannot establish causal relationships. A longitudinal study design is recommended to somewhat address this limitation. Second, the study was conducted in one district only and was limited to the lowest socio-economic groups, which limits the generalisability of our findings. Third, most of the information on feeding and health practices was obtained from mothers’ recall which is prone to recall bias and social desirability. Fourth, although we used the standard 24-h recall method to assess child feeding practices, dietary information may not represent the usual diet of children because certain foods may be infrequently consumed. For example, the consumption of animal source foods may be rare, whereas some fruits may be available only in certain seasons. Last, even though we controlled for several covariates, we cannot rule out the influence of unmeasured variables, such as hygiene and sanitation which are known to influence child nutritional status, particularly in resource-limited settings(Reference Onyango, Esrey and Kramer53).
Conclusion
Findings from the present study indicated that stunting among children 6–23 months in Rutsiro district remains of high public health concern and that child HAZ is associated with both pre- and postnatal factors. The results on the relationship between ICFI, HPI and child HAZ provided a mixed picture, making the overall conclusion regarding the role of care practices less clear cut. On one hand, the finding of a positive association between ICFI and child HAZ supports our assumption that better feeding practices are positively associated with better child HAZ in this setting. On the other hand, however, the fact that only a few components of the ICFI were significantly associated with child HAZ (findings also observed in other studies), and given the lack of a significant association between HPI and child HAZ, the findings of the present study raise questions on the usefulness of the composite indices and call for further research to validate such indices. Such research could help elucidate the strengths and weaknesses of using indices v. individual components. In the context of the present study, breast-feeding and child dietary diversity appear to have potential to improve child HAZ.
Acknowledgements
We are grateful to the mothers and their children involved in the study for their willingness to participate. We would like to thank the community health workers and Mr Jules Ihorere (research assistant) for their logistical support during data collection. This study was conducted as part of T. D.’s doctoral research funded by the New Zealand (NZ) Scholarships. Additional funding was obtained from Massey University. The funders had no role in the study design, analysis or writing of this article.
Authors’ contributions: T. D., J. L. W., T. R., P. O. I. and L. B. designed the study. T. D. collected data, analysed the data, interpreted the results and wrote the first draft of the manuscript. L. B., J. L. W. and T. R. advised on data analysis. All authors contributed to the results’ interpretation and critical revision of subsequent drafts of the manuscript. All authors approved the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114520004961







