INTRODUCTION
Porifera is the dominant phylum of sessile animals in marine cave environments (Sará, Reference Sará1962; Corriero et al., Reference Corriero, Liaci, Ruggiero and Pansini2000; Bell, Reference Bell2002). Marine caves are acknowledged for their rich assemblages and have been characterized as ‘sponge biodiversity reservoirs’ (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012). The majority of studies concerning cave sponge assemblages provide taxonomic descriptions, with special emphasis on the description of new species, as well as qualitative ecological information (e.g. Vacelet & Lévi, Reference Vacelet and Lévi1958; Boury-Esnault et al., Reference Boury-Esnault, Muricy, Gallissian and Vacelet1995; Vacelet & Boury-Esnault, Reference Vacelet and Boury-Esnault1996; Pisera & Vacelet, Reference Pisera and Vacelet2011; Rützler et al., Reference Rützler, Piantoni, Van Soest and Díaz2014). On the contrary, only a limited number of studies include quantitative data describing the variability of sponge coverage and diversity across the different cave sectors (Russ & Rützler, Reference Russ and Rützler1959; Pouliquen, Reference Pouliquen1972; Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989; Corriero et al., Reference Corriero, Liaci, Ruggiero and Pansini2000; Bell, Reference Bell2002; Parravicini et al., Reference Parravicini, Guidetti, Morri, Montefalcone, Donato and Bianchi2010).
Marine cave communities of the Mediterranean Sea have been studied more extensively than in any other marine area with Porifera being the most studied phylum in this ecosystem (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012, Reference Gerovasileiou and Voultsiadou2014). The majority of the relevant studies have taken place in shallow caves along the north-western and central Mediterranean rocky coasts. Interestingly, limited research in marine caves of the eastern basin has brought to light several new sponge species with a narrow distribution range (Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Vafidis, Koutsoubas, Sini, Dailianis, Issaris, Akritopoulou, Dimarchopoulou and Voultsiadou2015a and references therein). Information on the ecology of these species is scarce, while quantitative data describing the structure and diversity gradients of sponge assemblages in eastern Mediterranean marine caves is practically non-existent. This becomes critical given that the latter area is subjected to severe temperature alterations attributed to global climate change (Bianchi & Morri, Reference Bianchi and Morri2003), as well as to biological invasions (Gerovasileiou et al., Reference Gerovasileiou, Voultsiadou, Issaris and Zenetos2015b). Marine cave habitats are protected by the European and Mediterranean legislative framework due to their unique biological wealth (92/43/EEC and UNEP-MAP-RAC/SPA, 2015).
The study of marine caves involves several difficulties, related mainly to underwater fieldwork due to practical restrictions in space, time and visibility conditions. Therefore, the use of rapid and non-destructive photographic methods constitutes a valuable advantage for the quantitative study of cave communities. The main disadvantage of photographic methods are potential limitations as regards taxonomic identification of species (Bianchi et al., Reference Bianchi, Pronzato, Cattaneo-Vietti, Benedetti-Cecchi, Morri, Pansini, Chemello, Milazzo, Fraschetti, Terlizzi, Peirano, Salvati, Benzoni, Calcinai, Cerrano and Bavestrello2004), especially in areas where information on diversity is limited and light conditions are poor, which could affect animal colouration. To address such problems, a surrogate diversity measure might be a solution. Sponge morphological diversity (MD) is a good option, since it can be easily assessed during underwater fieldwork, even by non-specialists, and thus could be used in monitoring programmes (Bell & Barnes, Reference Bell and Barnes2001; Bell, Reference Bell2007; Schönberg & Fromont, Reference Schönberg, Fromont and Edwards2013). Bell & Barnes (Reference Bell and Barnes2001) showed that sponge MD could serve as a qualitative predictor of species diversity in open rocky habitats but no significant correlation was found in tropical marine caves (Bell & Barnes, Reference Bell and Barnes2002). The functionality of this model has not been examined either in temperate marine caves or in the Mediterranean ecosystem. The application of a photographic method along with diversity surrogates in eastern Mediterranean caves could both accelerate our knowledge of sponge communities in this area and check the effectiveness of these cost- and time-saving methods.
Under this scope, the aim of this study was to provide a quantitative description of sponge assemblages in two distinct marine caves of the eastern Mediterranean Sea, using non-destructive methods in order to examine (i) the specific composition of sponge assemblages and (ii) the spatial variability of sponge diversity in various cave microhabitats using several different diversity measures.
MATERIALS AND METHODS
Study areas
Two marine caves of the Aegean Sea (north-eastern Mediterranean) were studied. The caves represent distinct types, according to the morphological categorization proposed by Riedl (Reference Riedl1966), reflecting differences in the two critical abiotic factors, which generate ecological zonation in cave communities: (i) light and (ii) water movement. Both caves are situated off Lesvos Island, on the rocky islets of Agios Vasilios and Fara Bay.
The entrance of Agios Vasilios cave is located at a depth range of 24–40 m (average 32 m). Its large dimensions (16 m high and 7 m wide) result in a relatively well-lit zone within the first 10–15 m, giving the impression of a large cavern. The cave becomes narrower towards the interior, resembling a large blind funnel (Figure 1A, B). At a distance of 22 m from the entrance, the cave leads to a narrow dark tunnel ascending up to a depth of 15 m. Within the framework of this study only the first 20 m of this cave were surveyed.

Fig. 1. Scaled three-dimensional models of Agios Vasilios (A: lateral view, B: top view) and Fara cave (C: lateral view, D: top view) produced with ‘cavetopo’ software (Gerovasileiou et al., Reference Gerovasileiou, Trygonis, Sini, Koutsoubas and Voultsiadou2013).
The entrance of Fara cave is located at a depth of 18 m, while the average depth of the cave interior is 14 m. At its inner end, 32 m from the entrance, a narrow fissure connects this cave to a second smaller one on the other side of the islet; it actually constitutes a submerged tunnel (Figure 1C, D) penetrating the islet. The caves were mapped and three-dimensional depictions of their morphology were produced using ‘cavetopo’ software (Gerovasileiou et al., Reference Gerovasileiou, Trygonis, Sini, Koutsoubas and Voultsiadou2013).
Quantitative and qualitative sampling
Sampling was implemented with scuba diving. The sponge assemblages of the two caves were studied with 25 × 25 cm photoquadrats, as proposed by Kipson et al. (Reference Kipson, Fourt, Teixidó, Cebrian, Casas, Ballesteros, Zabala and Garrabou2011) for the study of sciaphilic sessile benthos. Three randomly placed replicate quadrats were photographed at 5 m intervals, from the entrance to the interior of the two caves, along three transects: one along the ceiling (C) and two along the opposite vertical walls (R: right and L: left). For comparison purposes, sampling was also performed on the adjacent side walls of the outer zone (Out) of the conic entrance of Fara cave, while this was not necessary for the entrance of Agios Vasilios cave, due to its specific morphology described above. In total, 72 quadrats were photographed in Fara and 45 in Agios Vasilios cave.
Additionally, about 80 sponge specimens were photographed in situ and collected from different zones of the two caves in order to identify the sponges present in the photoquadrats. Sponge specimens and spicule preparations were identified based on a large number of publications, in accordance with the classification proposed in Systema Porifera (Hooper & Van Soest, Reference Hooper and Van Soest2002) and the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015).
Image processing
Biotic per cent coverage of sponges present on the 117 photoquadrats was calculated using photoQuad; this advanced image processing software is dedicated to underwater ecological applications integrating various methods and tools for the accurate calculation of species coverage (Trygonis & Sini, Reference Trygonis and Sini2012). In each photoquadrat, the external outline around every sponge specimen was defined manually in detail using freehand drawing tools (i.e. freehand region method). All defined specimen surfaces (regions of interest) were assigned to the corresponding sponge species based on the in situ photographs of the identified qualitative samples. Furthermore, sponge specimens in each photoquadrat were assigned to eight morphological types, according to the relevant bibliography (i.e. Boury-Esnault & Rützler, Reference Boury-Esnault and Rützler1997; Bell & Barnes, Reference Bell and Barnes2001, Reference Bell and Barnes2002): massive, massive-tubular, arborescent, globular, encrusting, clathrate, boring and repent. Per cent coverage for every sponge species and morphological type was calculated automatically by photoQuad.
Assemblage structure and statistical analyses
Multivariate resemblance analysis of the quadrats photographed was performed separately for each marine cave with multidimensional scaling (MDS), based on the Bray–Curtis similarity index (fourth root transformed coverage data). The contribution of sponge species to Bray–Curtis dissimilarity among the resulting sample groups was estimated with SIMPER (SIMilarity PERcentages).
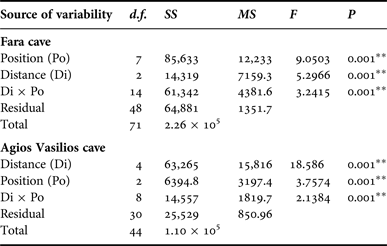
In order to assess the effect of internal cave topography on the sponge assemblage structure of each cave, two-way PERMANOVA was performed for two topographic factors: (i) Distance from entrance (Di), fixed with eight levels for Fara (Out, 0, 5, 10, 15, 20, 25 and 30 m) and five levels for Agios Vasilios cave (0, 5, 10, 15 and 20 m); and (ii) Position (Po), fixed with three levels for both caves (C: cave ceiling, L: left wall, R: right wall). The design was balanced with three replicates at each level of Po and crossed provided all levels of Po could be found at every level of Di and vice versa (Anderson et al., Reference Anderson, Gorley and Clarke2008).
Five diversity indices were calculated for the sponge fauna of each photoquadrat, based on coverage data: species richness (S), Shannon–Wiener diversity (H’), species evenness (J’), taxonomic (TD) and morphological diversity (MD). For the estimation of taxonomic diversity, taxonomic distinctness (Δ*) was chosen as a measure of taxonomic relatedness (Warwick & Clarke, Reference Warwick and Clarke1995; Clarke & Warwick, Reference Clarke and Warwick1998). Morphological diversity was calculated with the Shannon–Wiener function, as proposed by Maldonado & Young (Reference Maldonado and Young1996). Spearman's rank correlation coefficient was used to investigate relationships of MD with S and H’.
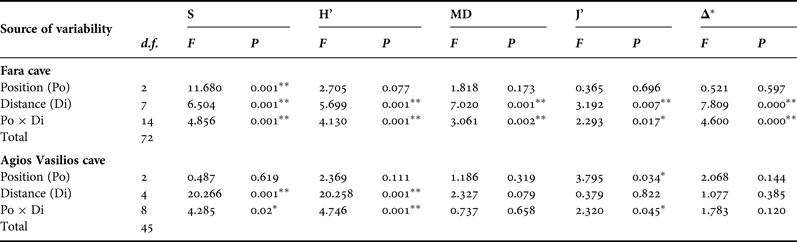
In order to assess the effect of the two aforementioned topographic factors (Di and Po) on the sponge diversity of the two caves, two-way ANOVA was performed for each diversity measure separately. Diversity data were checked for normality and homogeneity of variance with the Kolmogorov–Smirnov and Levene's tests, respectively, and were properly transformed, when these assumptions were not met. The Tukey's range post-hoc test was used in cases of significant differences between samples.
All statistical analyses were performed with the IBM SPSS Statistics 21 and PRIMER-E v6 software packages (Clarke & Gorley, Reference Clarke and Gorley2006), using the extracted sponge coverage data.
RESULTS
In total, 50 sponge species classified into three classes, 11 orders and 27 families were identified. Out of these, 46 species were recorded in Fara cave, 32 in Agios Vasilios cave and 28 were found in both caves (Table 1). One of the species found, Ircinia paucifilamentosa, is endemic to the Aegean ecoregion. In Fara cave, the dominant species in terms of spatial coverage were: Dendroxea lenis, Spirastrella cunctatrix, Agelas oroides, Phorbas tenacior, Diplastrella bistellata and Hexadella pruvoti. In Agios Vasilios cave, the dominant sponges were: Spirastrella cunctatrix, Plakina bowerbanki, Dendroxea lenis, Hexadella racovitzai, H. pruvoti and Aplysina aerophoba. In the latter cave, the orders Homosclerophorida (24.6%) and Verongida (23.2%) prevailed, while in the former these taxa occupied a notably smaller proportion (<5%) of total sponge coverage.
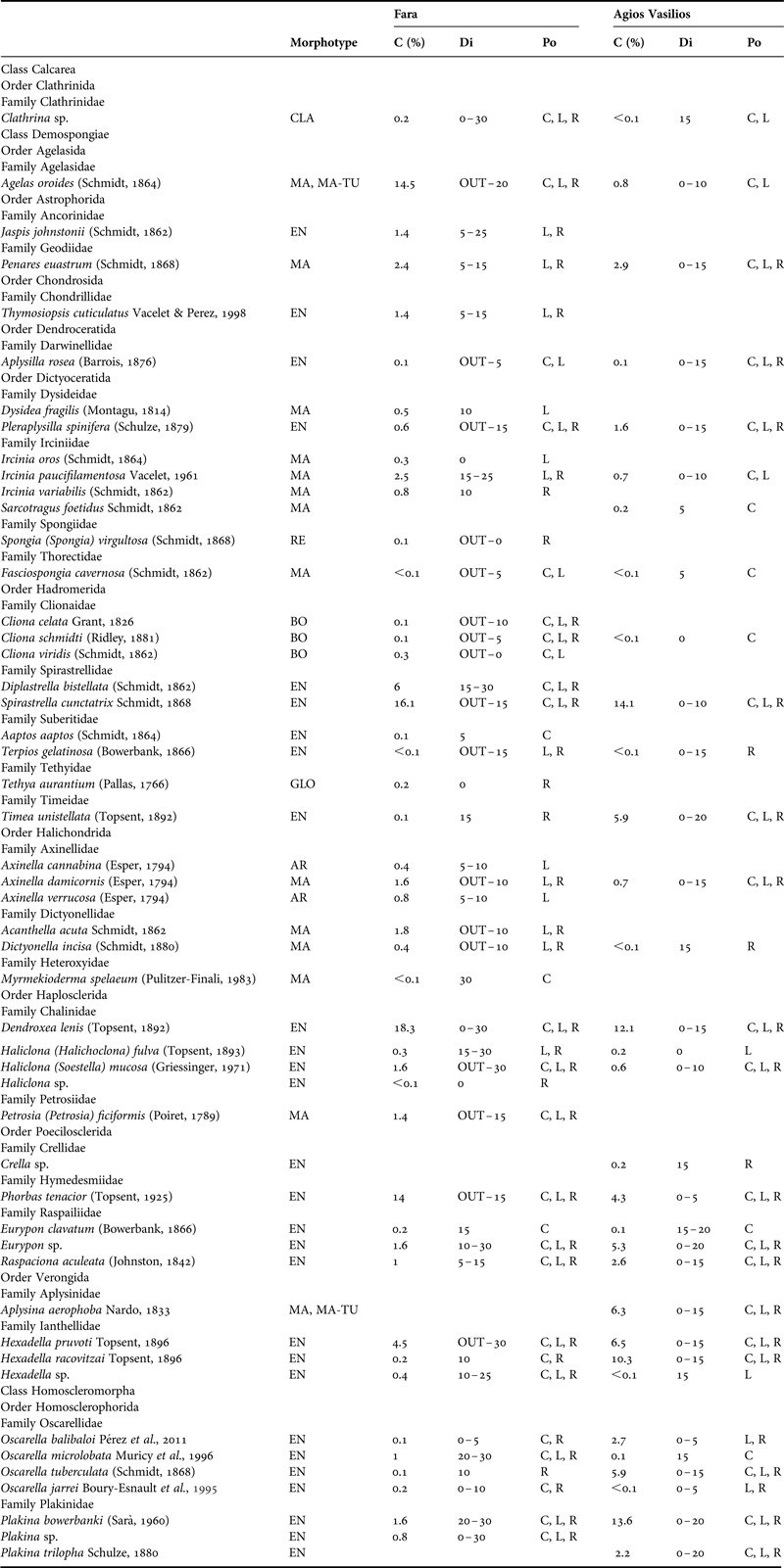
Table 1. Morphotype, per cent sponge coverage (C), distribution (Di) across the horizontal axis (OUT: outer zone, 0–30: distance from entrance in metres) and position (Po) of the sponge species recorded in the surveyed caves. EN, encrusting; MA, massive; MA-TU, massive-tubular; AR, arborescent; CLA, clathrate; BO, boring; RE, repent; GLO, globular; C, cave ceiling; L, left wall; R, right wall.

Multivariate resemblance analysis revealed two major groups of photoquadrat samples in both caves, one corresponding to the samples taken close to the cave entrance and the other to those of the internal cave sectors (Figure 2). Photoquadrats taken at different positions at the same distance level of Fara cave were often placed in different sub-groups (e.g. 5 and 10 m left wall vs right wall samples) or even major groups (e.g. photoquadrats from the 15 m ceiling zone were placed with the inner group, while the corresponding wall samples with the outer group). The Distance and Position factors, and their combined action had a significant effect on the assemblage structure in both caves (Table 2). However, pair-wise tests showed no significant differences in similarity among specific successive distance levels (i.e. 5 and 10 m; 25 and 30 m in Fara; 0 and 5 m in Agios Vasilios cave). Similarity of samples at different positions of each distance level generally increased towards the interior in both caves (Figure 3).

Fig. 2. Resemblance of sponge assemblages demonstrated as MDS plots for Fara (A) and Agios Vasilios caves (B). Numbers indicate distance from cave entrance.

Fig. 3. Similarity (Bray–Curtis index) of samples between the three positions (ceiling and two vertical walls) at each distance level towards the interior of Fara (F) and Agios Vasilios cave (V).
Table 2. Summary of results of two-way PERMANOVA with the Distance (Di) and Position (Po) as factors for the studied caves. Analyses were performed on fourth root transformed coverage data, based on the Bray–Curtis similarity index (** significance at the 0.01 level).

SIMPER analysis between the outer and inner groups of Fara cave showed that nine species (Spirastrella cunctatrix, Agelas oroides, Dendroxea lenis, Phorbas tenacior, Hexadella pruvoti, Eurypon sp., Diplastrella bistellata, Plakina bowerbanki and Haliclona mucosa) contributed 63% to the Bray–Curtis dissimilarity index. Similarly, in Agios Vasilios cave, Spirastrella cunctatrix, Plakina bowerbanki, Eurypon sp., Aplysina aerophoba, Dendroxea lenis, Oscarella tuberculata, Hexadella pruvoti, Phorbas tenacior and Timea unistellata contributed 63% to the Bray–Curtis dissimilarity index.
Concerning spatial variability of diversity, the S and H’ indices were generally higher in Agios Vasilios cave, at most distance levels. Overall, the examined diversity measures showed the same general trend in both caves (Figure 4A, E). Specifically, in Fara cave, an increase was observed from the entrance to the first 5 m, then a decrease towards the dark inner chamber, where few encrusting species prevailed (20 m), and finally an increase at the end of the chamber, close to the fissure that connects the cave to the other side of the islet (30 m); in this cave area, few massive and clathrate species developed, in addition to the typical encrusting sponges of the dark cave sectors. In Agios Vasilios cave, a general decrease was observed, with S and H’ presenting an increase at the 15 m distance level.

Fig. 4. Diversity indices across the horizontal axis (OUT: outer zone, 0–30: distance from entrance in metres) of Fara (A: total, B: left wall, C: right wall, D: ceiling) and Agios Vasilios (E: total, F: left wall, G: right wall, H: ceiling) caves.
This general pattern was differentiated when the two walls and the ceiling of the surveyed caves were examined separately. Thus, in Fara cave, S, H’ and MD on the opposite vertical walls followed the general trend described above (Figure 4B, C). Contrasting spatial patterns were observed for the opposite vertical walls of Agios Vasilios cave. Thus, on the left wall (Figure 4F) S, H’ and MD gradually decreased towards the dark interior. On the right cave wall (Figure 4F), these indices gained their highest values 15 m away from the entrance.
Along the ceiling of Fara cave, all diversity measures decreased towards the middle sector (15 m) but presented an increasing trend thereafter (Figure 4D). However, diversity values on the ceiling were lower compared with those on the vertical walls at the corresponding distance levels, with the exception of the entrance and internal cave sector. A different pattern was found for the ceiling of Agios Vasilios cave (Figure 4H). Species richness and H’ decreased towards the intermediate zone (10 m), increased again at 15 m from the entrance, and gained their lowest values in the inner dark zone. Morphological diversity and J’ decreased towards the cave interior, though not statistically significantly (Table 3).
Table 3. Summary of results of two-way ANOVA with the Distance (Di) and Position (Po) factors for each diversity measure of the studied caves. S, species richness; H’, Shannon–Wiener diversity; MD, morphological diversity; J’, species evenness; Δ*, taxonomic distinctness, ** significance at the 0.01 level, * significance at the 0.05 level.

In Fara cave, S was considerably higher on the walls (L: 34, R: 36) than on the ceiling (C: 27), while in Agios Vasilios approximately the same number of species was found in all positions (C: 25, L: 24, R: 21).
Morphological diversity was generally higher in Fara where all eight sponge morphotypes were found (C: 5, L: 6, R: 7), while five morphotypes were recorded in Agios Vasilios (C: 5, L: 4, R: 3). Morphological diversity values were found to be positively correlated with S and H’ indices in the two caves; however, a higher correlation coefficient value was found in Fara cave (Table 4).
Table 4. Spearman's rank correlation coefficient of morphological diversity with species richness (S) and diversity (H’) in the studied caves (** significance at the 0.01 level).

Taxonomic distinctness (Δ*) showed a slight increasing trend towards the inner zone of both caves (Figure 5). Interestingly, on the ceiling transect it steeply decreased 15 m away from the entrance; however, statistically significant variance was found only in Fara cave (Table 3).

Fig. 5. Phylogenetic diversity calculated as taxonomic distinctness (Δ*) along Fara (A) and Agios Vasilios caves (B).
Results of the two-way ANOVA showed that Distance from the entrance as well as its interaction with Position had a significant effect on the variance of all diversity measures in Fara but only of S and H’ in Agios Vasilios (Table 3). On the other hand, Position seemed to have a significant effect only on S in the former and J’ in the latter cave.
DISCUSSION
This study provided a first quantitative description of the diversity and spatial distribution of sponge assemblages in eastern Mediterranean marine caves, allowing recognition of distinct patterns. Analyses revealed that different sponge species dominated in each cave (e.g. Agelas oroides was abundant in Fara but was rare in Agios Vasilios). A considerable proportion of the recorded species (44%) were found in only one cave, although both were located in the same geographic area. This could be explained by the fact that the studied caves are characterized by different topographic features. Previous studies have underlined the high level of individuality of marine cave assemblages; different sessile species might dominate in equivalent sectors of even neighbouring caves with similar morphology (Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006). It should also be taken into account that the two caves are situated on different islets. Marine caves are considered fragmented habitats (Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985) and insular caves have been characterized as double-isolated ecosystems (Bibiloni et al., Reference Bibiloni, Uriz and Gili1989). A recent overview of the sponge diversity in Mediterranean marine caves found that 67% of the species were recorded in fewer than five caves and 34.5% in only one cave (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012). Furthermore, photographic approaches often underestimate the presence of cryptobiotic species, which may constitute a significant element of cave sponge assemblages (Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989; Corriero et al., Reference Corriero, Liaci, Ruggiero and Pansini2000). This has been witnessed during detailed sampling in the two Aegean marine caves considered, which revealed the presence of several species that were not encountered in the photoquadrats (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012).
Resemblance analysis for the surveyed caves revealed two major groups of samples corresponding to the shadowy outer and the darker internal cave sectors, in accordance with pioneer studies (Sará, Reference Sará1961). The dissimilarity among these two cave sectors in Fara cave was attributed to different species, which (according to the results of SIMPER analysis) could be assigned to the following categories: (i) species found exclusively in the outer sector (Spirastrella cunctatrix and Phorbas tenacior), (ii) species with higher coverage in the outer sector (Agelas oroides and Hexadella pruvoti), (iii) species with higher coverage in the inner sector (Dendroxea lenis, Eurypon sp., Diplastrella bistellata and Haliclona mucosa), and (iv) species found exclusively in the inner sector (Plakina bowerbanki). Respectively, in Agios Vasilios, the following categories were responsible for cave sector dissimilarity: (i) species with higher coverage in the outer sector (Spirastrella cunctatrix, Aplysina aerophoba, Oscarella tuberculata, Hexadella pruvoti, and Phorbas tenacior), (ii) species with higher coverage in the inner sector (Eurypon sp., Dendroxea lenis and Timea unistellata), and (iii) species found exclusively in the inner sector (Plakina bowerbanki). Previous studies have also reported on the preference of some species (e.g. Dendroxea lenis, Diplastrella bistellata and Timea unistellata) for dark conditions (Cinelli et al., Reference Cinelli, Fresi, Mazzella, Pansini, Pronzato, Svoboda, Keegan, Ceidigh and Boaden1977; Bibiloni et al., Reference Bibiloni, Uriz and Gili1989; Corriero et al., Reference Corriero, Liaci, Ruggiero and Pansini2000).
Besides the commonly examined effect of horizontal distance from entrance on cave communities, this study investigated the effect of position at each distance level (i.e. ceiling and opposite vertical walls) and showed that this topographic factor played a significant role in assemblage structure. Heterogeneity within particular cave sectors has been rarely investigated in the past. A limited number of studies has revealed differences in the structure of sessile communities between opposite vertical walls (Dellow & Cassie, Reference Dellow and Cassie1955), walls vs cave bottoms (Russ & Rützler, Reference Russ and Rützler1959), different depth levels on vertical walls (Bell, Reference Bell2002), or sites on the walls within cave sectors (Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006). In certain cases, the magnitude of such variations within a single cave was greater than that observed among different caves (Dellow & Cassie, Reference Dellow and Cassie1955; Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006), thus highlighting the effect of internal cave topography on the structure of cave assemblages.
Divergent patterns of sponge diversity were found in the surveyed caves. Previous studies concerning the animal-dominated communities of western Mediterranean caves also revealed divergent patterns of spatial variability: in some caves, S, H’ and sponge coverage decreased inwards (Pansini et al., Reference Pansini, Pronzato, Fresi, Cinelli, Mazzella, Ponticelli, Fresi and Cinelli1977; Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989; Marti et al., Reference Martí, Uriz, Ballesteros and Turón2004a, Reference Martì, Uriz, Ballesteros and Turonb), while in other cases the above measures increased from the entrance to the middle (Sará, Reference Sará1962; Cinelli et al., Reference Cinelli, Fresi, Mazzella, Pansini, Pronzato, Svoboda, Keegan, Ceidigh and Boaden1977; Corriero et al., Reference Corriero, Scalera Liaci, Gristina, Riggio and Mercurio1997, Reference Corriero, Liaci, Ruggiero and Pansini2000; Bell, Reference Bell2002), and even to the innermost cave sectors (Marti et al., Reference Martí, Uriz, Ballesteros and Turón2004a, Reference Martì, Uriz, Ballesteros and Turonb). The aforementioned researchers generally agree that the higher values of diversity in the middle cave sectors, compared with the outer entrance zone is related to the elimination of light and the resulting disappearance of space-competing macroalgae; the decreasing trend inwards, on the other hand, is probably related to the reduction in trophic resources due to weak water movement. Ultimately, differences in diversity patterns among caves are attributed to cave-specific topographic features generating gradients of abiotic and biotic parameters (Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989; Bell, Reference Bell2002; Marti et al., Reference Martí, Uriz, Ballesteros and Turón2004a, Reference Martì, Uriz, Ballesteros and Turonb; Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006).
The two surveyed caves are characterized by different topographic features (e.g. depth, shape, and size of entrance), which presumably affect light penetration and water circulation. It is noteworthy that in the tunnel-shaped Fara cave, all diversity measures steeply decreased between 5 and 20 m from the entrance and then slightly increased towards the inner cave end. The diversity patterns observed in Fara can be reasonably related to the variation of the cross-sectional area along the horizontal axis of the cave and the associated hydrodynamic factors. Underwater tunnels are characterized by stronger water flow compared with blind caves, and this situation is often reflected in the structure of their assemblages (Riedl, Reference Riedl1966; Harmelin, Reference Harmelin1969; Vacelet, Reference Vacelet1976; Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985). Indeed, in our case, during underwater fieldwork, the existence of a mild inward current (stronger during heavy sea conditions) was detected in the cave interior. Pansini et al. (Reference Pansini, Pronzato, Fresi, Cinelli, Mazzella, Ponticelli, Fresi and Cinelli1977) suggested that the dominance of a few encrusting sponges along the middle corridor of a semi-submerged cave on Ischia Island (Tyrrhenian Sea) manifested the increasing level of hydrodynamics due to the Venturi effect (i.e. velocity of the fluid increases as the cross-sectional area decreases). Bell (Reference Bell2002), in a study of the sponge assemblage of a temperate semi-submerged cave in Ireland, also suggested that spatial variation of S, H’, and MD was partially related to high flow rates, due to the cave narrowing. On the other hand, Agios Vasilios is a blind funnel-shaped cave exhibiting decreasing trends in diversity inwards, in accordance with previous studies in marine caves with similar morphology (e.g. Marti et al., Reference Martí, Uriz, Ballesteros and Turón2004a). Blind caves (also known as ‘cul-de-sac’ caves) are generally characterized by a notable depletion of trophic resources due to increasing water confinement inwards, which results in progressive decrease of biotic coverage, biomass and diversity and the disappearance of massive growth-forms (Bianchi & Morri, Reference Bianchi and Morri1994). Our results, coupled with the findings of the aforementioned studies, stress the necessity of establishing monitoring schemes for representative Mediterranean cave types in order to promote the understanding of the underlying factors that shape community structure divergence.
Species evenness in the inner parts of the two surveyed caves was generally higher compared with that in their outer sectors, indicating that sponge species were more evenly distributed in the cave interior and that no species dominated the corresponding assemblage, in accordance with other cave studies (Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989; Bell, Reference Bell2002; Barnes & Bell, Reference Barnes and Bell2002b). Interestingly, S and H’ were significantly positively correlated with MD in the studied caves in spite of their contrasting diversity patterns. Bell & Barnes (Reference Bell and Barnes2002) found no significant correlation between the aforementioned diversity measures in tropical caves of the West Indian Ocean, due to the dominance of encrusting forms. Nevertheless, this model had not been tested up to date in temperate marine caves. Filling this gap, our results suggest that MD could possibly be used as a surrogate measure for describing sponge diversity trends in Mediterranean marine caves. However, this should be examined in marine caves of different topography in various areas. The slight increment of TD towards the interior sectors of the studied caves corroborates that environmental stability in inner cave sectors might favour the development of a taxonomically broader sponge assemblage compared with exposed sublittoral substrates (Gerovasileiou & Voultsiadou, Reference Gerovasileiou and Voultsiadou2012).
Sponges of particular growth forms presented higher coverage in specific sectors of the studied marine caves, possibly reflecting adaptation to local environmental conditions. For example, it has been suggested that the dominance of encrusting sponges in the dark inner cave sectors (Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985; Corriero et al., Reference Corriero, Liaci, Ruggiero and Pansini2000; Barnes & Bell, Reference Barnes and Bell2002a; Marti et al., Reference Martí, Uriz, Ballesteros and Turón2004a; Bussotti et al., Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006; this study) reflects an adaptation to water confinement and reduced trophic resources; thinly encrusting forms exhibit a more effective filtration surface/volume ratio and can, therefore, exploit the rare particulate organic matter of the water (Bibiloni et al., Reference Bibiloni, Uriz and Gili1989). The arborescent species Axinella cannabina and A. verrucosa presented greater coverage at the 5–10 m distance levels, on the left wall of Fara cave. A thin layer of sediment was constantly observed in this area during the dives, due to a locally higher sedimentation rate resulting from the positive wall inclination and the proximity to the muddy bottom. Erect sponges can cope better with high sedimentation levels as this growth form prevents sediment settlement and clogging of their aquiferous system (Bell et al., Reference Bell, Barnes and Shaw2002 and references therein). Bibiloni et al. (Reference Bibiloni, Uriz and Gili1989) also reported the development of erect growth forms in cave sectors with high levels of sedimentation. On the other hand, boring species (Cliona spp.) were found only in the outer sectors of the studied caves, which were dominated by calcareous biogenic substrate (e.g. encrusting rhodophytes). Previous studies have shown that horizontal distribution of clionids in marine caves is related to the presence of suitable calcareous substrate (e.g. calcareous macroalgae, Balanus and Ostrea shells, and carbonate rocks) along the cave and the interactions with other organisms (Sará, Reference Sará1962; Pansini et al., Reference Pansini, Pronzato, Fresi, Cinelli, Mazzella, Ponticelli, Fresi and Cinelli1977; Balduzzi et al., Reference Balduzzi, Bianchi, Boero, Cattaneo Vietti, Pansini and Sarà1989).
In conclusion, this quantitative study of marine cave sponge assemblages in the eastern Mediterranean basin filled regional gaps for an understudied habitat of special conservation interest. The results of the study highlighted (a) the spatial heterogeneity in the composition of sponge assemblages and (b) the presence of divergent sponge diversity gradients among marine caves with different morphology as well as in various microhabitats within these caves. Given that heterogeneity constitutes an important parameter for conservation actions, further research in representative marine cave types of the eastern Mediterranean is needed to enhance scientific knowledge of these ecosystems.
ACKNOWLEDGEMENTS
The authors would like to thank Maria Sini for her help during fieldwork and Charalampos Dimitriadis for assistance in statistical analysis. The first author is grateful to the organizing committee of the 9th World Sponge Conference.
FINANCIAL SUPPORT
This research has been co-financed by the EU and Greek national funds through the Research Funding Program: Heracleitus II, Investing in knowledge society. Vasilis Gerovasileiou also benefited from an ‘Alexander S. Onassis Public Benefit Foundation’ fellowship for postgraduate studies.