Introduction
Urbanisation is dramatically affecting the world. Over the past 150 years, rapid urban growth has had profound environmental consequences due to the modification of natural habitats and the demand for resources (Carpenter et al. Reference Carpenter, Mooney, Agard, Capistrano, DeFries, Diaz, Dietz, Duraiappah, Oteng-Yeboah, Pereira, Perrings, Reid, Sarukhan, Scholes and Whyte2009, Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, McKinney Reference McKinney2006, Parés-Ramos et al. Reference Parés-Ramos, Álvarez-Berríos and Aide2013). The human population is projected to increase by one-third over the next 30 years, with cities accounting for the majority of this population (McKinney Reference McKinney2006). By 2050, urban growth will be concentrated in the least developed countries, with an estimated 95% of urban expansion in Africa, Asia and South America (Parés-Ramos et al. Reference Parés-Ramos, Álvarez-Berríos and Aide2013). Among the environmental problems, urbanisation leads to fragmentation (Kattan et al. Reference Kattan, Alvarez-Lopez and Giraldo1994), habitat loss (Magura & Lövei Reference Magura and Lövei2021), species extinction (Wagner Reference Wagner2008), establishment of exotic species (Wagner Reference Wagner2008), biotic homogenisation (McKinney Reference McKinney2006), urban ‘heat islands’ (Magura et al. Reference Magura, Ferrante and Lövei2020) and pollution (Magura & Lövei Reference Magura and Lövei2021). Environmental problems generated by urbanisation alter the structure and composition of biotic communities, affecting ecosystem services (Alarcon & Montlleó Reference Alarcon, Montlleó and Herce2010, Magura & Lövei Reference Magura and Lövei2021, Main & Jackson, Reference Main and Jackson2003, Santos & Tellería Reference Santos and Tellería2006), and despite their importance, there are few studies on these effects (Desaegher et al. Reference Desaegher, Nadot, Machon and Colas2019, Frey et al. Reference Frey, Vega, Zellweger, Ghazoul, Hansen and Moretti2018).
Predation is an ecosystem service that helps to shape urban biological communities but is itself highly altered by urbanisation (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Pena et al. Reference Pena, Aoki-Gonçalves, Dáttilo, Ribeiro and MacGregor-Fors2021). Predation pressure varies both within and between habitats due to differences in community and predator density (Cagnolo & Valladares Reference Cagnolo and Valladares2011, García et al. Reference García, Benítez and López-Ávila2007), fragmentation (Koh & Menge Reference Koh and Menge2006), vegetation structure and complexity (Nason et al. Reference Nason, Eason, Carreiro, Cherry and Lawson2021), substrate type (e.g., ground, leaves and stems) (Sinu et al. Reference Sinu, Viswan, Fahira, Rajesh, Manoj, Hariraveendra and Jose2021, Tvardikova & Novotny Reference Tvardikova and Novotny2012), seasonal (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014), disturbances (e.g., traffic volume, high temperature and noise) (Pena et al. Reference Pena, Aoki-Gonçalves, Dáttilo, Ribeiro and MacGregor-Fors2021), prey coloration (Ferrante et al. Reference Ferrante, Barone, Kiss, Bozóné-Borbáth and Lövei2017a) and levels of urbanisation (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Posa et al. Reference Posa, Sodhi and Koh2007). Tropical forests are key in the global carbon cycle and are home to more than half of the world’s species (Taubert et al. Reference Taubert, Fischer, Groeneveld, Lehmann, Müller, Rödig, Wiegand and Huth2018). Ecosystem services in forests depend to a large extent on insects, and these are highly sensitive to fragmentation (Didham et al. Reference Didham, Ghazoul, Stork and Davis1996); therefore, the study of invertebrate predation in tropical forests, with different degrees of urbanisation, is relevant to understand the changes in biological processes in these habitats. Compared to the forest interior, the edges and patches of forest fragments are considered the areas of greatest predatory risk for many species (Bustamante & Grez Reference Bustamante and Grez1995, Main & Jackson Reference Main and Jackson2003, Posa et al. Reference Posa, Sodhi and Koh2007, Richards & Coley Reference Richards and Coley2007). In forest clearings, herbivores and their predators can be more abundant, since these sites show increased leaf and plant growth compared to the understory (Richards & Coley Reference Richards and Coley2007). The degree of prey exposure also influences detection by predators. For example, predation risk was found to be greater for artificial caterpillars on exposed leaves compared to hidden ones (Tvardikova & Novotny Reference Tvardikova and Novotny2012). Predation preference depends on the degree of prey exposure; however, the predator community and associated foraging strategies also affect substrate preference (Maas et al. Reference Maas, Tscharntke, Saleh, Dwi Putra and Clough2015, Philpott et al. Reference Philpott, Soong, Lowenstein, Pulido, Lopez, Flynn and DeClerck2009, Sinu et al. Reference Sinu, Viswan, Fahira, Rajesh, Manoj, Hariraveendra and Jose2021). Given the variety of biotic and abiotic factors affecting predation, results on arthropod predation in urban areas have not been consistent (Pena et al. Reference Pena, Aoki-Gonçalves, Dáttilo, Ribeiro and MacGregor-Fors2021). In some instances, rather than an increase (Kozlov et al. Reference Kozlov, Lanta, Zverev, Rainio, Kunavin and Zvereva2017, Posa et al. Reference Posa, Sodhi and Koh2007) a decrease in predation (Eötvös et al. Reference Eötvös, Magura and Lövei2018, Reference Eötvös, Lövei and Magura2020, Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Pena et al. Reference Pena, Aoki-Gonçalves, Dáttilo, Ribeiro and MacGregor-Fors2021, Sinu et al. Reference Sinu, Viswan, Fahira, Rajesh, Manoj, Hariraveendra and Jose2021) has been found, as the intensity of the anthropogenic disturbance increases.
Typically, predatory events happen quickly and are often hard to measure because predators may be nocturnal, or hide while consuming prey, thereby leading to reduced detection (Howe et al. Reference Howe, Lövei and Nachman2009). Similarly, predation intensity is difficult to measure because predation events will often leave only fragments of the consumed prey, or no trace at all. The sentinel prey method is an alternative way to measure predation (Ferrante et al. Reference Ferrante, Möller, Möller, Menares, Lubin and Segoli2021, Howe et al. Reference Howe, Lövei and Nachman2009). This technique consists of manipulating prey availability by locating a known number of prey (artificial or live) and recording the rate of disappearance or traces of predation after a given period of exposure (Ferrante et al. Reference Ferrante, Möller, Möller, Menares, Lubin and Segoli2021, Lövei & Ferrante Reference Lövei and Ferrante2017). This method has been successfully used to estimate predation pressure on caterpillars (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Howe et al. Reference Howe, Lövei and Nachman2009, Reference Howe, Nachman and Lövei2015, Loiselle & Farji-Brener Reference Loiselle and Farji-Brener2002, Richards & Coley Reference Richards and Coley2007, Tvardikova & Novotny Reference Tvardikova and Novotny2012). The majority of these studies were carried out in wooded areas with a different level of succession, whereas only a few studies have evaluated predation pressure in urban and suburban environments (Eötvös et al. Reference Eötvös, Magura and Lövei2018, Reference Eötvös, Lövei and Magura2020, Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Kozlov et al. Reference Kozlov, Lanta, Zverev, Rainio, Kunavin and Zvereva2017, Long & Frank Reference Long and Frank2020, Roels et al. Reference Roels, Porter and Lindell2018). Although it has been suggested that generalist predators are similarly attracted by chemical cues of artificial and real caterpillars (Ferrante et al. Reference Ferrante, Barone and Lövei2017b, Richards & Coley Reference Richards and Coley2007), this method does not measure actual predation rates (Lövei & Ferrante Reference Lövei and Ferrante2017). Several anti-predator strategies (e.g., aggregation, sounds, olfactory and visual cues, etc.) are difficult to control under field assays (Witz Reference Witz1990). In the case of visual cues, colour, model posture and markings can influence the predation rate of artificial larvae (Hernández-Agüero et al. Reference Hernández-Agüero, Polo, García, Simón, Ruiz-Tapiador and Cayuela2020, Hossie & Sherratt Reference Hossie and Sherratt2012, Reference Hossie and Sherratt2013, Oliveira et al. Reference Oliveira, Diniz, Araujo-Lima, Rosário and Duca2020). For example, the aposematic coloration of Harmonia axyridis beetle larvae deters birds from preying on them. A lower predation rate was found in artificial larvae with a similar colour to H. axyridis compared to green and black models (Aslam et al. Reference Aslam, Nedvěd and Sam2020). Therefore, given the limitations of using artificial larvae, absolute estimations of predation cannot be obtained. However, useful comparisons between habitats can be made by this method (Lövei & Ferrante Reference Lövei and Ferrante2017).
Establishing how urbanisation affects the incidence of predation provides knowledge regarding the dynamics of urban ecological interactions and offers a tool for the management of the populations involved. The main purpose of the present study was to determine whether predation pressure (estimated using artificial models) varies as a result of urbanisation degree (urban and suburban). The study also sought to establish whether substrate type (leaves and stems) influences predation incidence on artificial lepidopteran caterpillar models. According to the increasing disturbance hypothesis (Gray Reference Gray1989), predator abundance decreases with increasing urbanisation, leading to lower predation pressure. Hence, we expected to detect a lower predation rate in urban rather than suburban habitats. Furthermore, we hypothesised that the leaves are the substrate where the highest predation occurs because they are an important source of nutrients for arthropods, which can influence their abundance on this substrate (Kwok Reference Kwok2009, Laxton Reference Laxton2005).
Materials and methods
Study site
This study was carried out in the city of Santiago de Cali, Department of Valle del Cauca, Colombia (3°32ʼ33ʼʼ N, 76°31ʼ58ʼʼ W; 995 a.s.l.) between August 2015 and August 2016. The city has a mean annual temperature of 24.1 °C, relative humidity of 73 % and rainfall of 1481 mm (ranging between 1000 and 2000 mm). A bimodal rainfall pattern is present with dry periods during January–February and July–August and rainy periods during March–June and September–December (IDEAM 2015). According to Holdridge (Espinal Reference Espinal1968), these climatic characteristics correspond to the dry tropical forest life zone (df-T). Historically, this eco-area represents a deciduous dry forest mixed with evergreen dry forest and a gallery forest along the Cauca River. On either side of the valley, the dry forests give way to another eco-zone (moist montane forest) along the slopes of the Central and Western Andean ranges. Fabaceae is the most dominant vegetal family, including Pithecellobium dulce, Gliricidia sepium, Samanea saman, Bauhinia spp., Cassia spp. the most typical tree species. Other native species such as Crescentia cujete (Bignoniaceae), Ceiba pentandra (Malvaceae), Guazuma ulmifolia (Malvaceae) and Spondias mombin (Anacardaceae) are used as ornamental species. The entire region has been severely transformed by human settlements and activities (mainly, agriculture), only a narrow strip of forest remains.
The percentage of urban construction for each study area matrix was considered to determine the degree of urbanisation. Suburban areas were those ubicated in the periphery of the city where there are all kinds of human activities, including private clubs, urbanisation and crops (Rivera-Gutiérrez Reference Rivera-Gutiérrez2006), which occupied less than 65% of the area. Urban areas were those ubicated within the city where civil constructions occupied ≥ 65% of the area. A minimum of 65% urban constructions was determined to be considered an urban area. Two different areas were defined according to the degree of human disturbance: urban and suburban, and two sites were chosen in each one: Universidad del Valle Campus (Urban 1) (3°22ʼ26.6ʼʼ N, 76°31ʼ51.1ʼʼ W) and Constructora Limonar (Urban 2) (3°23ʼ39.6ʼʼ N, 76°31ʼ21.0ʼʼ W) in the urban area, and Parque de las Garzas (Suburban 1) (3°19ʼ56.3ʼʼN,76°32ʼ13.3ʼʼW) and Hacienda Cañasgordas (Suburban 2) (3°21ʼ17.7ʼʼ N, 76°31ʼ31.9ʼʼ W) in the suburban area. Suburban 1 and Suburban 2 are two suburban areas surrounded by agricultural crops, country parks and tiny dry forest remnants. Suburban 1 has an artificial lagoon ecosystem with regenerative vegetation (c.a. 4.7 ha) and 70 % vegetation coverage. Suburban 2 is a rural village (29 ha) with a wide and open pasture area and 54 % vegetation cover. It has an extensive park area with abundant herbaceous shrubs and 38 % vegetation cover. Urban 1 corresponds to a green area of 100 ha, surrounded by commercial malls, residential houses (88.29% built-in area) and a tiny tree corridor in the verge of the Meléndez River. Urban 2 corresponds to an area of 24 ha, mainly composed of human dwellings. ‘Chiminango’ (P. dulce) and ‘Saman’ (S. saman) are the dominant trees in all the study areas.
Artificial lepidopteran caterpillars
Based on natural caterpillars of Phoebis sennae, artificial models (40 mm long and 6 mm wide) were made with green, odourless and non-toxic plasticine (School Smart, 88678) (Figure 1a). P. sennae was chosen because it is one of the most common species in Cali suburban and urban areas (Ramírez et al. Reference Ramírez, Chacón and Constantino2007, Dolores Heredia, pers. comm.). In addition, because the green colour of its larvae (Minno et al. Reference Minno, Butler and Hall2005), it is widely used in studies on predation and herbivory (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Posa et al. Reference Posa, Sodhi and Koh2007, Richards & Coley Reference Richards and Coley2007, Tvardikova & Novotny Reference Tvardikova and Novotny2012).

Figure 1. Predator marks registered on the lepidopteran larvae models. a artificial caterpillar model; b, c and d bird beak; e and f ants; g, h and i wasp jaw; j wasp sting; k chewing arthropod; and l mammal. Southwestern Colombia. Photo taken by Image Laboratory at the Graduate School of Biological Sciences, Universidad del Valle.
Predation tests
On August 2015, a pilot test was carried out in order to evaluate whether model shape can affect the incidence of attacks. For this, two types of model were made using green plasticine. The first model was an artificial caterpillar (Figure 1a). The second was a solid sphere (10 mm × 10 mm × 10 mm), representing any common form present in nature (fruits, adult insects or pupae). The models were fastened to the trees using wires. For this test, 20 P. dulce trees were used in each zone (suburban–urban) separated by at least 30 m from each other; the total number of trees used for this test was 40. In order to avoid learning, these trees were different from those of the main experiment. Each zone had 10 trees with caterpillar models (five trees with caterpillars installed on leaves and five trees with caterpillars installed on stems) and 10 trees with sphere models (five trees with spheres installed on leaves and five trees with spheres installed on stems). The experiment tested by shape (caterpillar-like/spherical-like), substrate (stem-leaves) and zone (suburban–urban) in 40 trees. The total number of models used in this experiment was 200. The test lasted for 30 days, and each site was visited twice a week. During each visit, the models were moved to a different place on the same tree to avoid predator bias through learning, and all of the models with attack marks were replaced (Tvardikova & Novotny Reference Tvardikova and Novotny2012). Models with evidence of attack marks were collected, and the marks were subsequently analysed and identified in the laboratory (Section 2.3).
For the main predation test, 10 trees were selected per site (Suburban 1, Suburban 2, Urban 1 and Urban 2), and based on the results of the pilot experiment, only the caterpillar models were used. Five artificial caterpillars were placed at heights of between 1.5 and 2 m, separated by at least 250 mm and distributed according to the substrate. Five trees had caterpillars on their leaves and five on the stems. The test lasted for 30 days with two repetitions (October–November 2015 and January–February 2016). The same scheme (checking, replacing and moving models) was followed as in the pilot test. The total number of caterpillars models used in this experiment was 200 per repetition.
Identification of potential predators
Based on the results obtained in the pilot experiment (Section 3.1), it was determined that the main predators of artificial caterpillars are birds, so a census was conducted in the study area to identify potential predators and assess the effect of their abundance by habitat (suburban–urban) on predation. The bird census was achieved through 10 point counts in each site; each point was 15 m in diameter and on average 93 m apart from each other. Each point count was visited and inspected for 15 min twice/day (between 0700–1000 h and 1400–1700 h). This activity was carried out once at each site during each experiment. The bird species observed were identified using specialised keys (Hilty & Brown Reference Hilty and Brown2001, Remsen et al. Reference Remsen, Cadena, Nores, Pacheco, Pérez and Robbins2020).
The marks or signs found on the models were photographed (Figure 1) and compared with those obtained in pilot tests and by other researchers (Low et al. Reference Low, Sam, McArthur, Posa and Hochuli2014, Tvardikova & Novotny Reference Tvardikova and Novotny2012). The ‘attacked’ models were used as templates to generate a guide to identify marks of potential predators (birds or arthropods). Though limited, this type of guide provides a reliable, useful taxonomical description (Low et al. Reference Low, Sam, McArthur, Posa and Hochuli2014, Tvardikova & Novotny Reference Tvardikova and Novotny2012).
Statistical analysis
For the pilot study, a generalised linear mixed model (GLMM) was used with predation (total of attacked models/tree/week) as a binomial response variable. Prey shape (spherical–caterpillar), habitat (suburban–urban) and substrate (stem–leaf) were used as explanatory variables, and site as a random factor. Similarly, GLMM analysis was used for the main predation test with predation as a binomial response variable response, and habitat (suburban–urban), substrate (stem–leaf) and season (dry–rainy) as explanatory variables. Weekly predation rates were calculated as the number of plasticine models with attack marks per week divided by the total number of plasticine models, multiplied by 100. R software was used for all the analyses (R Core Team, 2016).
Species accumulation curves were used to evaluate the representativeness of the bird sampling, and the ACE estimator using the EstimateS 9 Program (Colwell Reference Colwell2016) was used to measure expected richness. Probabilities of less than or equal to 0.05 were considered significant.
Results
Predation tests
Pilot test: The total number of models with attack marks was 58, corresponding to 22.48% of the models. There were no models with multiple marks nor lost models, and including replacements, a total of 258 models were used. Of the attacked marks, 86.21% corresponded to birds, and 13.79% to arthropods, all of which were found on artificial caterpillars. The weekly predation rate on artificial caterpillars (x̄ = 9%, SD = 5.59%, n = 4) was higher than that on spherical-like models (x̄ = 5.5%, SD = 7.04%, n = 4). Although no significant statistical differences were detected, a strong trend was observed suggesting that artificial caterpillar models were attacked more (P = 0.06, χ 2 = 3.59, df = 1, GLMM). In the suburban area (x̄ = 8.75%, SD = 5.61%, n = 4), the weekly predation rate was higher than in the urban area (x̄ = 5.75%, SD = 7.67%, n = 4), but no significant differences were found between habitats (P = 0.59, χ 2 = 0.29, df = 1, GLMM) or substrate types (P = 0.19, χ 2 = 1.63, df = 1, GLMM).
Main predation test: In the main test, 125 (23.8%), artificial models showed evidence of predator attacks: 77.6% (97) were identified as bird attacks, while 22.4% (28) corresponded to arthropods (Figure 2). A total of 525 models were used in the experiment. No models were lost, and no models with multiple marks were found. The incidence of predation was greater in the urban area which displayed a weekly predation rate of (x̄ = 9.88%, SD = 5.13 %, n = 8) compared to (x̄ = 5.75%, SD = 4.2%, n = 8) for the suburban area, and this difference was significant (P = 0.01, χ 2 = 5.65, Table 1). Leaf substrate showed a greater number of attacks than the stem substrate, from the 125 marks on the models, 60% (75) were found on leaves vs. 40% (50) on stems, and this difference was significant (P = 0.05, χ 2 = 3.82, Table 1) with the weekly predation rate on leaves (x̄ = 9.63%, SD = 5.95%, n = 8) higher than that of stems (x̄ = 6%, SD = 4.2%, n = 8). Season was found to have no effect on the incidence of predation, with total predation similar in both the dry (55.2 %; n = 69) and rainy (44.8 %; n = 56) seasons, and this difference was not significant (P = 0.15, χ 2 = 1.97, Table 1). There was no interaction between the variables (Table 1).
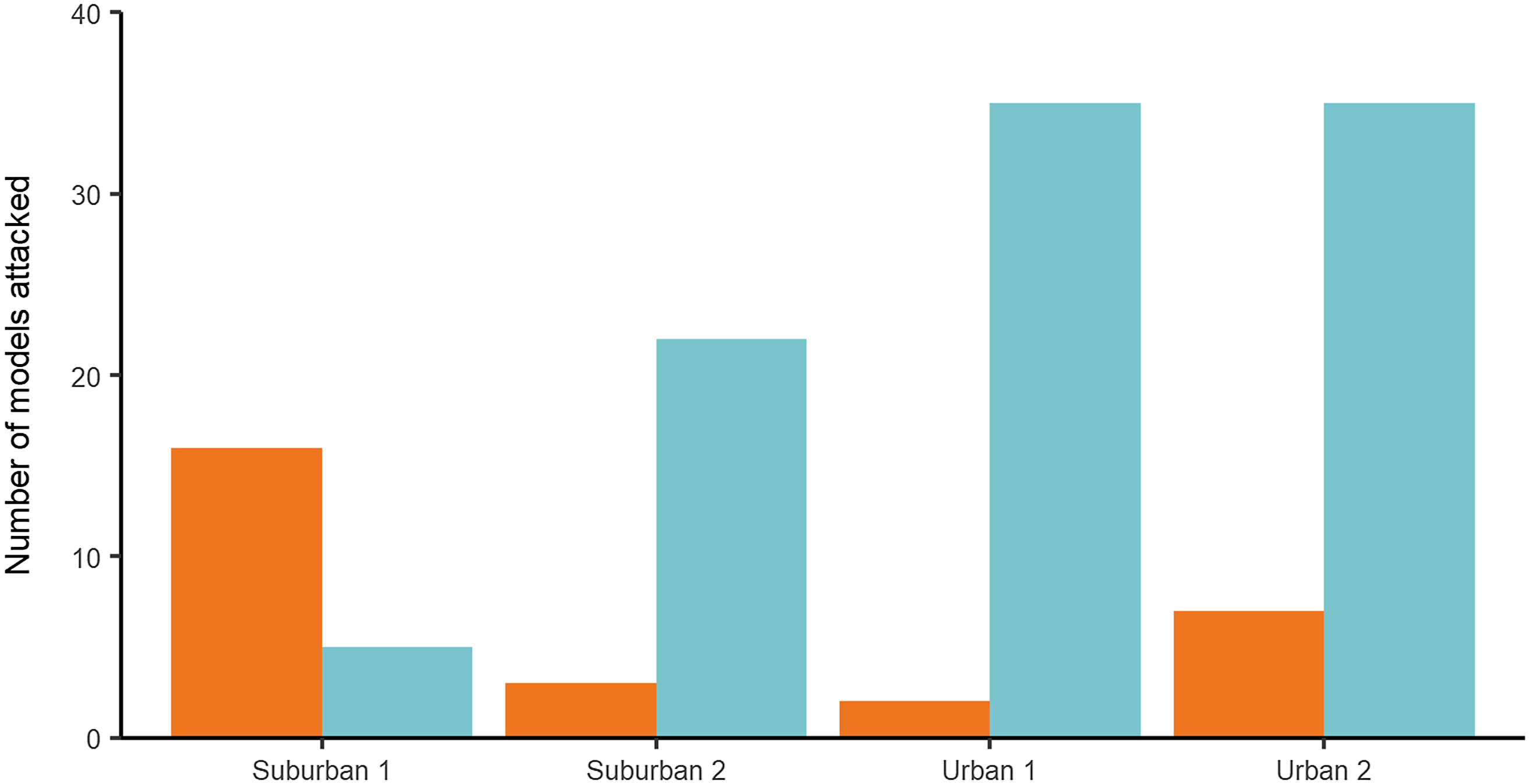
Figure 2. Number of models attacked by arthropods (orange) and birds (blue) at each site: Suburban 1 (Parque de las Garzas), Suburban 2 (Hacienda Cañasgordas), Urban 1 (Universidad del Valle Campus) and Urban 2 (Constructora Limonar). A total of 50 artificial caterpillars were used per site in each climatic season (rainy/dry). The test lasted for 30 days with two repetitions (October–November 2015 and January–February 2016).
Table 1. Generalised linear mixed model (GLMM) where predation models of lepidopteran caterpillars were evaluated according to the degree of urbanisation (suburban and urban), substrate (stem and leaf) and season (rainy and dry) in southwestern Colombia

DU, degree of urbanisation; Sub, substrate.
Potential predators
A total of 74 bird species grouped in 30 families were found, Tyrannidae being the most representative. Species accumulation curves show 78.15% (suburban environment) and 75.13% (urban) efficiency in the avifauna sampled (Figure S1). Of the total number of species, 42 were considered potential predators of lepidopteran caterpillars based on their foraging habits (Del Hoyo et al. Reference Del Hoyo, Elliott and Christie1996, Hilty & Brown Reference Hilty and Brown2001) (Table S1).
The abundance of potential predator birds (Section 3.2) varied significantly with the degree of urbanisation. The urban area had the greatest number of individuals (χ2 = 49.80; df = 1; P < 0.0001) (Figure 3). There were no statistically significant differences between species richness in suburban and urban areas (χ2 = 0.10; df = 1; P = 0.74).

Figure 3. Abundance of predator birds and number of models attacked by birds per each site: Suburban 1 (Parque de las Garzas), Suburban 2 (Hacienda Cañasgordas), Urban 1 (Universidad del Valle Campus) and Urban 2 (Constructora Limonar), in southwestern Colombia.). Prediction line equation: y = 0.3063x – 31.339. R 2 = 0.8771.
Discussion
Degree of urbanisation
Our results suggest that predation increases with the degree of urbanisation. Given that the weekly predation rate in urban areas was higher than in suburban areas, the level of habitat disturbance may have a significant effect on herbivorous insect predation (Posa et al. Reference Posa, Sodhi and Koh2007, Richards & Coley Reference Richards and Coley2007, Seifert et al. Reference Seifert, Lehner, Adams and Fiedler2015, Tvardikova & Novotny Reference Tvardikova and Novotny2012). Similar patterns of arthropod predation were observed in other tropical habitats. For instance, in Panama, Richards and Coley (Reference Richards and Coley2007) found that the incidence of predation on artificial larvae in forest clearings is significantly higher than in closed forest, due to the high primary productivity of the forest, which harbours a large number of herbivorous insects that are controlled by their predators. In the Philippines, the incidence of predation on herbivores was significantly higher in rural areas (59.4 %) compared to closed canopy forest (46.1 %) (Posa et al. Reference Posa, Sodhi and Koh2007). In Costa Rica, predation pressure on herbivores was found to be twice as high in open fields (mean attack frequency per caterpillar: 1.11 ± 0.08) as in forest (0.66 ± 0.07) (Seifert et al. Reference Seifert, Lehner, Adams and Fiedler2015).
The greater incidence of predation on artificial caterpillars in the urban area when compared to the suburban area could be related to the increased abundance of birds associated with urbanisation and allows for rejection of the increasing disturbance hypothesis (Gray Reference Gray1989). Results on abundance are in agreement with those reported by Kale and colleagues (Reference Kale, Dudhe, Ferrante, Ivanova, Kasambe, Trukhanova, Kasambe, Trukhanova, Bhattacharya and Lövei2018a, Reference Kale, Ferrante, Dudhe, Kasambe, Trukhanova, Ivanova, Bhattacharya and Lövei2018b) in India, who found higher individual concentrations in urban zones. We found that the abundance of birds was significantly higher in the urban environment compared to the suburban one, and marks of this group were more commonly observed on the attacked models. Also, our results are in line with those obtained by Roels and colleagues (Reference Roels, Porter and Lindell2018) in a Panamanian forest, and by Posa and colleagues (Reference Posa, Sodhi and Koh2007) in a Philippine forest reserve, where areas with higher human disturbance (residential countryside and rural areas, respectively) showed the highest bird predation rates. In urban settings, the abundance of some bird species increases due to the absence or reduction of the predators that control them; in these environments, the survival of predators such as snakes or birds of prey diminishes. Although the presence of other predators such as dogs and cats increases, the pressure exerted by them does not significantly affect the population density of urban birds (Fischer et al. Reference Fischer, Cleeton, Lyons and Miller2012). Lower predation pressure on birds can increase arthropod predation as there is a greater abundance of insectivorous birds in urban environments (Fischer et al. Reference Fischer, Cleeton, Lyons and Miller2012, Shochat et al. Reference Shochat, Lerman, Katti and Lewis2004). Additionally, areas of urban vegetation favour the increased abundance of birds since these patches act as connecting points between suburban and urban areas and offer food resources for exploitation by birds (Caicedo-Argüelles & Cruz-Bernate Reference Caicedo-Argüelles and Cruz-Bernate2014, Torres et al. Reference Torres, Vargas, Guevara Llano, Orrego, Duque, Moreno and Ruiz2014). Also, it has been observed that the degree of urbanisation affects species richness. The greater the urban development, the greater the decrease in the number of species and the abundance of a reduced group of species (Kale et al. Reference Kale, Dudhe, Ferrante, Ivanova, Kasambe, Trukhanova, Kasambe, Trukhanova, Bhattacharya and Lövei2018a). In our case, for the insectivorous guild, five species represented 47.93% of the individuals observed, and these species occurred in both urban areas.
In areas with higher degrees of urbanisation, Lepidoptera larvae could be considered a valuable resource, especially for bird reproduction (Schwagmeyer & Mock Reference Schwagmeyer and Mock2008). Arthropod size is one of the traits most affected by habitat disturbance, both in terms of survival (Seress & Liker Reference Seress and Liker2015) and a reduction in the size of individuals (Niemelä & Kotze Reference Niemelä and Kotze2009, Zvereva & Kozlov Reference Zvereva and Kozlov2010). The artificial caterpillars used correspond in size to the last instar, which may increase predation pressure in urban areas, where the probability of finding large lepidopteran larvae may be lower, since this represents a scarce and valuable resource compared to areas with less disturbance,
Although the phenological state of P. dulce was not evaluated in the present study, it is known to have constant phenophases (Cárdenas-Henao et al. Reference Cárdenas-Henao, Londoño-Lemos, Llano-Almario, González-Colorado, Rivera-Hernández, Vargas-Figueroa, Duque-Palacio, Torres-González, Jiménez-Taquinas and Moreno-Cavazos2015) producing floral buds and flowers throughout the year. Nevertheless, it is not known whether subtle differences exist in the phenological state of some trees according to the level of habitat disturbance, and whether, in this case, trees with a greater supply of fruits would be found in the urban areas, thus stimulating both an increase in generalist bird visits as well as the probability of contact with caterpillars. It would be advisable for future studies to include this variable in order to determine whether the observed response is due solely to the disturbance factor and not to the visit of generalist birds attracted to fruiting trees.
Substrate
The higher predation found for prey exposed on leaves (60%) than on stems (40%) could be related to the foraging of the community of predators present in the habitats studied (Gutiérrez Reference Gutiérrez, Ardila, López, Pérez, Quiñones and Reyes1998, Morse Reference Morse1990). In a Puerto Rican novel Prosopis-Leucaena woodland (Beltran & Wunderle Reference Beltran and Wunderle2013), birds preferred to forage for food on P. dulce, since this tree houses a large quantity of arthropods associated with its foliage due to the high nitrogen content and small amount of hemicellulose in its leaves. At Universidad del Valle, this plant is frequented by both resident and migratory birds, and their main feeding activity is the foraging and consumption of insects (61.5%) compared to seed (29.7%), flower (4.4%), nectar (2.2%) and leaf (2.2%) consumption (Caicedo-Argüelles & Cruz-Bernate Reference Caicedo-Argüelles and Cruz-Bernate2014, Torres et al. Reference Torres, Vargas, Guevara Llano, Orrego, Duque, Moreno and Ruiz2014). The bird response detected in this study might be a consequence of specific preferences of various groups of birds for substrate (Gunnarsson et al. Reference Gunnarsson, Wallin and Klingberg2018). For example, when assessing the foraging strategies of Tyrannidae in Brazil, a marked preference was found for foraging in the air or on living leaves, and none of the 28 species assessed showed a preference for branches (Gabriel & Pizo Reference Gabriel and Pizo2005).
In the case of the arthropods, different foraging strategies are exhibited that may affect predation on a specific substrate, for example, carabids may be generalists, staphylinids are facultative predators, spiders and opilions may have specialised hunting strategies, and ants are social insects that hunt from the ground to the treetops depending on the species (Vehviläinen et al. Reference Vehviläinen, Koricheva and Ruohomäki2008). Although in this study models were not placed at ground level, some work has found that at that level, arthropod predation may be more significant than other predator groups (Eötvös et al. Reference Eötvös, Lövei and Magura2020, Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Reference Ferrante, Barone, Kiss, Bozóné-Borbáth and Lövei2017a, Reference Ferrante, Lövei, Magagnoli, Minarcikova, Tomescu, Burgio, Cagan and Ichi2019, Mansion-Vaquié et al. Reference Mansion-Vaquié, Ferrante, Cook, Pell and Lövei2017). This may explain why arthropod markings were less common in this study.
Although the results indicate a preference for leaves, some limitations of the experimental design must be considered. Only considering artificial caterpillar size and not assessing other variables such as the height at which birds prey (Mansor & Mohd Sah Reference Mansor and Mohd Sah2012) may hinder the ability to draw specific conclusions about foraging strategies and preferences in this community. There are still gaps on how different ecological aspects affect the interactions between insectivorous birds and arthropods (Gunnarsson et al. Reference Gunnarsson, Wallin and Klingberg2018). Tree diversity and structure (Robinson & Holmes Reference Robinson and Holmes1984, Unno Reference Unno2002) as well as arthropod abundance (Unno Reference Unno2002) are known to shape the foraging strategy of a predator community (Robinson & Holmes Reference Robinson and Holmes1984). When assessing predation with artificial models, predation preferences vary by substrate (Koh & Menge Reference Koh and Menge2006, Maas et al. Reference Maas, Tscharntke, Saleh, Dwi Putra and Clough2015, Philpott et al. Reference Philpott, Soong, Lowenstein, Pulido, Lopez, Flynn and DeClerck2009, Sinu et al. Reference Sinu, Viswan, Fahira, Rajesh, Manoj, Hariraveendra and Jose2021); however, studies based on the observation of insectivorous birds show a tendency to forage on leaves over other substrates (Gabriel & Pizo Reference Gabriel and Pizo2005, Kwok Reference Kwok2009, Mansor & Mohd Sah Reference Mansor and Mohd Sah2012). This is probably because it is an abundant substrate and a good source of nutrients, which may in turn lead to a higher presence of arthropods (Kwok Reference Kwok2009. Laxton Reference Laxton2005). Increased predation on leaves may indicate greater visual exposure of prey on this substrate to birds (Tvardikova & Novotny Reference Tvardikova and Novotny2012), or be a reflection of the leaf preference of lepidopteran larvae in P. dulce.
Potential predators
Most marks were made by birds. These results are in agreement with those reported by Ferrante et al. (Reference Ferrante, Nunes, Lamelas-López, Lövei and Borges2022) and Sam et al. (Reference Sam, Koane and Novotny2015) in other experiments using artificial larvae. Despite that, the general trend is for arthropods to be the main predators (Ferrante et al. Reference Ferrante, Lo Cacciato and Lovei2014, Reference Ferrante, Barone, Kiss, Bozóné-Borbáth and Lövei2017a, Reference Ferrante, Lövei, Magagnoli, Minarcikova, Tomescu, Burgio, Cagan and Ichi2019, Reference Ferrante, Möller, Möller, Menares, Lubin and Segoli2021, Magagnoli et al. Reference Magagnoli, Masetti, Depalo, Sommaggio, Campanelli, Leteo, Lövei and Burgio2018, Molleman et al. Reference Molleman, Remmel and Sam2016, Pena et al. Reference Pena, Aoki-Gonçalves, Dáttilo, Ribeiro and MacGregor-Fors2021). The fact that the most common markings found on models fitted those of birds does not mean that these are the main predators in urban environments. One possibility is that the size of the model may favour attacks by birds. It has been observed that birds respond positively to increased prey size (Postema Reference Postema2021), having a strong impact at the end of the larval period; whilst, the effect is the opposite in arthropods since these mainly attack small individuals (Feeny et al. Reference Feeny, Blau and Kareiva1985, Lövei & Ferrante Reference Lövei and Ferrante2017, Remmel & Tammaru Reference Remmel and Tammaru2009, Remmel et al. Reference Remmel, Davison and Tammaru2011). Barton (Reference Barton1986) observed that ants attack eggs and small larvae of P. sennae, while avoiding lepidopteran larvae larger than 10 mm. In the case of the artificial larvae in the experiment, they were made of a length (40 mm) that corresponds to the last instar of P. sennae (35–45 mm) (Barton Reference Barton1986); therefore, it can be assumed that the results may be reflecting aspects of the natural history of lepidopteran larvae, where vertebrates, in this case birds, are the major threat to the larvae in their last instar.
The results might also reflect the limitations of using an artificial caterpillar method which may underestimate the risk of predation on real caterpillars. Detection of prey in many predator arthropods is guided by a combination of olfactory and visual cues as well as sensing of substrate vibration caused by feeding prey (Agrawal Reference Agrawal1998, Ferrante et al. Reference Ferrante, Barone and Lövei2017b, Reference Ferrante, Nunes, Lamelas-López, Lövei and Borges2022, Howe et al. Reference Howe, Lövei and Nachman2009, Mäntylä et al. Reference Mäntylä, Alessio, Blande, Heijari, Holopainen, Laaksonen, Piirtola and Klemola2008, Sam et al. Reference Sam, Koane and Novotny2015), characteristics absent in artificial caterpillars. In addition, another factor to be considered is the reliability of identification. The accuracy was 76% for identification in the categories of arthropods, birds and mammals by scientists with no previous experience (Valdés-Correcher et al. Reference Valdés-Correcher, Mäntylä, Barbaro, Damestoy, Sam and Castagneyrol2022). Hence, it cannot be concluded that birds are the main herbivore predators in urban settings. Furthermore, although the use of artificial models has certain disadvantages compared to the use of real caterpillars, these disadvantages do not make the method any less valid, and in some comparative studies, no significant differences have been found (Ferrante et al. Reference Ferrante, Barone and Lövei2017b, Richards & Coley Reference Richards and Coley2007).
Seasons
During periods of higher primary productivity, such as the rainy season, herbivore insect populations experiment peaks of maximum abundance that coincide with the breeding season of some predators such as birds (Atlegrim Reference Atlegrim1992, Langen & Berg Reference Langen and Berg2016, Richards & Coley Reference Richards and Coley2007). We did not find increased predation of artificial larvae in the rainy season, as already mentioned, the phenology of P. dulce is not marked in Cali (Cárdenas-Henao et al. Reference Cárdenas-Henao, Londoño-Lemos, Llano-Almario, González-Colorado, Rivera-Hernández, Vargas-Figueroa, Duque-Palacio, Torres-González, Jiménez-Taquinas and Moreno-Cavazos2015); therefore, the density of lepidopteran larvae may not present strong variations throughout the year, a determining factor in predation pressure (Molleman et al. Reference Molleman, Remmel and Sam2016), hereby explaining why seasons do not affect predation as occurs in other research in the tropics (Molleman et al. Reference Molleman, Remmel and Sam2016, Pan et al. Reference Pan, Mizuno, Ito, Ohsugi, Nishimichi, Nomiya, Ohno, Yamawo and Nakamura2020, Richards & Coley Reference Richards and Coley2007, Tiede et al. Reference Tiede, Schlautmann, Donoso, Wallis, Bendix, Brandl and Farwig2017).
In conclusion, our results suggest that predation pressure on a prey organism can vary significantly according to level of disturbance and the substrate location. A higher level of disturbance increases the abundance of some predators such as birds and thus increases the possibility of caterpillars being preyed upon. The substrate where prey is found becomes a key aspect for their detection and will depend on the specific foraging behaviour of their predators.
Geolocation information
3°22ʼ26.6ʼʼ N, 76°31ʼ51.1ʼʼ W; Santiago de Cali, Valle del Cauca, Colombia.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S026646742300007X
Acknowledgements
Thanks to K. Sam for methodological advice and to A W. Torres for statistical advice. Thanks to I. Castro, H. Alvarez-López, M. D. Heredia and and C. Espinosa for their valuable recommendations and to Marcia Dittmann, H. Burnham, I. Castro, and N. Bansal for help with the English translation. Finally, thanks to Constructora Limonar, Administradora de Vallados, Fundación Cañasgordas, and to the Department and Graduate School of Biology, Universidad del Valle for providing access to study sites. The project received permission (Res. 1070) from the Environmental Licence Authority [Autoridad Ambiental de Licencias Ambientales – ANLA], Colombia.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
The authors declare none.
Ethical statement
None.







