Prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of health-promoting bacteria in the intestinal tract( Reference Gibson 1 ). Fructo-oligosaccharide (FOS or oligofructose) is a fructan with a degree of polymerisation (2–20) that is obtained by enzymatic hydrolysis of inulin( Reference Mahious, Van Loo and Ollevier 2 ). FOS is present in a number of common foods such as garlic, onion, artichoke and asparagus( Reference Van Loo, Cummings and Delzenne 3 ), and dietary FOS has received great attention as a prebiotic for aquatic animals( Reference Ringø, Dimitroglou, Hoseinifar, Merrifield and Ringø 4 , Reference Ringø, Olsen and Gifstad 5 ). Despite some contradictory results( Reference Ringø, Olsen and Gifstad 5 – Reference Anguiano, Pohlenz and Buentello 7 ), beneficial effects of dietary FOS supplementation on growth performance and survival( Reference Mahious and Ollevier 8 – Reference Akrami, Iri and Khoshbavar Rostami 14 ), gut microbiota( Reference Akrami, Iri and Khoshbavar Rostami 14 , Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ), immune response( Reference Soleimani, Hoseinifar and Merrifield 12 , Reference Hoseinifar, Mirvaghefi and Merrifield 16 , Reference Buentello, Neill and Gatlin 17 ) and digestive enzyme activity( Reference Soleimani, Hoseinifar and Merrifield 12 , Reference Wu, Liu and Li 13 ) have been reported in several fish species.
Common carp (Cyprinus carpio) is a widespread freshwater fish and is the main aquaculture species in many European and Asian countries( Reference Naylor, Goldburg and Primavera 18 ). Considering the progress in culture methodologies, specifically elevation of the production by intensification of culture, which causes deterioration of water quality, stress to cultured organisms and outbreaks of infectious diseases, elevation of fish resistance and improvement of fish health status through dietary supplements are of importance in commercial carp aquaculture, especially in sensitive periods (i.e. larval and fry culture)( Reference Zhou, Li and Jun 19 ). However, despite the well-documented beneficial effects of prebiotics in finfish( Reference Ringø, Dimitroglou, Hoseinifar, Merrifield and Ringø 4 , Reference Ringø, Olsen and Gifstad 5 ), to our knowledge, there is no available information on the effects of dietary FOS supplementation in carp fry.
Therefore, the aim of the present study was to investigate the effects of dietary FOS supplementation on the growth performance, haemato-immunological parameters, culturable gut microbiota and stress resistance of common carp fry.
Experimental methods
Prebiotic
FOS used in the present study was kindly provided by Orafti (Raffinerie Tirlemontoise). According to the manufacturer, the chemical composition of the product was 98·2 % DM and 1·8 % crude ash.
Experimental diets
Experimental diets were prepared by supplementing a basal formulated diet with different levels (0 % (control), 1 %, 2 % and 3 %) of FOS (Table 1). The ingredients were blended thoroughly in a mixer and pelleted using a meat grinder equipped with a 2 mm die. The pelleted diets were air-dried and stored in plastic bags at 4°C until further use.
Table 1 Formulation (%) and proximate composition of the experimental diets
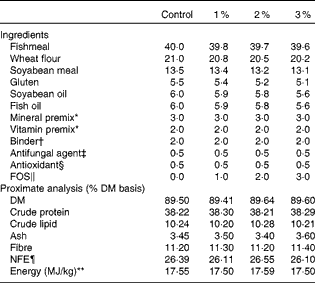
FOS, fructo-oligosaccharide; NFE, nitrogen-free extracts.
* Premix detailed by Hoseinifar et al. ( Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ).
† Amet binder™; Mehr Taban-e-Yazd.
‡ ToxiBan antifungal agent (Vet-A-Mix; Shenandoah).
§ Butylated hydroxytoluene (Merck).
∥ FOS; Raffinerie Tirlemontoise (FOS content >93 %).
¶ NFE = DM − (crude protein+crude lipid+ash+fibre).
** Gross energy (MJ/kg) calculated according to 23·6 kJ/g for protein, 39·5 kJ/g for lipid and 17·0 kJ/g for NFE.
Fish culture and feeding trial
A total of 480 common carp fry (3·23 (sem 0·14) g) obtained from the Siowal Caspian sea teleost fish propagation and cultivation centre (Golestan province, Iran) were acclimatised to the experimental conditions for 1 week. Thereafter, fish were randomly allocated to twelve 500-litre tanks (forty fish per tank and triplicate tanks per treatment). Water temperature, dissolved oxygen content and pH were monitored daily and maintained at 25·74 ± 1·79°C, 7·13 (sem 0·23) mg/l and 7·11 (sem 0·18), respectively. Each tank was aerated continuously through an airstone connected to a central air compressor. During the rearing experiment (7 weeks), fish were hand-fed to apparent satiation twice a day (09.00 and 15.00 hours). Uneaten feed was collected 1 h after feeding and dried at 60°C( Reference Hoseinifar, Khalili and Khoshbavar Rostami 20 ).
Determination of growth performance and survival rate
The growth performance parameters including weight gain (%), specific growth rates (SGR), condition factor (CF), feed conversion ratio (FCR) and survival rate were calculated according to the following formulae:
where W 1 is the initial weight (g), W 2 is the final weight (g), T is time (d), FO is the feed provided (g) and WG is the weight gain (g),
where N 0 is the initial number of fish and N f is the final number of fish.
Haemato-immunological analysis
At the end of the feeding trial, after 7 weeks, blood was collected from the caudal vein of carp fry (three fish per tank) for haemato-immunological analysis. Whole blood was suspended in the Natt & Herrick diluent( Reference Natt and Herrick 21 ) for performing erythrocyte counts and total leucocyte counts (WBC) using a haemocytometer. Haematocrit (Ht) was determined using the micro-Ht method as described by Brown( Reference Brown 22 ), and Ht values are reported as packed cell volume percentage. Hb levels were estimated using Sahli's method( Reference Blaxhall and Daisley 23 ). Differential WBC (neutrophils, lymphocytes and monocytes) were performed using May–Grunwald–Giemsa-stained blood smears.
The respiratory burst activity was measured in triplicate by chemiluminescent assay (measurement of light emission) based on the modified protocol of Mathews et al. ( Reference Mathews, Warinner, Weeks, Stolen, Fletcher, Anderson, Roberson and van Muiswinkel 24 ) as described by Khoshbavar-Rostami et al. ( Reference Khoshbavar-Rostami, Soltani and Hassan 25 ) using an automated system for chemiluminescent analysis (Luminoskan Ascent T392; Thermo Fisher Scientific, Inc.).
Quantification of autochthonous intestinal microbiota
Samples of the entire intestine of fifteen fish were used for quantifying total viable autochthonous heterotrophic aerobic bacteria and lactic acid bacteria (LAB) at the start and end of the experiment as described previously( Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ). Samples were processed on an individual basis and were not pooled. Dilutions (100 μl) were spread in triplicate onto Plate Count Agar medium (Merck) and de Man, Rogosa and Sharpe medium (Merck) for quantifying total viable heterotrophic aerobic bacteria and LAB, respectively. The plates were incubated at room temperature (25°C) for 5 d and colony-forming units (CFU)/g were calculated from statistically viable plates (i.e. plates containing thirty to 300 colonies)( Reference Rawling, Merrifield and Davies 26 ).
Salinity stress challenge test
Salinity stress challenge test was carried out at the end of the feeding trial according to the method of Hoseinifar et al. ( Reference Hoseinifar, Khalili and Khoshbavar Rostami 20 ). A total of fifteen fish were sampled from each tank and subjected to salinity stress challenge. The fry were exposed to 15 g/l salinity in triplicate (fifteen fish per tank). The survival rates of fish were calculated 72 h after the challenge.
Statistical analyses
Statistical analyses were conducted using SPSS statistical package version 17.0 (SPSS, Inc.). Each tank served as the unit of analysis. All data were subjected to a one-way ANOVA to test the effects of dietary FOS supplementation on the haemato-immunological parameters, growth performance, culturable gut microbiota and stress resistance of carp fry (C. carpio). Duncan's multiple range test was carried out when the differences were significant( Reference Zar 27 ). P values < 0·05 were considered to be statistically significant. Data are presented as mean values with their standard errors.
Results
The growth performance parameters of carp fry fed experimental diets containing different levels of FOS are summarised in Table 2. There were no significant differences in the initial weight of the treatment groups (P>0·05). Similarly, at the end of the feeding trial (7 weeks), no significant differences were observed in the final weights, WG, SGR, CF and FCR of common carp fry fed the FOS-supplemented diets and the control diet (P>0·05; Table 2).
Table 2 Growth performance parameters and survival rate of common carp fry fed diets supplemented with varying levels of fructo-oligosaccharide for 7 weeks (Mean values with their standard errors)
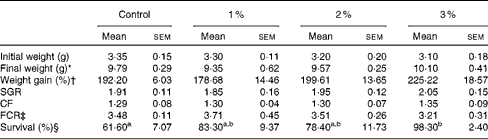
SGR, specific growth rates; CF, condition factor; FCR, feed conversion ratio.
a,bMean values within a row with unlike superscript letters were significantly different (P>0·05).
* α = 0·05; β = 0·17.
† α = 0·05; β = 0·15.
‡ α = 0·05; β = 0·11.
§ α = 0·05; β = 0·16.
Calculation of survival rates at the end of the experiment revealed that dietary FOS supplementation significantly increased the survival rate of carp fry (P< 0·05; Table 2). Carp fry fed the 3 % FOS diet exhibited the highest survival rate (98·3 (sem 2·4)%).
The effects of dietary FOS supplementation on the haemato-immunological parameters of carp fry are summarised in Tables 3 and 4. Statistical analyses revealed that erythrocyte count, mean corpuscular volume, mean corpuscular Hb content, mean corpuscular Hb concentration, Hb, Ht and differential leucocyte counts were not significantly affected by dietary FOS supplementation (P>0·05; Table 3). WBC were significantly affected by 3 % FOS supplementation (P< 0·05; Table 4), while no effect of FOS supplementation was observed on lymphocyte, neutrophil and monocyte counts.
Table 3 Effects of dietary fructo-oligosaccharide (FOS) supplementation on the haematological parameters of common carp fry (Mean values with their standard errors)
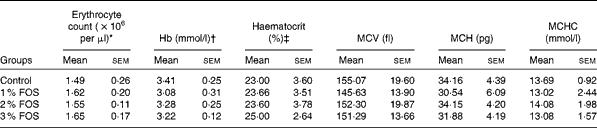
MCV, mean corpuscular volume; MCH, mean corpuscular Hb content; MCHC, mean corpuscular Hb concentration.
* α = 0·05; β = 0·10.
† α = 0·05; β = 0·14.
‡ α = 0·05; β = 0·13.
Table 4 Differential leucocyte counts of common carp fry fed diets supplemented with different levels of fructo-oligosaccharide (FOS) for 7 weeks (Mean values with their standard errors)
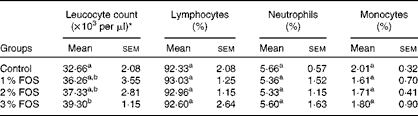
a,bMean values within a column with unlike superscript letters were significantly different (P>0·05).
* α = 0·05; β = 0·12.
Dietary FOS supplementation significantly increased the respiratory burst activity compared with the control treatment (P< 0·05; Fig. 1).
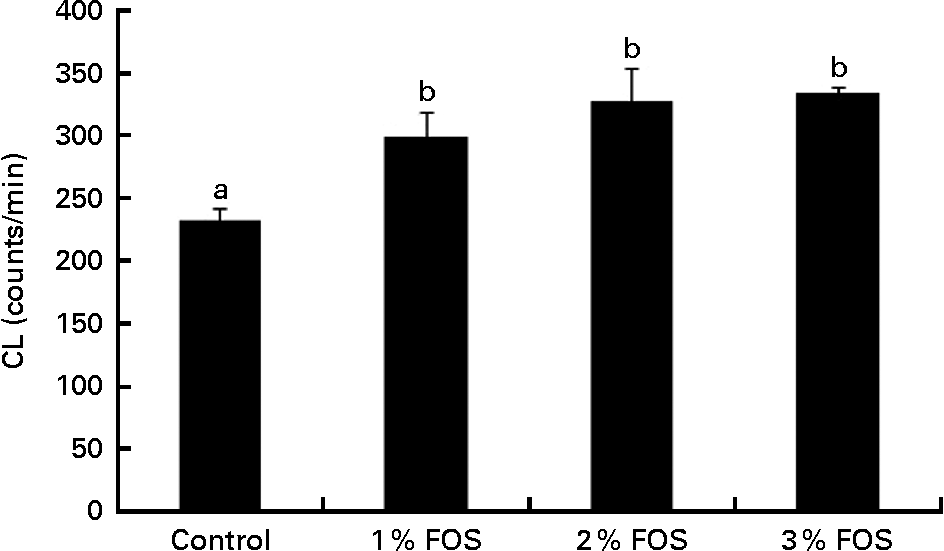
Fig. 1 Respiratory burst activity (chemiluminescence (CL) response; light emission count/min) (α = 0·05; β = 0·17) of carp fry fed diets supplemented with different levels (0 % (control), 1 %, 2 % and 3 %) of fructo-oligosaccharide (FOS) for 7 weeks. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05).
The levels of total heterotrophic autochthonous gut bacteria in the control group (4·05 (sem 0·07) CFU/g) were significantly lower than those in carp fry fed the FOS-supplemented diets (P< 0·05; Fig. 2). Total autochthonous intestinal heterotrophic bacteria levels were significantly higher in the 2 and 3 % FOS groups (5·68 (sem 0·10) and 5·57 (sem 0·24) CFU/g, respectively). However, no cultivable LAB were detected in the control and 1 % FOS groups; the 2 and 3 % FOS groups had 1·50 (sem 0·04) and 2·42 (sem 0·06) CFU/g of autochthonous LAB, respectively (Fig. 2).
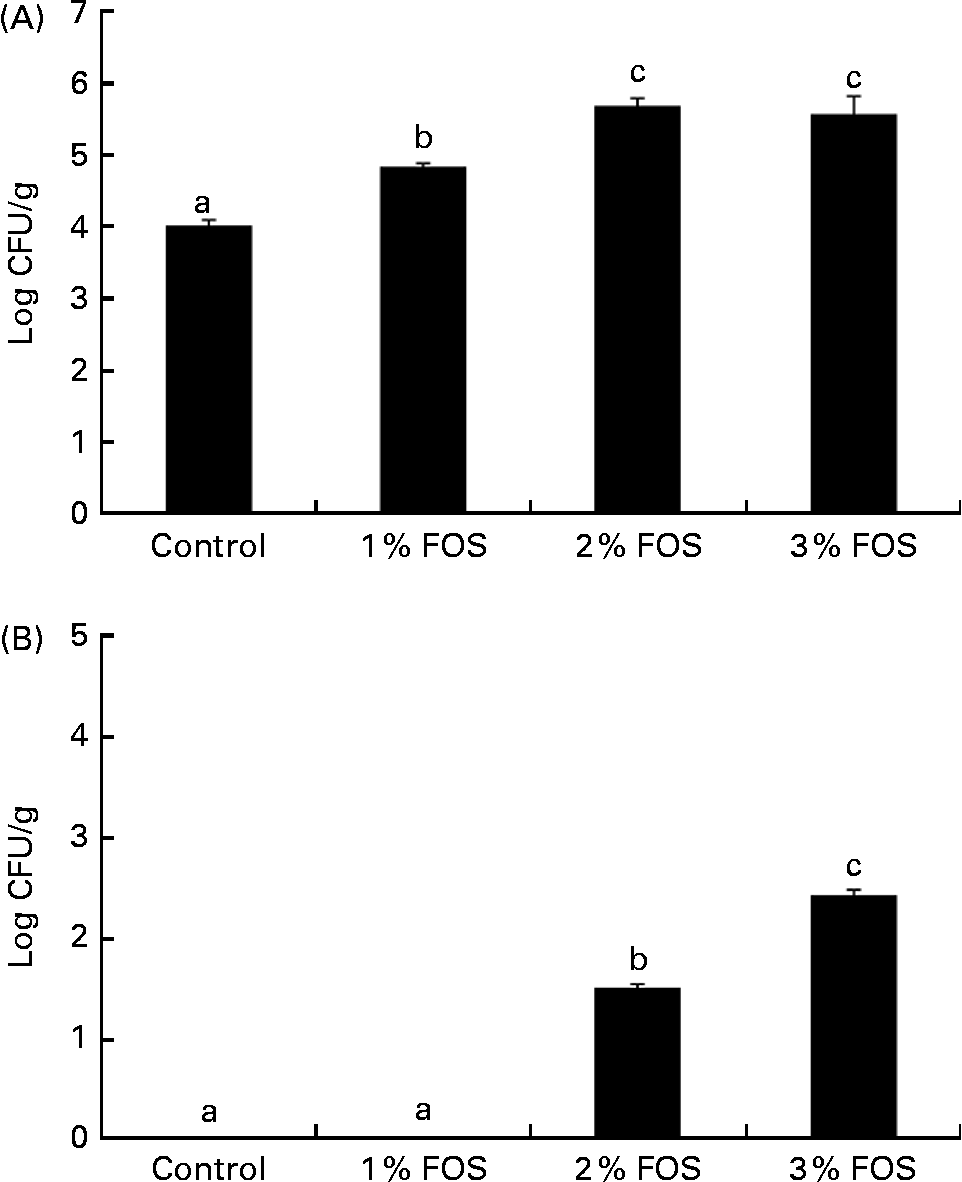
Fig. 2 Levels of total culturable autochthonous bacteria (A) (α = 0·05; β = 0·14) and autochthonous lactic acid bacteria (B) (α = 0·05; β = 0·12) (log colony-forming units (CFU)/g intestine) of carp fry fed diets supplemented with different levels (0 % (control), 1 %, 2 % and 3 %) of fructo-oligosaccharide (FOS) for 7 weeks. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05).
The results of the salinity challenge test are shown in Fig. 3. Low survival rates were observed 72 h after the challenge in the control group, while carp fry fed the FOS-supplemented diets had significantly improved the survival rate (P< 0·05; Fig. 3). The survival rates of the 1, 2 and 3 % FOS groups were 53·4 (sem 3·9), 57·7 (sem 4·52) and 59·6 (sem 8·16) %, respectively.
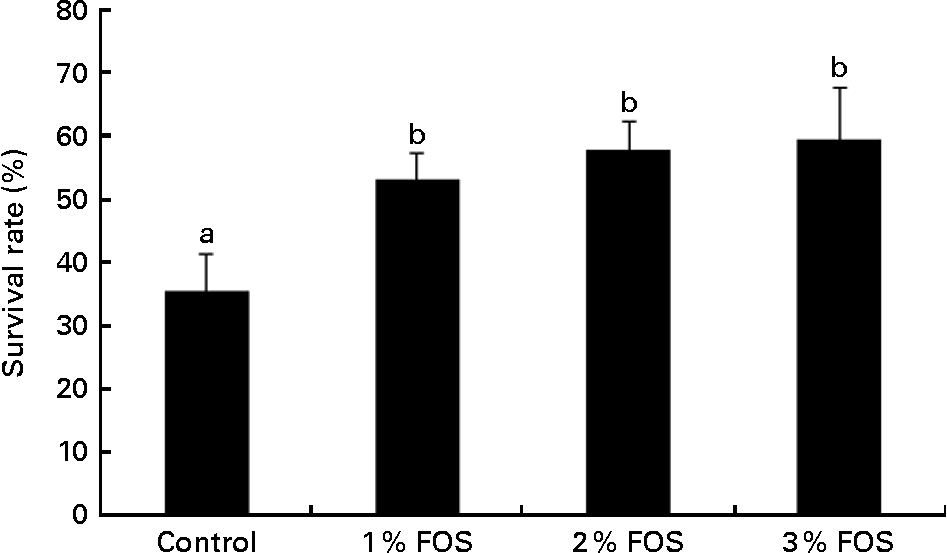
Fig. 3 Survival rate of carp fry fed diets supplemented with different levels (0 % (control), 1 %, 2 % and 3 %) of fructo-oligosaccharide (FOS) for 7 weeks after exposure to salinity stress challenge (exposure to 15 g/l water salinity for 72 h) (α = 0·05; β = 0·19). Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05).
Discussion
To our knowledge, this is the first study to investigate the effects of dietary FOS supplementation on the growth performance, haemato-immunological parameters, cultivable gut microbiota and stress resistance of common carp fry. The results of the present study showed that 1, 2 or 3 % FOS supplementation had no significant effects on the growth performance of carp fry. These results are not in accordance with those of previous studies in blunt snout bream (Megalobrama amblycephala) fingerlings( Reference Wu, Liu and Li 13 ), stellate sturgeon (Acipenser stellatus) juveniles( Reference Akrami, Iri and Khoshbavar Rostami 14 ), farmed rainbow trout (Oncorhynchus mykiss)( Reference Ortiz, Rebolé and Velasco 28 ) and red drum (Sciaenops ocellatus)( Reference Buentello, Neill and Gatlin 17 ). However, Hoseinifar et al. ( Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ) and Grisdale-Helland et al. ( Reference Grisdale-Helland, Helland and Gatlin 29 ) reported that dietary FOS supplementation had no significant effects on the growth performance of beluga (Huso huso) juveniles and Atlantic salmon (Salmo salar). The differences observed in the results of these studies might be due to the different methods of prebiotic administration, dosage levels, different intestinal morphology, gut microbiota and life stages( Reference Hoseinifar, Zare and Merrifield 11 ).
Dietary FOS supplementation significantly increased the survival rate of common carp fry compared with the control treatment. Similarly, improved survival of cobia (Rachycentron canadum) larvae( Reference Salze, McLean and Schwarz 30 ), rainbow trout( Reference Staykov, Spring and Denev 31 ) and beluga juveniles( Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ) has been observed upon prebiotic administration. Akrami et al. ( Reference Akrami, Iri and Khoshbavar Rostami 14 ) reported that the survival rate of stellate sturgeon juveniles was not affected by 1 or 2 % dietary FOS supplementation. The improved survival rate of prebiotic-fed cultured organisms might be due to improved general health or immune status, as reported previously( Reference Soleimani, Hoseinifar and Merrifield 12 ), but further investigations are needed to clarify the underlying mechanisms.
Haemato-immunological parameters are valuable tools for the determination of cultured organism health status( Reference Cnaani, Tinman and Avidar 32 ). These parameters have been widely used in prebiotic studies for the evaluation of changes in health status as a result of prebiotic administration( Reference Ringø, Dimitroglou, Hoseinifar, Merrifield and Ringø 4 , Reference Merrifield, Dimitroglou and Foey 6 ). The results of the present study showed that dietary FOS supplementation had no significant effects on haematological parameters including erythrocyte count, mean corpuscular volume, mean corpuscular Hb content, mean corpuscular Hb concentration, Hb, Ht and differential leucocyte counts, but the significantly higher WBC values in the prebiotic-fed fish are in accordance with the results of the study carried out by Akrami et al. ( Reference Akrami, Iri and Khoshbavar Rostami 14 ). Similar elevation of WBC has been observed in beluga (H. huso) juveniles fed FOS( Reference Hoseinifar, Mirvaghefi and Merrifield 16 ). In the present study, dietary FOS supplementation increased the respiratory burst activity compared with the control treatment. Similarly, previous studies on the effects of synbiotic administration in large yellow croaker (Larimichthys crocea)( Reference Ai, Xu and Mai 33 ), gilthead sea bream (Sparus aurata L.)( Reference Cerezuela, Cuesta and Meseguer 34 ) and koi (C. carpio koi)( Reference Lin, Mao and Guan 35 ) revealed a significantly higher respiratory burst activity. In contrast to these results, Akrami et al. ( Reference Akrami, Iri and Khoshbavar Rostami 14 ) reported that FOS supplementation had no effect on the respiratory burst activity of stellate sturgeon. Respiratory bursts, produced by phagocytes to attack invasive pathogens during phagocytosis, have been widely used for the evaluation of defence ability against pathogens( Reference Chiu, Guu and Liu 36 ). Elevation of WBC and respiratory burst activity observed in the present study is probably caused by the stimulation of carp immune response due to FOS supplementation.
The intestinal microbiota of fish is a complex and dynamic ecosystem containing a diverse community of micro-organism( Reference Ringø and Birkbeck 37 , Reference Ringø, Strøm and Tabachek 38 ), and genetic, nutritional and environmental factors are the main factors that modulate the intestinal microbiota( Reference Pérez, Balcázar and Ruiz-Zarzuela 39 ). Although LAB are considered a minor constitute of the intestinal microbiota of several fish species, they have been considered to be beneficial components of the fish intestine( Reference Ringø and Gatesoupe 40 ). Modulation of the intestinal microbiota of fish towards beneficial communities (i.e. LAB) has been studied through the administration of probiotics, prebiotics and synbiotics( Reference Ringø, Dimitroglou, Hoseinifar, Merrifield and Ringø 4 , Reference Dimitroglou, Merrifield and Carnevali 41 ). The levels of cultivable autochthonous intestinal microbiota observed in the present study revealed that dietary FOS supplementation significantly increased the levels of total autochthonous intestinal heterotrophic bacteria. In addition, the levels of LAB were significantly elevated in common carp fed the 2 and 3 % FOS diets. However, further studies using more sensitive molecular methods such as denaturing gradient gel electrophoresis (DGGE) and 16S rRNA sequencing are required to identify the LAB species to confirm their beneficial effects in common carp fry. Similar to the present results, FOS has been reported to increase the levels of total cultivable autochthonous intestinal heterotrophic bacteria and LAB in stellate sturgeon( Reference Akrami, Iri and Khoshbavar Rostami 14 ) and beluga juveniles( Reference Hoseinifar, Mirvaghefi and Mojazi Amiri 15 ). The results of the present study confirmed that modulation of the intestinal microbiota of carp can be achieved through dietary FOS supplementation, but further investigations are needed.
Resistance during exposure to abrupt changes in water salinity (salinity stress test) has been considered an important indicator of fry quality in nutrition trials, especially in prebiotic studies( Reference Hoseinifar, Khalili and Khoshbavar Rostami 20 , Reference Salze, McLean and Schwarz 30 , Reference Smith, Kane and Popper 42 , Reference Taoka, Maeda and Jo 43 ). The results of the present study revealed that dietary FOS supplementation significantly increased fish resistance in the salinity stress challenge test compared with the control treatment. Similarly, Soleimani et al. ( Reference Soleimani, Hoseinifar and Merrifield 12 ) reported that Caspian roach (Rutilus rutilus) fry fed 3 % dietary FOS exhibited significantly higher resistance in salinity stress challenge test, and this is in accordance with previous reports on Caspian roach fed galacto-oligosaccharide( Reference Hoseinifar, Khalili and Khoshbavar Rostami 20 ) and cobia( Reference Salze, McLean and Schwarz 30 ) and white sea bream larvae (Diplodus sargus L.)( Reference Dimitroglou, Davies and Sweetman 44 ) fed mannan oligosaccharide. Although not confirmed in the present study, the main reason for the prebiotic effects on salinity stress resistance has been suggested to be the improved alignment of microvilli( Reference Dimitroglou, Davies and Sweetman 44 ), and evaluation of microvillus alignment will be included in further prebiotic studies.
In conclusion, dietary FOS supplementation had no significant effects on the growth performance and haematological parameters of carp fry, but significantly increased WBC and respiratory burst activity and modulated cultivable autochthonous gut microbiota levels and stress resistance. The positive results obtained encourage conducting further research on the administration of FOS and other prebiotics in common carp fry studies. Determination of the mechanisms of action and optimal inclusion levels is a topic that merits further research.
Acknowledgements
The authors are grateful to Orafti Company (Raffinerie Tirlemontoise) for its support and provision of FOS and to Professor Van Loo and Dr Mahious for their kind help and guidance. They also thank Dr Khoshbavar Rostami, Dr Yelghi, Mr Kor, Mr Fazel, Mr Binaii, and the staff at Gharesoo Research Station for their support during the study.
The present study was supported by grants from the Gorgan University of Agricultural Sciences and Natural Resources. The funder had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: S. H. H. contributed to the study design, conducted the study and analysed the samples, interpreted the data and wrote a draft of the manuscript; N. S. contributed to the conduction of the study; E. R. contributed to the interpretation of the results and improved the manuscript by critical comments.
None of the authors has any conflicts of interest to declare.









