Introduction
Porphyrins are planar macrocyclic molecules containing four pyrroles connected in ring fashion through four methine carbons at their α-positions (Figure 1). These ubiquitous molecules are widely present in almost all living organisms in one form or another, the best known being chlorophyll and heme (or hemoglobin).Reference Barber1,Reference Ostfeld and Tsutsui2 It is self-evident that porphyrins are important participants in the energy supply system for both earth and life via photosynthesis and oxygen transport.

Figure 1. Three-dimensional molecular structure of (a) porphyrin and (b) metalloporphyrin.
Porphyrins are basically cyclic tetrapyrrole derivatives with highly delocalized π electrons that form a planar conjugated framework. This is an 18-π electron system that exhibits aromaticity (Figure 1a). The highly conjugated π-system is the origin of the strong color of these compounds as well as their characteristic electronic and redox properties. Indeed, the word “porphyrin” is derived from the Greek word for “purple.”
Porphyrins mostly exist in the form of derivatives of the basic molecules due to their chemical properties. On one hand, substitution can be performed at all of the peripheral eight pyrrole β-carbon atoms and the four meso-carbon centers; this would make porphyrin exist in varied forms with structural variations. On the other hand, the porphyrin ring provides a vacant site at its center, which is ideally suited for metalloporphyrin incorporation (Figure 1b). The NH protons inside the ring of porphyrins possess acidic character and can become deprotonated, forming dianion species. These dianion species exhibit remarkable coordination characteristics toward metal ions and mostly act as a tetradentate ligand with metal ions. The common coordination number of the metal ion in a metalloporphyrin is possibly four. The electronic delocalization leads to substantial planarity of the macrocycle and a square planar environment for the metal ion in four-coordinate complexes. A coordination number (five or six) greater than four is also possible through ligation of suitable moieties either neutral or anionic.
Meanwhile, the extensive electronic delocalization that occurs in the porphyrin ring leads to substantial planarity of the macrocycle and an essentially square planar environment for the metal ion in four-coordinate complexes. The bonding between a central metal ion and the porphyrin occurs via two types of primary interactions—σ-coordination of nitrogen lone pairs directed toward the central metal atom, and π-interaction of metal pπ and dπ orbitals with nitrogen pπ orbitals. This electron system results in visible light absorption of metalloporphyrin, the normal spectrum for which shows an intense B (Soret) band (strong transition from the base to the second excited state (S0 → S2)) at ∼420 nm and two weaker Q bands (transition to the first excited state (S0 → S1)) at ∼550–600 nm.Reference Suslick, Rakow, Kosal and Chou3
In nature, porphyrin-based biological functional materials are mostly constructed by metalloporphyrins with protein.Reference Denisov, Makris, Sligar and Schlichting4,Reference Suga, Akita, Hirata, Ueno, Murakami, Nakajima, Shimizu, Yamashita, Yamamoto, Ago and Shen5 The best example is heme, which is an iron complex of porphyrin. Heme plays a key role in various proteins and enzymes in life, such as for O2 transport and storage (hemoglobin and myoglobin), electron transfer (cytochrome), and O2 activation and utilization (cytochrome oxidase and cytochrome P450).Reference Shaik, Kumar, de Visser, Altun and Thiel6 The versatile characteristics of these porphyrin molecules can be attributed to both the electronically sensitive and tunable delocalized π-systems and the affinity of the center metal atom to diatomic gases.
Chlorophyll, a magnesium complex of porphyrin, is another attractive porphyrin-base nature compound. Chlorophyll plays a key role as a reaction center to absorb and transfer light energy to other parts of the photosystem. Chlorophyll donates a high-energy electron to a series of molecular intermediates through aggregates of magnesium-porphyrin. The charged reaction center of the chlorophyll (P680+, a chlorophyll-protein complex in the photosynthesis process) is then reduced back to its ground state by accepting an electron stripped from water. The electron that reduces P680+ ultimately comes from oxidation of water into O2 and H+ through several intermediates. This reaction process demonstrates how photosynthetic organisms (e.g., plants), which is the source for practically all of the oxygen in Earth’s atmosphere currently, produce oxygen.Reference Najafpour, Renger, Holynska, Moghaddam, Aro, Carpentier, Nishihara, Eaton-Rye, Shen and Allakhverdiev7
Along with the continuous exploration of functional natural porphyrin compounds, as well as the combination of porphyrins and nanotechnology, various artificial porphyrin compounds and porphyrin-based nanomaterials have been synthesized for numerous application fields, including solar-energy harvesting,Reference Zhang, Lai and Cao8,Reference Kundu and Patra9 chemical sensors,Reference Paolesse, Nardis, Monti, Stefanelli and Di Natale10,Reference Ding, Zhu and Xie11 photodynamic therapy (PDT),Reference Dougherty, Gomer, Henderson, Jori, Kessel, Korbelik, Moan and Peng12,Reference Abrahamse and Hamblin13 and self-assembly.Reference Chen, Li, Huang, Wang and Kang14,Reference Zhong, Wang, Zhang, Bai, Wu, Haddad and Fan15
Self-assembly of porphyrins
Self-assembly of functional molecular materials into well-defined nanostructures depending on various noncovalent interactions has attracted increasing research interest both for materials synthesis as well as for device integration.Reference Hasobe16,Reference Chakrabarty, Mukherjee and Stang17 As typical representatives of functional molecular materials with highly conjugated structures, porphyrins hold advantages of chemical stability and easy structure manipulation, making them attractive for organization into nano- and microscale structures using self-assembly techniques especially for highly functional optoelectronic applications. Upon aggregation, the molecular properties of the building blocks are significantly altered.
More sophisticated studies of the electronic properties of J-aggregates, a red-shift in absorption, reveal that excited states are formed by extended domains of coherently coupled molecular-transition dipoles. This suggests that J-aggregation can be the archetypical phenomenon for emergent systems with properties imparted on the supramolecular or nanoscale level.Reference Vasilopoulou, Douvas, Georgiadou, Constantoudis, Davazoglou, Kennou, Palilis, Daphnomili, Coutsolelos and Argitis18 Arrangeing porphyrin molecules in certain ways can improve the degree of conjugation and widen the intensity of absorption range of visible light. The ordered arrangement can form an internal electric field, which promotes the generation and separation of photogenerated carriers, inhibits recombination of photogenerated carriers, and thus improves photocatalytic performance.
Porphyrins and their derivatives can be self-assembled into one-dimensional–three-dimensional (1D–3D) nanostructured materials through noncovalent molecular interactions using different methods, depending on their solubility in water and their electrostatic properties (Figure 2). The formed nanostructures influence their optical properties and thereby functional applications.Reference Wang, Zhong, Wang, Zhan, Cao, Bian, Alarid, Haddad, Bai and Fan19,Reference Zhong, Wang, Zhang, Wei, Wang, Lu, Bai, Wu, Haddad and Fan20 Synthesis and assembly of these materials are conducted at two length scales—the nanoscale and the macroscale. At the nanoscale, porphyrins and their derivatives with well-defined size and chemistry possess unique optical, electrical, and photocatalytic properties, providing both structure and function. Essential understanding of porphyrin chemistry and associated noncovalent interactions such as hydrogen bonding and aromatic π−π stacking that drives them to self-assemble into 1D–3D nanostructures will be discussed in this issue.
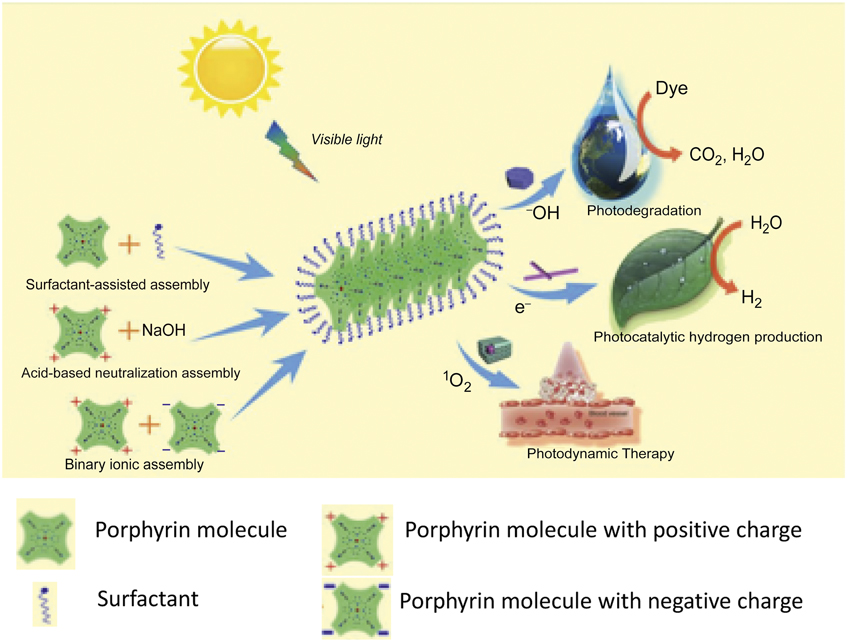
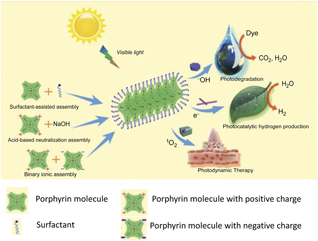
Figure 2. Self-assembly of porphyrins and their applications. The formation process of porphyrin nanostructures prepared from porphyrin molecules assisted with a surfactant and/or ionic interaction. These porphyrin nanostructures can be applied in water purification, water splitting, and photodynamic therapy. Note: 1O2, singlet oxygen; –OH, hydroxyl radical.
The articles in this issue address controlled assembly and formation of 1D–3D nanostructures with precisely defined size, shape, and spatial arrangement of individual porphyrins and their derivatives. The articles survey how self-assembly enables molecular coupling and facilitates intermolecular charge separation, and energy transfer or delocalization. The corresponding collective optical properties induced by controlled self-assembly such as absorption and photoluminescence are also covered. On the macroscale, the resulting self-assembled nanostructures with well-defined hierarchical structures and functions provide an ideal model material system for the fundamental understanding of structure–property relationships for complex advanced materials. We discuss major applications, including photodegradation of organic pollutants, photocatalytic water splitting and hydrogen production, photosynthesis of fuel-cell catalysts, and PDT. The articles in this issue provide an overview of the field for future opportunities, in particular, those associated with photoactive pigment materials and their applications in the phototransduction process for energy applications.
Solar-energy harvesting
Inspired by natural photosynthesis, researchers have made extensive efforts to utilize artificial chlorophylls based on porphyrins and their derivatives for converting solar energy to chemical energy with the production of hydrogen from water splitting as a clean and renewable energy source alternative to fossil fuels and their consequent environmental issues. The key is to develop efficient and robust catalysts as reaction centers. Metalloporphyrins, which are highly effective in stabilizing high-valent metal centers, are able to offer the highly dispersed metal ion that act on the catalytic active site.Reference Shaik, Hirao and Kumar21 These features are crucial in the oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), and oxygen evolution reaction (OER) catalysis.
Another significant feature of porphyrins is that the abundant substituents on the macrocycle greatly enrich the redox chemistry of their metal complexes. In addition, electron-withdrawing substituents on porphyrins can decrease the electron density on the macrocycle and thus increase its stability during catalysis. These substituents can cause the reduction potentials to shift in the positive direction, which decreases the over potential for ORR and HER catalysts and increases the oxidizing power of OER catalysts.Reference Zhang, Lai and Cao8 Porphyrins have also been shown to be good photosensitizers with high absorbance coefficient under visible light due to their delocalized π-systems. Mathew and co-workers reengineered the prototypical structure of donor–π-bridge–acceptor and demonstrated panchromatic light harvesting without the use of co-sensitization, resulting in a power-conversion efficiency of 13% at full sun illumination by functionalizing the porphyrin core with the bulky bis(2′, 4′-bis(hexyloxy)-[1,1′-biphenyl]-4-yl)amine donor and proquinoidal benzothiadiazole.Reference Mathew, Yella, Gao, Humphry-Baker, Curchod, Ashari-Astani, Tavernelli, Rothlisberger, Nazeeruddin and Graetzel22
The planar and aromatic porphyrin rings not only result in good light absorption capacity, but also enable strong aggregation of these molecules to form self-assembled arrays through noncovalent π−π interactions, which can further increase their catalytic activity and stability.Reference Zhong, Wang, Zhang, Wei, Wang, Lu, Bai, Wu, Haddad and Fan20 The absorption spectra of self-assembled porphyrin arrays display complicated signals compared to those of individual molecules due to the coupling of the conjugated π−π structure from individual porphyrins within the ordered assemblies. For example, assembled zinc-porphyrins produce much broader UV−visible absorption bands that are red-shifted and enhanced from the corresponding bands of the monomeric porphyrins. This red-shifted assembly (J-aggregates) suggests molecular coupling of the porphyrins.Reference Wang, Zhong, Wang, Zhan, Cao, Bian, Alarid, Haddad, Bai and Fan19 These materials exhibit enhanced photocatalytic activity for both photodegradation of organic dyes and the production of hydrogen. Additionally, the formation of a J-aggregate activates the porphyrin core by increasing the electronic density to a large extent, which helps stabilize the photoexcited electron holes for enhanced hydrogen production. Such self-assembly is similar to the role of a light-harvesting complex in natural photosynthesis systems that contain chlorophyll aggregates, in which chromophores form J-aggregates along the head-to-tail arrangement via a stronger transition-dipole moment for harvesting solar energy.Reference Zhang, Wang, Wang, Cao, Wang, Bai and Fan23
Benefiting from their relatively strong absorption in the visible light spectrum and efficient electron-transport through a well-ordered crystal, self-assembled porphyrin nanomaterials show great potential in solar-energy harvesting. The articles in this issue summarize recent research breakthroughs in the interdependent relationship of well-defined porphyrin self-assembled nanostructures and their visible-light catalytic processes in water splitting and organic pollutants degradation.
Photodynamic therapy
Photodynamic therapy (PDT) was discovered more than 100 years ago.Reference Moan and Peng24 While new methods are still emerging, PDT is already a successful and clinically approved therapy for cancer and nonmalignant diseases, including infections.Reference Agostinis, Berg, Cengel, Foster, Girotti, Gollnick, Hahn, Hamblin, Juzeniene, Kessel, Korbelik, Moan, Mroz, Nowis, Piette, Wilson and Golab25 PDT consists of three essential components—photosensitizer (PS), light, and oxygen. During PDT, PSs are activated by absorption of visible light to form the excited singlet state, followed by transition to the long-lived excited triplet state. This triplet state can undergo photochemical reactions in the presence of oxygen to form reactive oxygen species (singlet oxygen, 1O2). The 1O2 can rapidly cause significant toxicity leading to cell death via apoptosis or necrosis. 1O2 has a short lifetime in biologic systems (<0.04 μs) and a short radius of action (<0.02 mm) for precision therapy. PDT can be used either before or after chemotherapy, radiotherapy, or surgery without compromising these therapeutic modalities.
Most of the PSs used in cancer therapy are based on porphyrin, such as hematoporphyrin derivative (HPD).Reference Abrahamse and Hamblin13 The most widely used photosensitizer in clinical PDT today is Photofrin, which is made by partially purifying HPD to remove less-active porphyrin monomers.Reference Dougherty26 An ideal PS agent should be a single pure compound to achieve highly efficient PDT with low manufacturing cost and good stability during storage. It should have a high absorption peak between 600–800 nm (red to deep red), because absorption of photons with wavelengths longer than 800 nm does not provide enough energy to excite oxygen to its singlet state and to form a substantial yield of reactive oxygen species.Reference Allison and Sibata27
To improve the effectiveness of PDT, two issues must be addressed: accumulation of the PS in diseased tissues and localized light delivery. These demands require materials scientists to prepare red absorption PS with a proper size, which should be between 50–100 nm for long residence time in tissue.Reference Nam, Won, Bang, Jin, Park, Jung, Jung, Park and Kim28 Nanotechnology has made a significant contributions, providing approaches such as nanoparticle delivery, fullerene-based PS, and the use of upconverting nanoparticles to increase light penetration into tissues. Wang and co-workers recently discovered that core−shell structured porphyrin−silica nanocomposites with 80-nm diameter show high-yield generation of 1O2 for PDT, paving a new way for the preparation of efficient PS.Reference Wang, Zhong, Wang, Yang, Bai, Zhang, Alarid, Bian and Fan29 The Yang and Zhang articleReference Yang and Zhang30 in this issue discusses the development of porphyrin photosensitizers, characterization methods of singlet oxygen, strategies of porphyrin delivery, as well as the challenges and outlook in porphyrin-based nanocomposites-mediated PDT.
In this issue
The articles in this issue focus on the recent developments of porphyrin nanomaterials and their applications in both energy harvesting and biomedicine. In their article in this issue, Li et al.Reference Li, Zhao and Bai31 discuss the interdependent relationship of well-defined porphyrin self-assembled nanostructures and their visible light catalytic processes. The porphyrin self-assembly, which assembles the modular units into specific spatial arrangements and facilitates their working cooperatively, exhibits more complicated absorption and emission spectra than those of the monomers. Rapid photo-induced charge separation and relatively slow charge recombination act as a novel structure and improve the photocatalytic activity.
Yang and Zhang,Reference Yang and Zhang30 in their article, provide a comprehensive review introducing the development of porphyrin photosensitizers and characterization methods for singlet oxygen (1O2), followed by strategies for porphyrin delivery, including encapsulation, covalent conjugation, and self-assembly for PDT. The challenges and outlook in porphyrin-based nanocomposite-mediated PDTs are also discussed.
Zhong et al.Reference Zhong, Wang and Tian32 cover the concept of binary ionic self-assembly and ionized forms of porphyrins, including tuning of compositions, control of hierarchical structures, and related electronic and light-harvesting properties in preparing porphyrin functional nanomaterials.
Wei et al.Reference Wei, Sun and Fan33 provide general perspectives on porphyrin and derivative chemistry and discussions on surfactant-assisted cooperative self-assembly using amphiphilic surfactants and functional porphyrins and derivatives. Through kinetic control, this method readily allows fine tuning of the nanostructure and function on multiple length scales and multiple locations. These structure-controlled porphyrin nanomaterials provide a controllable model system to correlate synthesis–structure–property relationships and amplify the intrinsic advantages of individual porphyrins for their application in light harvesting and energy storage.
Summary
In summary, porphyrin, as a planar macrocyclic molecule widely present in nature, has many potential applications ranging from the energy conversion, catalysis, and sensors to nanomedicine. This issue highlights the synthesis of porphyrin nanostructures by self-assembly, the formation mechanisms for these nanostructures, and their applications in the water splitting, dye degradation, and photodynamic therapy. These research areas point to the potential new near future directions.

Ying-Bing Jiang is the founder and CEO of Angstrom Thin Film Technologies LLC. He is also the manager of the transmission electron microscope and focused ion beam laboratories at The University of New Mexico. He received his BS and MS degrees in materials science and engineering from the Northeast Institute of Heavy Machineries, China, followed by research in thin-film materials at the Institute of Physics, Chinese Academy of Sciences, China, and the Polish Academy of Sciences, Poland. He received his PhD degree in chemical engineering from The University of New Mexico. Jiang completed postdoctoral research at Sandia National Laboratories. He received two R&D 100 Awards and is an author/co-author of more than 100 peer-reviewed publications. His research interests focus on nanostructured materials, ultrathin membranes for carbon capture and energy conversions, and atomic-layer deposition of thin-film materials. Jiang can be reached by email at [email protected].

Zaicheng Sun has been a professor of chemistry at Beijing University of Technology, China, since 2016. He received his BS degree from Jilin University, China, and his PhD degree from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (CAS), China, in 1999. In 2001, he became an Alexander von Humboldt Research Fellow. He joined Changchun Institute of Optics, Fine Mechanics and Physics, CAS, China, in 2010. He obtained R&D 100 Awards in 2010 on thin-film optical coating. He has published more than 100 SCI papers, two book chapters, 19 patents, more than 4600 citations, and has an H-index of 35. His research interests focus on nanomaterials for energy, environment, and nanomedicine. Sun can be reached by email at [email protected].