The complex interrelationships between physical and psychiatric illnesses have been the focus of much research. Reference Lawrence, Hancock and Kisely1 Depression, for example, is not only a serious chronic illness but also a major risk factor for heart disease and cancer. At the same time there is strong evidence that mental health is important for maintaining good physical health and healthy lifestyle practices. However, the relationship between oral and mental health is a relatively neglected area. In the only meta-analysis of the association between the two, patients with psychiatric disorders such as schizophrenia, bipolar disorder and dementia were over three times as likely to have lost all their teeth. Reference Kisely, Quek, Pais, Lalloo, Johnson and Lawrence2 However, this study did not include eating disorders.
Eating disorders are divided into three main diagnoses. Reference Romanos, Javed, Romanos and Williams3 Anorexia nervosa is characterised by low body weight and food restriction. Bulimia nervosa is characterised by binge eating and inappropriate compensatory behaviours such as self-induced vomiting, use of laxatives and excessive exercise. Eating disorder not otherwise specified includes a mixture of anorexia- and bulimia-like atypical disorders. Reference Romanos, Javed, Romanos and Williams3 The impact of eating disorders on oral health was initially reported by Hellstrom and Hurst et al in the late 1970s. Reference Hellstrom4,Reference Hurst, Lacey and Crisp5 There are three main types of oral pathology. Reference Romanos, Javed, Romanos and Williams3,Reference Milosevic6–Reference Lo Russo, Campisi, Di Fede, Di Liberto, Panzarella and Lo Muzio9 Dental erosion or pathological wear on tooth surfaces is defined as loss of dental tissue without the involvement of bacteria; Reference Bretz8 risk factors include the consumption of large amounts of citrus fruit, soft drinks and sports drinks, as well as the presence of gastric reflux or frequent vomiting. In contrast, dental caries is the result of bacterial action; Reference Bretz8 organic acids produced by microorganisms in dental plaque cause decalcification of the tooth enamel and subsequent destruction of enamel and dentin. Finally, self-induced vomiting or starvation can lead to hyposalivation and xerostomia (dry mouth). This may be accentuated by psychotropic medication. Reference Bretz8 Hyposalivation and xerostomia are risk factors for both dental caries and erosion.
The association between oral pathology and eating disorders is most clearly established in cases with frequent self-induced vomiting, regardless of whether the diagnosis is anorexia or bulimia, and is characterised by dental erosion on palatal surfaces (the inner surfaces of teeth in the upper jaw). Reference Frydrych, Davies and McDermott7,Reference Bretz8 Dental caries and dry mouth secondary to salivary gland dysfunction also occur. Oral pathology is less clearly established in patients with an eating disorder but without self-induced vomiting, particularly in the case of dental caries. Reference Frydrych, Davies and McDermott7 On one hand, people with anorexia without self-induced vomiting might be more at risk through additional factors such as nutritional deficiency or the use of carbonated drinks as appetite suppressants; Reference Milosevic6 on the other hand, the frequent co-occurrence of obsessional personality traits might mean such people are more fastidious in their oral hygiene. Reference Milosevic6 We therefore undertook a meta-analysis to determine the association between eating disorders and poor oral health including any differences between patients with and without self-induced vomiting. Although there have been several systematic reviews, none included any meta-analysis. Reference Romanos, Javed, Romanos and Williams3,Reference Frydrych, Davies and McDermott7,Reference Bretz8
Method
The review was registered with PROSPERO, an international database of prospectively registered systematic reviews in health and social care based in the UK. Reference Booth, Clarke, Dooley, Ghersi, Moher and Petticrew10 In addition, we followed recommendations for the reporting of Meta-analyses of Observational Studies in Epidemiology (MOOSE), including background, search strategy, methods, results, discussion and conclusions. Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie11
Oral health outcomes
The primary outcome of this study was dental erosion. This can be expressed as either a continuous or a dichotomous variable. In either situation, the area of the mouth that is worst affected determines the overall score. The pattern of erosion is described by the direction in which the tooth surface faces (see online Fig. DS1). In terms of the outer surfaces, buccal surfaces are adjacent to the cheeks, whereas labial ones face the lips. Inner surfaces adjacent to the tongue are called lingual; Reference Metivier and Bland12 sometimes these surfaces are called palatal when referring to the upper teeth adjacent to the palate. The top areas are divided into the occlusal surfaces (the chewing surfaces of posterior teeth) and the incisal edges (the biting edge of anterior teeth). Reference Metivier and Bland12
Secondary outcomes were dental decay and salivary gland function. Dental caries was assessed by the number of decayed, missing and filled teeth or surfaces. Both scores are expressed as a continuous variable that accumulates over a person's lifetime, reflecting the individual's overall experience of dental caries. Reference Slade, Spencer and Roberts-Thomson13 This is because both dental decay and its treatment leave permanent marks, either through the continued presence of carious lesions, the presence of fillings or the loss of affected teeth by extraction. The total number of teeth (T) and surfaces (S) that are decayed (D), missing because of pathology (M) or filled (F) are measures referred to as the DMFT and DMFS respectively. In both measures an increase in score means greater cumulative dental decay; however, DMFS scores are higher than DMFT scores because the former measure counts damage to each surface of every tooth rather than counting the tooth as a single unit. This can be four or five surfaces depending on the tooth. The maximum possible DMFT score is therefore 32, whereas the maximum DMFS score is 148. Salivary gland function was assessed where possible by measurement of unstimulated salivary flow, otherwise by report of dry mouth by the patient. Both outcomes are usually reported as dichotomous variables; in the case of saliva flow, cut-off values can be either less than 0.1 ml/min or less than 0.2 ml/min. Reference Dawes14
Inclusion and exclusion criteria
We included studies of the oral health of people with eating disorders that included a control group of people without eating disorders, ideally matched by age, gender, socioeconomic status and education level. Psychiatric status could be determined by clinical diagnosis or diagnostic criteria. Studies of people with severe mental illness, primary alcohol or substance use disorders, intellectual disability and other psychological disorders were excluded. As our focus was on dental erosion, decay and salivary gland function, we excluded studies of other dental outcomes such as poor oral hygiene.
Search strategy
We searched Medline, PsycINFO and EMBASE from January 1951 until June 2014 using the following text, MeSH or Emtree terms as appropriate: bulimia, binge eating, eating disorder, overeating, appetite disorder, binge eating disorder, binge eating disorders, anorexia, anorexia nervosa, oral health, dental health survey, dental care, dental health services, edentulous mouth, dental caries, dental erosion, toothloss and tooth wear. Other descriptive words associated with the above MeSH terms were also used as key terms. We searched for further publications by scrutinising the reference lists of initial studies identified and other relevant review papers. We made attempts to contact selected authors and experts. Two reviewers (H.B. and S.K.) independently assessed titles, abstracts and papers, as well as extracting and checking the data for accuracy. In cases of disagreement consensus was reached on all occasions. Authors R.L. and N.W.J. provided content expertise, especially in relation to oral and dental health issues.
Study quality
We assessed the quality of included studies using the Newcastle–Ottawa Scale (NOS). Reference Wells, Shea, O'Connell, Peterson, Welch and Losos15 This assesses the quality of non-randomised studies in meta-analyses in the three following areas:
-
(a) selection of the study groups in terms of case definition, representativeness (e.g. all eligible cases with the outcome of interest over a defined period of time or from a defined catchment area), source of controls (ideally the community) and checks that the controls did not have an eating disorder;
-
(b) comparability of the groups such as the use of matching or multivariate techniques;
-
(c) ascertainment of outcome such as the use of standardised or validated measures with masking to psychiatric status.
Statistical analysis
We used Review Manager version 5.0, a statistical software package for analysing a Cochrane Collaboration systematic review, for our analysis. For each outcome we divided the eating disorder group into three subgroups regardless of whether the diagnosis was anorexia or bulimia: one in which self-induced vomiting was frequent, a second in which it was absent and a third group that had a mixture or where it was not explicitly stated. Where we encountered a situation where data for the same outcome were presented in some studies as dichotomous data and in others as continuous data, we combined them using statistical approaches as recommended in the Cochrane Handbook. Reference Higgins and Green16 These techniques re-express odds ratios as standardised mean differences (and vice versa), allowing dichotomous and continuous data to be pooled. Once standardised mean differences (or log odds ratios) and their standard errors were computed for all studies in the meta-analysis, they were combined using the generic inverse variance method in RevMan. For dental decay and salivary flow, most studies reported dichotomous outcomes. We therefore converted continuous data to dichotomous variables and then calculated odds ratios given that the studies were a cross-sectional design. Odds ratios also have the advantage of being easier to understand and more clinically meaningful than standardised mean differences (SMD). Dental decay was consistently reported using continuous data. We calculated the mean differences (as opposed to SMD) for studies that used the same scale for each outcome (e.g. DMFT, DMFS).
We assessed heterogeneity by using the I 2 statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. An I 2 estimate of 50% or above indicates possible heterogeneity, and scores of 75–100% indicate considerable heterogeneity. Reference Higgins and Green16 The I 2 statistic is calculated using the chi-squared statistic (Q) and its degrees of freedom. It has several advantages over the Q statistic alone in that it does not depend on the number of studies in the meta-analysis and so has greater power to detect heterogeneity where the number of studies is relatively low. Reference Higgins and Green16 The I 2 statistic can also be interpreted similarly irrespective of whether outcome data are dichotomous or continuous.
We used a random effects model throughout as there was significant heterogeneity in the majority of our analyses. This model assumes that variations in effect among different studies are due to differences in samples or paradigms and have a normal distribution, i.e. that heterogeneity exists. In addition, where possible, we investigated heterogeneity in sensitivity analyses omitting each study in turn. Other sensitivity analyses included investigating the effects of setting (e.g. in-patient or out-patient) and of excluding studies where there were concerns about data quality. Where there were at least 10 studies we tested for publication bias using both the fail-safe N statistic and funnel plot asymmetry. We used WinPepi version 11.34. Reference Abramson17 The fail-safe N statistic is the number of non-significant studies that would be necessary to reduce the odds ratio or effect size to a negligible value. In tests for a skewed funnel plot, low P-values suggest publication bias.
Results
We found 1085 citations of interest in the initial electronic searches, from which 112 abstracts were screened. Of these, 39 full-text papers were potentially relevant and assessed for eligibility. Assessing the references in all papers yielded one more full-text paper that was deemed relevant, giving a total of 40 full-text papers. Of these, 30 papers were excluded, most because they were not prevalence studies of oral health and eating disorder, or did not include a relevant dental outcome (Fig. 1). This left 10 studies that could be included in the meta-analysis. Four were from Nordic countries, Reference Dynesen, Bardow, Petersson, Nielsen and Nauntofte18–Reference Rytomaa, Jarvinen, Kanerva and Heinonen21 two studies were from England, Reference Milosevic and Slade22,Reference Robb, Smith and Geidrys-Leeper23 and there was one study each from Australia, Reference Liew, Frisken, Touyz, Beumont and Williams24 Israel, Reference Emodi-Perlman, Yoffe, Rosenberg, Eli, Alter and Winocur25 Germany, Reference Philipp, Willershausen-Zonnchen, Hamm and Pirke26 and the USA. Reference Altshuler, Dechow, Waller and Hardy27 The most common diagnosis was bulimia, followed by anorexia and eating disorders not otherwise specified. Ages ranged from 10 years to 50 years. The studies are summarised in online Table DS1.
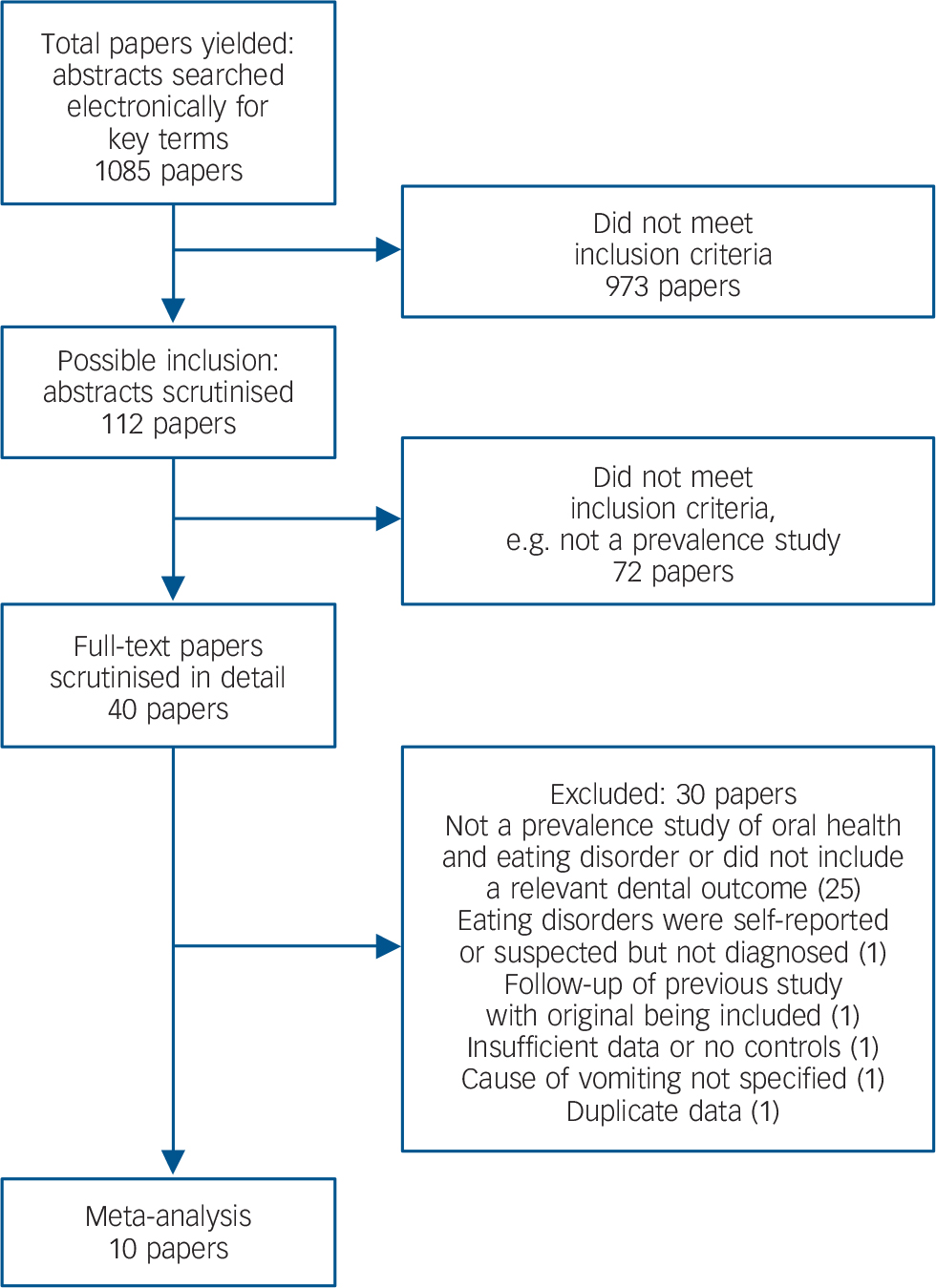
Fig. 1 Number of papers yielded by search strategy.
Study quality was not optimal, particularly in the areas of selection and ascertainment of outcome. Only one study stated that eating disorder cases were consecutive admissions, Reference Liew, Frisken, Touyz, Beumont and Williams24 and no study gave details about participation rates. Six studies defined psychiatric caseness using diagnostic criteria such as the DSM or ICD, but this was by clinical assessment not a standardised psychiatric interview (Table DS1). One further study assessed the presence of morbidity using the Eating Disorders Inventory and Examination, as well as the Eating Attitudes Test. Reference Liew, Frisken, Touyz, Beumont and Williams24 None of the studies used community controls; three recruited dental patients, Reference Johansson, Norring, Unell and Johansson19,Reference Robb, Smith and Geidrys-Leeper23,Reference Altshuler, Dechow, Waller and Hardy27 and the remainder used staff or university students. In one study the source of controls was not stated. Reference Philipp, Willershausen-Zonnchen, Hamm and Pirke26 One study excluded the presence of eating disorders in the control group through the use of a standardised questionnaire, Reference Johansson, Norring, Unell and Johansson19 whereas another asked about past psychiatric history. Reference Philipp, Willershausen-Zonnchen, Hamm and Pirke26
In terms of group comparability, all the studies either used age- and gender-matched controls or checked that there was no significant difference between the two groups at baseline. Two studies checked that participants in the eating disorder cases and control groups were of similar socioeconomic status, Reference Milosevic and Slade22,Reference Robb, Smith and Geidrys-Leeper23 and a third that they were similar in terms of ethnicity and medical history. Reference Altshuler, Dechow, Waller and Hardy27
Ascertainment of oral status in all the studies was by trained dental examiners. In the case of erosion, this was a clinical assessment sometimes guided by an established classification. Two studies used the Tooth Wear Index. Reference Smith and Knight28 Five studies supplemented the clinical examination with dental impressions, radiographs and/or intraoral photographs. In the case of caries, all the studies used some or all of the Decayed, Missing and Filled classification. 29 In two studies, radiographs and/or intraoral photographs were also taken. However, only one study made specific mention of assessor calibration and the measurement of interrater reliability, and another measured agreement on a random subsample of 10 participants. Reference Johansson, Norring, Unell and Johansson19,Reference Rytomaa, Jarvinen, Kanerva and Heinonen21 The dental assessor was masked to psychiatric status in four studies. Reference Dynesen, Bardow, Petersson, Nielsen and Nauntofte18,Reference Johansson, Norring, Unell and Johansson19,Reference Rytomaa, Jarvinen, Kanerva and Heinonen21,Reference Milosevic and Slade22 Five studies assessed salivary gland function, and saliva flow was measured in all cases. (Full details are given in Table DS1.)
Data for meta-analyses were available for 556 patients with eating disorder and 556 controls (total n = 1112). Gender data were available for 868 participants, of whom 852 (98%) were female. There were two studies where a mean and range were reported. Reference Dynesen, Bardow, Petersson, Nielsen and Nauntofte18,Reference Johansson, Norring, Unell and Johansson19 In this situation we used the range rule to estimate the standard deviation through dividing the range by four. Reference Taylor30 However, given this is not universally accepted, Reference Higgins and Green16 we did a sensitivity analysis of the effect of excluding these two studies.
Dental erosion
Participants with an eating disorder had five times the odds of dental erosion (95% CI 3.31–7.58) compared with controls (Fig. 2). Patients with self-induced vomiting had the highest likelihood (odds ratio (OR) = 7.32) whereas those without vomiting had the lowest (OR = 3.10), although this still remained significantly greater than for controls (95% CI 1.67–5.77). Excluding the two studies where the s.d. was estimated from ranges made no difference to these results. The same applied when we included only studies that used diagnostic criteria to define the psychiatric cases (OR = 4.95, 95% CI 3.13–7.84). In terms of sensitivity analyses of the effect of setting, it was not always clear whether studies were of in-patients, day patients or out-patients. However, limiting the analyses to studies that were clearly restricted to outpatients made no difference to the results (OR = 3.75, 95% CI 2.11–6.70). We undertook two sensitivity analyses of study quality. Including only studies that matched or checked for confounding variables such as socioeconomic status, ethnicity and medical history, Reference Milosevic and Slade22,Reference Robb, Smith and Geidrys-Leeper23,Reference Altshuler, Dechow, Waller and Hardy27 or those where the dental assessment was made masked to psychiatric status, Reference Dynesen, Bardow, Petersson, Nielsen and Nauntofte18,Reference Johansson, Norring, Unell and Johansson19,Reference Rytomaa, Jarvinen, Kanerva and Heinonen21,Reference Milosevic and Slade22 also did not alter the results.

Fig. 2 Dental erosion. AN, anorexia nervosa; BN, bulimia nervosa.
Dental caries
Four studies used some or all of the DMFS classification, and a fifth used the DMFT (Fig. 3). Patients with an eating disorder had significantly more decayed, missing and filled surfaces than controls. The study that used the DMFT reported no difference but this was confined to non-vomiting patients. There were insufficient studies to undertake any sensitivity analyses.

Fig. 3 Dental caries (tooth decay). DMFS/T, decayed, missing and filled surfaces/teeth.
Salivary gland function
Five studies assessed salivary gland function in terms of dry mouth or reduced salivary flow and there was a significant association with eating disorders (Fig. 4). Again, there were insufficient studies to undertake any sensitivity analyses.
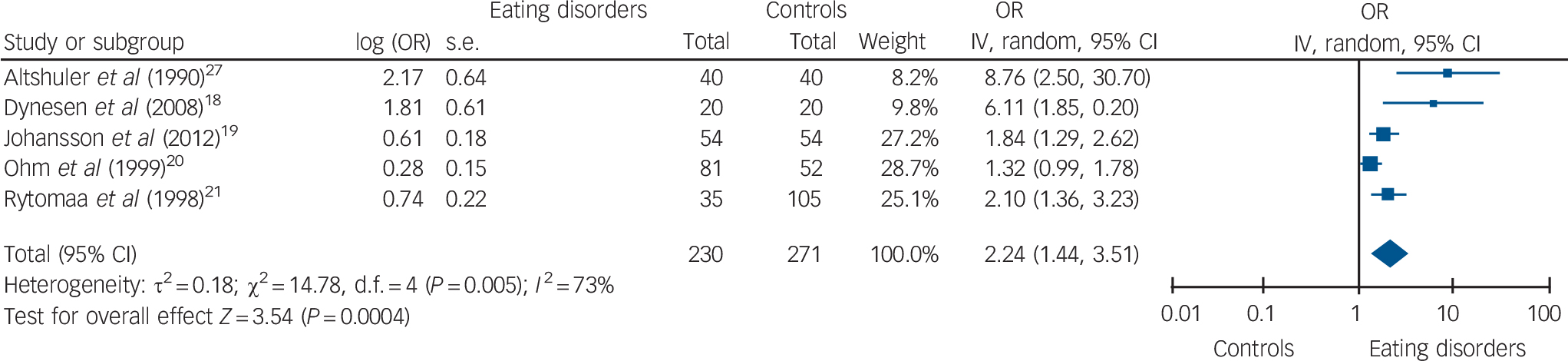
Fig. 4 Dry mouth or reduced salivary flow.
Heterogeneity
All but two of the results had an I 2 estimate of 50% or more, indicating possible heterogeneity. The two exceptions were erosion in the absence of self-induced vomiting and DMFS scores. There was no difference in I 2 values in sensitivity analyses of the effect of omitting each study in turn.
Publication bias
We were only able to test for publication bias for dental erosion as there were insufficient studies for the other two outcomes. The fail-safe N of additional ‘null’ studies needed to reduce the overall odds ratio to 1.1 was 201, suggesting that the findings for erosion were reasonably robust against publication bias. Tests for funnel plot asymmetry gave a P-value of 0.35 (Fig. 5).
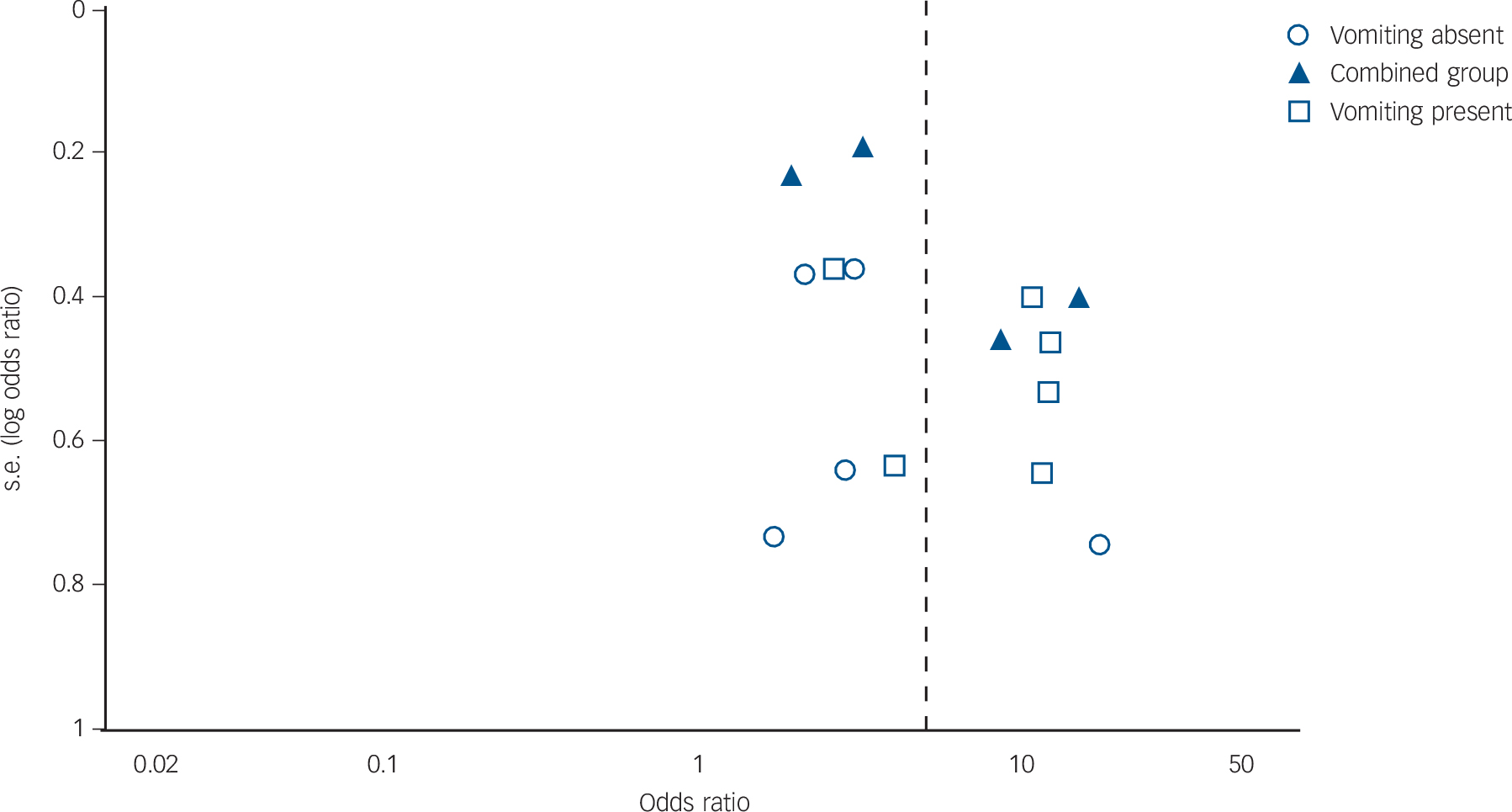
Fig. 5 Funnel plot of studies on dental erosion.
Discussion
To our knowledge, this is the first meta-analysis of the association between eating disorders and poor oral health including any difference between patients with and without self-induced vomiting. The most frequent finding was the presence of erosion or pathological wear on tooth surfaces. The risk of dental erosion is increased both in individuals who consume large amounts of citrus fruits, soft drinks or sports drinks, and in the presence of gastric reflux or vomiting. Reference Bretz8 This is consistent with our finding that patients with self-induced vomiting have the greatest degree of oral pathology, but even in patients in whom vomiting is not a prominent symptom erosion still occurs. Dental caries and reduced salivary gland function, although less marked, remained significantly greater than in controls.
Early reports of the oral consequences of eating disorders emphasised the role of self-induced vomiting as an aetiological factor, with wear occurring especially on palatal (inner) surfaces of teeth in the upper (maxillary) arch. Reference Hellstrom4–Reference Milosevic6,Reference Roberts and Li31 However, the relationship is not simple because the frequency, duration and total number of vomiting episodes were not linearly associated with erosion. Reference Milosevic6 In addition, later work has shown that erosion occurs in patients with an eating disorder who do not vomit, although the pattern is different with greater involvement of buccal and or labial sites (i.e. the outer surfaces of the teeth facing the cheeks and lips). Reference Milosevic6 It may therefore be simplistic to ascribe all dental effects as being secondary to vomiting when other risk factors such as acidic food and drink, and the pattern of their consumption, have not been studied. Reference Milosevic6,Reference Frydrych, Davies and McDermott7 One theory is that intrinsic (gastric) acid results in palatal erosion, whereas extrinsic (dietary) acids from fruit or carbonated drinks lead to labial erosion. Reference Milosevic6
Dental decay and salivary gland dysfunction are closely related. Dry mouth is a major risk factor for decay, possibly compounded by an increased risk of opportunistic infections as a result of nutritional deficiencies. Reference Lo Russo, Campisi, Di Fede, Di Liberto, Panzarella and Lo Muzio9 Changes in salivary secretion may be secondary to structural change within the gland, and benign parotid enlargement has been frequently described in patients with bulimia. Reference Milosevic6 Dry mouth may also be a side-effect of commonly used psychotropic medications. Reference Milosevic6,Reference Bretz8
Poor dental health can have major consequences for patients with eating disorder. These include oral function impairment, oral discomfort or pain, poor aesthetic quality and reduced quality of life. Reference Lo Russo, Campisi, Di Fede, Di Liberto, Panzarella and Lo Muzio9 In turn, deteriorating facial appearance may further alter body perception and/or self-esteem and hence contribute to a dangerous vicious cycle. Reference Lo Russo, Campisi, Di Fede, Di Liberto, Panzarella and Lo Muzio9
Limitations
There are a number of limitations to our study. Study quality was not optimal. For instance, none of the studies established psychiatric caseness using the gold standard of a structured standardised interview. Although all the studies used age- and gender-matched controls, only three matched or checked for other potential confounding variables such as socioeconomic status, ethnicity and medical history. Reference Milosevic and Slade22,Reference Robb, Smith and Geidrys-Leeper23,Reference Altshuler, Dechow, Waller and Hardy27 However, restricting the analyses to just these three studies did not change the results for our primary outcome. Although dental status in all the studies was assessed by trained examiners, in only four was this done masked to psychiatric status. Reference Dynesen, Bardow, Petersson, Nielsen and Nauntofte18,Reference Johansson, Norring, Unell and Johansson19,Reference Rytomaa, Jarvinen, Kanerva and Heinonen21,Reference Milosevic and Slade22 Again, a sensitivity analysis of the effects of only including masked (blinded) outcomes made no difference to the erosion results. Unfortunately, there were insufficient studies for sensitivity analyses of the secondary outcomes.
There were other limitations in study quality that we could not attempt to address using sensitivity analyses, such as the calibration or standardisation of dental assessments. In addition, many of our results showed heterogeneity. We explored this further through sensitivity analyses of the effect of omitting each study in turn, but this made no difference to the results. Accordingly, we used a random effects model throughout to incorporate heterogeneity into our analyses. However, although we have tried to minimise the effects of heterogeneity, our results should still be treated with caution. Finally, we cannot exclude the possibility of publication bias even though the fail-safe N for the primary outcome was 201. This is because tests for funnel plot asymmetry tend to be underpowered when the number of studies is relatively low. There were also insufficient studies to test for publication bias for the other two outcomes.
Implications
These findings highlight the importance of collaboration between dental and other health workers such as dieticians, general practitioners, psychiatrists and other mental health clinicians. This applies to all patients with eating disorders, not just those who present with self-induced vomiting. Dentists may be the first clinicians to suspect the diagnosis, given the reluctance of some people with eating disorders to present for treatment. Reference Lo Russo, Campisi, Di Fede, Di Liberto, Panzarella and Lo Muzio9 In established cases collaboration might help to minimise the harmful effects of inappropriate diet and self-induced vomiting; Reference Milosevic6 for instance, patients should be advised to reduce their intake of acidic drinks and food such as citrus fruit, as well as alcohol. After episodes of self-induced vomiting they should chew gum and rinse their mouth with water, milk or an antacid preparation. Reference Milosevic6 They should brush their teeth gently with a small amount of desensitising or bicarbonate toothpaste; vigorous brushing after self-induced vomiting is inadvisable as the softened, demineralised surface is more susceptible to toothbrush abrasion. Reference Milosevic and Slade22 Finally, medical practitioners should be aware that many psychotropic medications can exacerbate dry mouth with consequent adverse effects on oral health. Reference Milosevic6 If this is unavoidable, they should prescribe neutral artificial saliva or sialogogue pastilles. Reference Milosevic6
The increased focus on physical and psychiatric comorbidity should include consideration of oral health. Policy makers should consider providing free, accessible dental care for people with eating disorders. For example, Queensland's strategy to improve the physical health of people with psychiatric illness (Activate: Mind and Body) includes both the promotion of oral hygiene and regular care from a dentist. 32
Funding
This project was supported by the University of Queensland Winter Research programme.








eLetters
No eLetters have been published for this article.