Introduction
“Weedy” red rice, Oryza spp., is a problematic weed species because of its conspecificity with cultivated rice. Red rice has several traits that make this pest undesirable for cultivation, for example, high seed shattering and dormancy (Kanapeckas et al. Reference Kanapeckas, Tseng, Vigueira, Ortiz, Bridges, Burgos, Fischer and Lawton-Rauh2018), and poor cooking quality (Gealy and Bryant Reference Gealy and Bryant2009). It is more competitive than its cultivated relatives (Karn et al. Reference Karn, De Leon, Espino, Al-Khatib and Brim-DeForest2020) and increases milling costs if found in harvested grain (Ottis et al. Reference Ottis, Smith, Scott and Talbert2005). Durand-Morat et al. (Reference Durand-Morat, Nalley and Thoma2018) estimated economic losses of US$38 million per year under a moderate red rice infestation scenario. The most common method of red rice removal in California is hand pulling because of the limited number of herbicides available for selectively managing red rice in cultivated rice fields (Espino et al. Reference Espino, Brim-DeForest and Johnson2018). As a result of these constraints, nonchemical control methods must be developed to manage red rice in California rice cropping systems.
Early-season control of all weed species is critical to reduce competition with cultivated rice. One opportunity for early-season management of red rice is with the stale (“false”) seedbed methodology (Espino et al. Reference Espino, Leinfelder-Miles, Brim-DeForest, Al-Khatib and Linquist2016). The foundational goal of this technique is to germinate as many weed seeds as possible from the seedbank and eliminate them with nonselective preplant control strategies. This method is implemented in rice by flooding the field to some degree for some amount of time (Ceseski et al. Reference Ceseski, Godar and Al-Khatib2022; Dilipkumar et al. Reference Dilipkumar, Mohamad-Ghazali, Shari, Chuen, Chauhan and Chuah2022; Singh et al. Reference Singh, Bhullar and Gill2018). Flooding ceases after weed seeds germinate and seedlings emerge. Once the field is accessible by machinery, either mechanical or chemical control strategies can be implemented to destroy weed seedlings (Singh et al. Reference Singh, Bhullar and Gill2018). The amount of water and time needed for the floodwaters to remain on the field to induce germination are not well documented for water-seeded rice systems.
Weed seed germination is a response to ideal environmental conditions, including temperature, moisture, oxygen, light or solar radiation, and nitrogen availability (Travlos et al. Reference Travlos, Gazoulis, Kanatas, Tsekoura, Zannopoulos and Papastylianou2020). California red rice can germinate in the dark without the addition of nitrogen (Galvin et al. Reference Galvin, Inci, Mesgaran, Brim-Deforest and Al-Khatib2022), while preliminary studies suggest that accessions can germinate anaerobically. Thus the most influential factors over red rice germination are temperature and moisture availability. Some accessions that are believed to have been present in the California environment for some time (Kanapeckas et al. Reference Kanapeckas, Vigueira, Ortiz, Gettler, Burgos, Fischer and Lawton-Rauh2016) have likely adapted to water-seeded conditions and are heavily influenced by moisture availability. Regardless of these assumptions, the range of temperatures necessary and the amount of soil moisture for germinability of California red rice were previously unknown.
Temperature and water potential were chosen for this study to determine germinability because they are grower-accessible metrics and can be controlled to some degree. Floodwaters used by rice producers in California are usually sourced from deepwater reservoirs and are often too cold for rice production (Roel et al. Reference Roel, Mutters, Eckert and Plant2005). Most growers utilize warming basins to increase water temperatures and, consequently, soil temperatures (Al-Kayssi et al. Reference Al-Kayssi, Al-Karaghouli, Hasson and Beker1990). Knowing how much water to apply for how long at a given temperature would allow growers to have greater control over red rice seed germination. The objective of this study was to determine total germination of California red rice under a range of temperatures and water potentials.
Materials and Methods
Seed Multiplication Protocols
California red rice were first identified during a 2016 survey of infested fields, and were differentiated by phenotypic traits (Espino et al. Reference Espino, Brim-DeForest and Johnson2018). Differences were later confirmed by phylogenetic heredity; accessions were simply referred to as 1, 2, 3, and 5 (De Leon et al. Reference De Leon, Karn, Al-Khatib, Espino, Blank, Andaya, Andaya and Brim-DeForest2019). Seed multiplication was conducted from 2016 to 2019 in a controlled greenhouse on the University of California, Davis campus with accessions identified during the 2016 survey. Plants were grown in 3.8-L pots that were submerged in trays holding 5 cm of standing water through the plant’s life cycle. Plants were kept separate once flowering commenced to reduce cross-pollination between distinct accessions. Upon maturity, seeds were harvested and stored at 0 C until experimentation began. Table 1 describes each accession as well as ‘M206’, a medium-maturity, medium-grain variety utilized frequently in California rice production.
Table 1. Description of cultivar ‘M206’ and red rice accessions used for experimentation.a
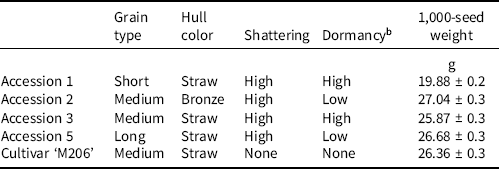
a Standard error is presented with average observed 1,000-seed weight (Galvin et al. Reference Galvin, Inci, Mesgaran, Brim-Deforest and Al-Khatib2022).
b High dormancy is defined as 5 yr or more, and low dormancy is less than 5 yr.
Germination Experiments
Red rice seeds were primed with a heat treatment of 50 C for 120 hr in a dry, dark incubation chamber (Model 320, National Appliance Co. [NAPCO], Portland, OR, USA) to break dormancy prior to experimentation (Galvin et al. Reference Galvin, Inci, Mesgaran, Brim-Deforest and Al-Khatib2022). ‘M206’ is nondormant and was not subjected to the priming treatment. Water potential solutions of −0.2, −0.4, and −0.8 MPa were made using polyethylene glycol (PEG 8000, Research Products International, Mt. Prospect, IL, USA) and 500 mL of deionized water (Boddy et al. Reference Boddy, Bradford and Fischer2012; Hardegree and Emmerich Reference Hardegree and Emmerich1990). The −0.2-MPa solutions were mixed for a minimum of 12 hr using a stir plate and magnetic rod, while the −0.4-MPa and −0.8-MPa solutions were mixed for a minimum of 24 hr. Solutions were measured for accuracy with a frequently calibrated WP4-T Dewpoint PotentiaMeter (Meter Group, Pullman, WA, USA) to ensure proper mixture of the solutions. A control treatment of 0 MPa, that is, deionized water, was also incorporated into the experiment. Once solutions were mixed and measured, 25 sheets of 90-mm-diameter filter paper (GE, Waukesha, WI, USA) were submerged in beakers of each water potential solution for 24 hr prior to experimentation to ensure full saturation of the filter paper (Hardegree and Emmerich Reference Hardegree and Emmerich1990). Beakers containing solutions were sealed with at least three layers of parafilm (Thermo Fisher Scientific, Waltham, MA, USA) at all times to prevent evaporation of deionized water from PEG solutions.
A single saturated filter paper was placed into each 100 × 15 mm petri dish (Thermo Fisher Scientific), then 25 primed seeds of a given accession were placed on top of the filter paper and wetted with an additional 5 mL of the respective water potential solution. Petri dishes were wrapped with parafilm and checked frequently for cracks. Petri dishes exposed to temperatures at and above 30 C received more parafilm compared to those exposed to cooler temperatures and were rewrapped when necessary. There were four petri dishes of each accession by water potential combination for each temperature treatment.
Petri dishes were placed into dark growth chambers (CMP6050 or CMP6010, Conviron, Pembina, ND, USA) and kept at one of the following constant temperatures for the duration of the experiment: 10 C, 15 C, 20 C, 25 C, 30 C, 35 C, or 40 C. Each temperature treatment containing all accession by water potential treatment combinations was repeated three times. Seed germination was evaluated daily, and petri dishes were not opened for the duration of the experiment to prevent contamination. A seed was considered germinated when the radicle reached 1 mm or longer. Seed decay was defined by the presence of mold, which appeared more rapidly at higher temperatures. Germination counts ceased once seed decay or mold was observed; thus the length of each study was defined by the experimental temperature, either 7, 14, 21, or 28 d.
Data Analysis
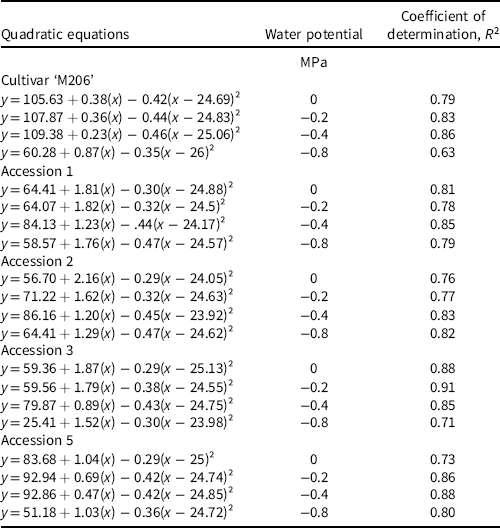
This study was arranged in a split-split plot design with temperature representing the whole plot and water potential representing the subplot factor, both applied in combination to each accession (Altman and Krzywinski Reference Altman and Krzywinski2015). A Welch’s t-test was used to compare experimental replicates to one another and ensure homogeneity of variance between sample means, for example, accession 1 exposed to 10 C and 0 MPa in Experimental Replicate 1 was compared to the same treatment combination in Experimental Replicate 2. Any experimental replicate that did not meet homogeneity of variance with other replicates was not included in the analysis. Data that did meet homogeneity of variance were pooled for analysis of variance (ANOVA). Temperature, water potential, accession, and their interactions were incorporated into a statistical model using JMP® (Pro version 16, SAS Institute, Cary, NC, USA) and subjected to ANOVA. Statistically significant treatment means were separated with Fisher’s least significant difference test (α = 0.05). Models with two, three, four, and five parameters were fit to the data to determine the ideal number of coefficients, and R 2 values were used to compare these models to one another (Chicco et al. Reference Chicco, Warrens and Jurman2021). A four-parameter quadratic regression model was fit to the water potential by temperature data to best represent the response of each accession using the following equation:
where y represents the germination percentage at a given water potential; x represents the experimental temperature; and a, b, c, and d are coefficients produced from the JMP output. The quadratic term is centered to provide a better estimate of model coefficients and to reduce collinearity of the higher-order terms.
Results and Discussion
Water potential, temperature, and accession (each P < 0.0001), as well as a three-way interaction between these variables (P < 0.0001) significantly influenced germination. Table 2 lists equations for each regression model to depict similarities between responses to water potential for each accession across temperature. R² values are also listed to elucidate the model fit to the data. Results of the three-way interaction are described by their means to give a general depiction of seed germination responses.
Table 2. Fitted quadratic models using Equation 1 and associated R² values for each accession by water potential combination, where y is the germination response and x is the experimental temperature.
Germination Responses under Ideal Conditions
Seeds exposed to 0 or −0.2 MPa in combination with temperatures between 20 and 35 C had an average of 95% or greater total germination regardless of accession (Figure 1). All seeds began to germinate after 3 to 4 d and reached maximum germination within 10 d under these conditions. Average air temperatures from 2018 to 2020 during the rice planting period, April, May, and June, were 16 C, 21 C, and 24 C, respectively (UCIPM 2022). Previous research suggests that red rice seedlings within the top 2.5 cm of the soil surface will emerge through both the soil and 15 cm of floodwaters (Galvin et al. Reference Galvin, Inci, Mesgaran, Brim-Deforest and Al-Khatib2022). Considering historical temperature ranges and standard flooding practices, red rice seeds within the top 2.5 cm of the soil would be expected to germinate and emerge in May and June, with full field saturation maintained for a minimum of 7 d.
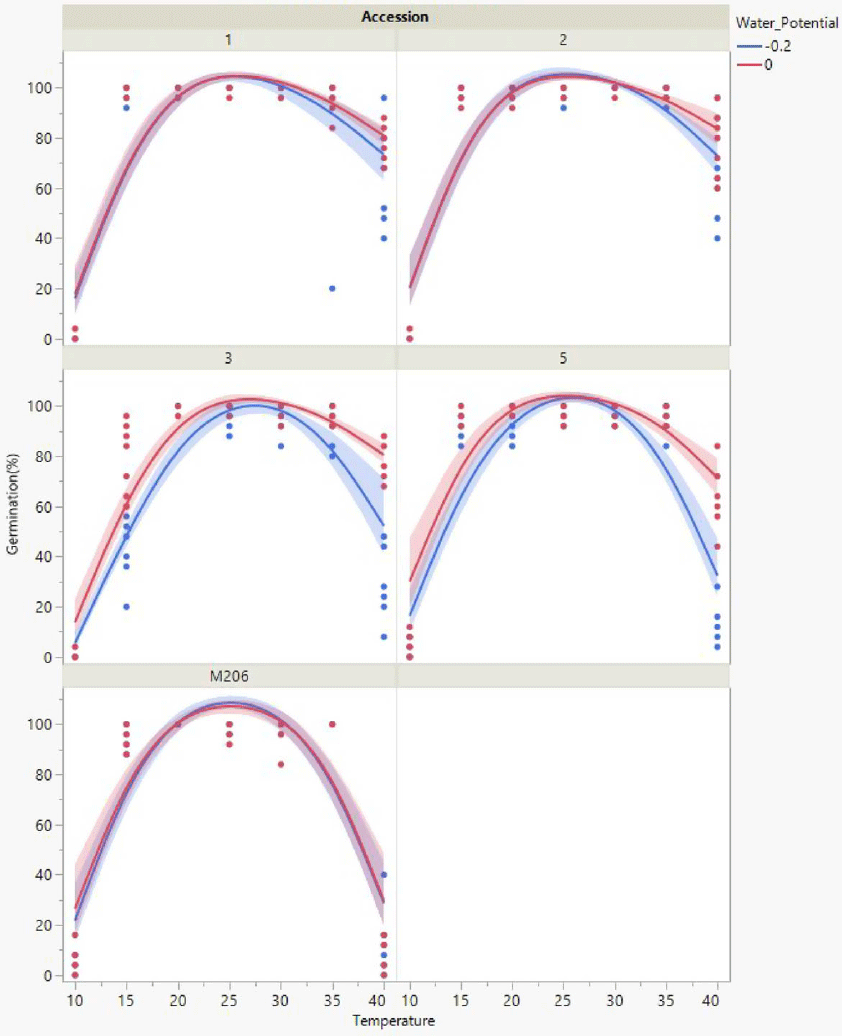
Figure 1. Total germination response for each accession across the temperature range assessed when exposed to adequate moisture (0, −0.2 MPa) conditions. Scatter points represent raw data from each replicate, lines represent a quadratic model fit for the data, and shaded areas are confidence of fit.
All California red rice seeds exposed to 0 or −0.2 MPa germinated more often (Figure 1) compared with seeds exposed to −0.4 or −0.8 MPa (Figure 2). Year-round flooding strategies that are foundational to the California rice growing culture (Brim-DeForest et al. Reference Brim-DeForest, Al-Khatib, Linquist and Fischer2017) have likely had significant selection pressure for flooding-tolerant red rice (Evans and Etherington Reference Evans and Etherington1990). Growers who are implementing the stale seedbed methodology to control California red rice should be applying ample floodwaters to obtain the best outcomes.
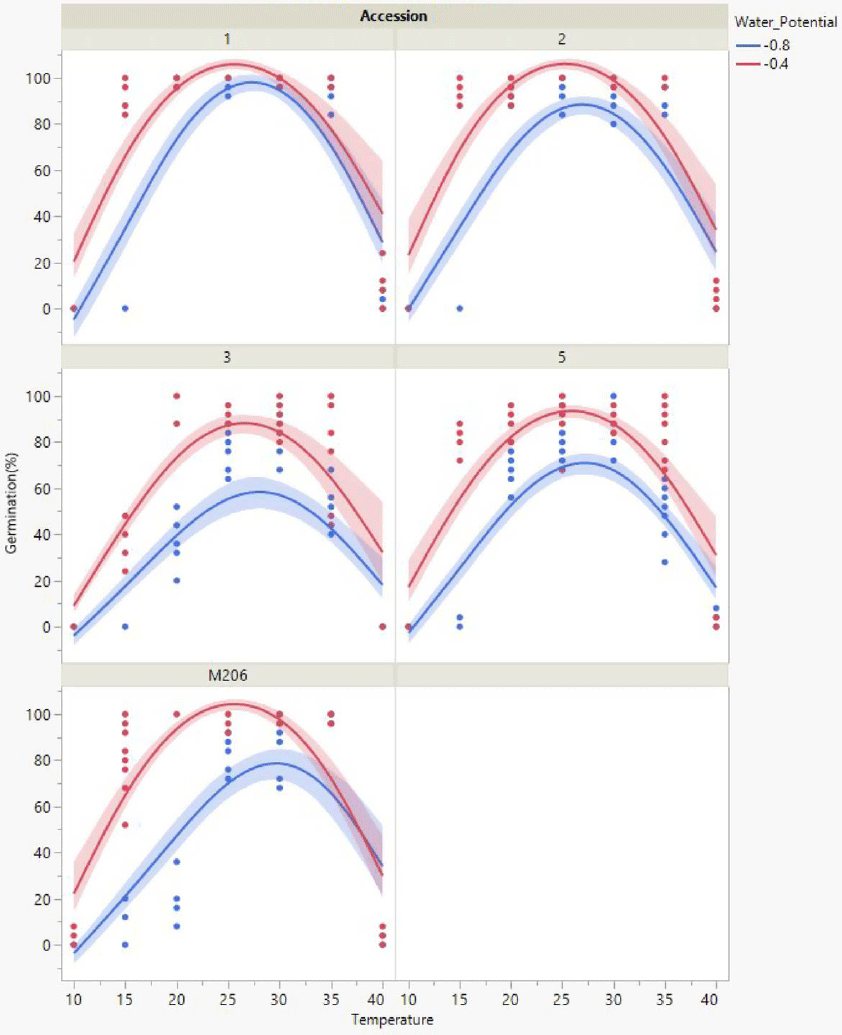
Figure 2. Total germination response for each accession across the temperature range assessed when exposed to water-stressed (−0.8, −0.4 MPa) conditions. Scatter points represent raw data from each replicate, lines represent a quadratic model fit for the data, and shaded areas are confidence of fit.
Germination Responses to Temperature Stress
Total germination decreased when seeds of any accession were exposed to temperatures colder than 20 C. Accession 5 followed by ‘M206,’ and accessions 2, 1, and 3 were the least to the most sensitive to cold. Accession 5 and ‘M206’ seeds exposed to 10 C had an average of 6% or less total germination regardless of water potential (Figures 1 and 2). Accessions 2, 1, and 3 did not germinate at this temperature. Accession 3 seeds had a total average germination of 80% at 15 C and 0 MPa; all other accessions had a total average seed germination of 91% or more when water potential was −0.2 or 0 MPa (Figure 1). California rice irrigation water is sourced from snowmelt and is often stored in deepwater reservoirs (Hill et al. Reference Hill, Williams, Mutters and Greer2006), and thus many cultivars are bred to have some cold tolerance (Shakiba et al. Reference Shakiba, Edwards, Jodari, Duke, Baldo, Korniliev, McCouch and Eizenga2017). Growers can utilize cold-tolerant cultivars in tandem with cooler headwaters as a means of reducing germination of cold-sensitive accessions 1, 2, and 3.
Total germination decreased and seeds of all accessions decayed quickly when exposed to temperatures warmer than 35 C. ‘M206’ followed by accessions 5, 3, 1, and 2 were the least to the most heat tolerant. ‘M206’ had the lowest total seed germination at 40 C, 5%, when exposed to 0 MPa compared with an average 63% to 79% total germination for red rice accessions under the same treatment combinations (Figure 1). Air temperatures for the first 9 d of September 2022 in the California rice growing region were 39 to 47 C (UCIPM 2022). This heat wave was preceded by 25 mm of rain. Although 39 to 47 C followed by rain is considered abnormal during the harvest period, these conditions would have been ideal for weedy rice decay due to high heat exposure and subsequent moisture. Increases in severe weather patterns and surface temperatures due to climate change could provide suitable conditions for growth and development of red rice (Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferriera, Marchesan, Menezes, Cohn, Linscombe, Carmona, Tang and Merotto2015). Growers who are aware of red rice in their fields should consider postharvest seedbank management options when ecological conditions are ideal for both growth and decay.
Germination Responses to Moisture Stress
Accession 1 followed by accessions 2 and 5, ‘M206’, and accession 3 were the least to the most sensitive to moisture stress (data not shown). Cooler temperatures in April, for example, at or less than 15 C, would require ample floodwater for achieving maximum germination (Figures 1 and 2). Red rice seeds exposed to cooler temperatures were observed to require longer amounts of time to germinate, regardless of accession. The amount of time a field is maintained at fully saturated conditions at 15 C would need to be more than 10 d to achieve maximum germination from the soil seedbank, especially for accessions 1, 2, and 3, which were more sensitive to cold compared with ‘M206’ and accession 5. If preseason temperatures are low and water is scarce, the stale seedbed methodology may be ineffective for controlling California red rice. The research outcomes from this article demonstrate the necessity for understanding the climatic factors that influence the phenological progression of red rice. This knowledge can be utilized during the weed management decision-making process when nonchemical control strategies are required.
Wholistic Stale Seedbed Applications for Water-Seeded Rice
The stale seedbed technique works best when germination and subsequent destruction of the greatest number of problematic weed seeds is maximized (Travlos et al. Reference Travlos, Gazoulis, Kanatas, Tsekoura, Zannopoulos and Papastylianou2020). This allows for destruction of weed seedlings before the crop is planted and, consequently, withdrawal from the soil weed seedbank (Rao et al. Reference Rao, Johnson, Sivaprasad, Ladha and Mortimer2007). Grassweed species barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], late watergrass [Echinochloa oryzicola (Vasinger) Vasinger], and bearded sprangletop [Leptochloa fusca (L.) Kunth ssp. fascicularis (Lam.) N. Snow] are highly problematic due to resistance to most available rice herbicides in California (Becerra-Alvarez and Al-Khatib Reference Becerra-Alvarez and Al-Khatib2022; Hill et al. Reference Hill, Williams, Mutters and Greer2006). These species require alternative control methods, for example, stale seedbed application incorporating glyphosate, to maximize control (Ceseski et al. Reference Ceseski, Godar and Al-Khatib2022).
Boddy et al. (Reference Boddy, Bradford and Fischer2012) found that late watergrass had greatest germination under high moisture availability and when temperatures were 35 C, with some germination occurring between 15 and 31 C. Barnyardgrass has similar moisture and temperature requirements as late watergrass (Song et al. Reference Song, Shi and Song2015). Conversely, bearded sprangletop requires 2 wk of wet-chilling to germinate (Driver et al. Reference Driver, Al-Khatib and Godar2020). Both Echinochloa species as well as red rice would likely germinate and emerge in a stale seedbed application during May or June of a given year. A stale seedbed that takes into consideration the 2-wk wet-chilling requirements for sprangletop seed germination would likely encompass the moisture requirements for other grassweed species. However, 2 wk of flooding may not be practical when considering temporal restraints between the soil drying period and preparation for planting. Managers should consider the germination requirements of their most problematic species before implementing this strategy to optimize the time and effort required for maximum germination and, consequently, efficacy.
In conclusion, flooding a field to full soil capacity for a minimum of 7 d when average water temperatures are 20 to 35 C will allow for the maximum number of red rice seeds to germinate, regardless of accessions present in the field. Historical average temperatures from April through June, the time when stale seedbed applications are made, will likely fall between 20 and 35 C. Cooler temperatures (<20 C) will begin to affect accessions differently and may result in suboptimal germination, especially if water is scarce. Growers can use the biological information detailed in this research to determine if a stale seedbed is the best option given the accessions present and the precipitation, temperature, and water availability each year.
Acknowledgments
The authors acknowledge the Henry A. Jastro Graduate Research Award as a source of funding for this research as well as financial support from the Melvin Androus Endowment, facilitated by the California Rice Research Board. The authors declare no conflicts of interest.







