Zn has a widespread role in cellular metabolic processes, immunity, neurological function and sexual development, as well as a fundamental role in growth and development( Reference King and Cousins 1 , Reference Samman 2 ). Zn supplementation may be beneficial for those who consume low bioavailability diets that contain high levels of phytic acid, e.g. vegetarians( Reference Foster, Chu and Petocz 3 ), those at risk of diarrhoeal disease( Reference Brown, Peerson and Baker 4 ) and during periods of complementary feeding( Reference Allen 5 ). However, Zn intake above the Upper Limit (UL) increases the risk of adverse health consequences( 6 ). The acute response to high Zn intakes may include nausea, vomiting, loss of appetite, abdominal cramps, diarrhoea and headaches( Reference Samman and Roberts 7 ). If maintained for an extended period, Zn intake above the UL may result in decreased Cu absorption, altered Fe metabolism and reduced immune function( 6 , Reference Samman and Roberts 7 ).
The nutrient reference values for Zn for Australia and New Zealand including an Estimated Average Requirement (EAR) and a UL( 8 ) are the same as those used in the USA but slightly different from those used in Canada( 9 ) and the UK( 10 ). The main dietary sources of Zn in the Australian diet include meat, poultry, dairy products and fortified cereals( Reference McLennan and Podger 11 , Reference Rangan and Samman 12 ).
The 2007 Australian Children’s Nutrition and Physical Activity Survey (ACNPAS) is the most recent national survey of food and nutrient intake of children( 13 ). A secondary analysis of ACNPAS showed that boys and girls aged 2–8 years were at an increased risk of adverse health effects resulting from excessive Zn intake, whether from diet alone or diet plus supplements( Reference Rangan and Samman 12 ). Zn supplements were used by 5·4 % of children, and contributed to 2 % of total Zn intake. While supplementary Zn intake was low (median intake per user was 2·0 mg), the Zn content of the supplements ranged from 0·1 to 129 mg. The objectives of the present paper were to elucidate these findings by: (i) examining the characteristics and Zn intakes of supplement users compared with non-users; and (ii) undertaking a survey of commercially available Zn supplements to compare Zn content and target audience.
Methods
2007 Australian Children’s Nutrition and Physical Activity Survey
The 2007 ACNPAS was undertaken by the Commonwealth Scientific and Industrial Research Organisation and the University of South Australia. Permission to use data was obtained from the Australian Social Science Archives( 14 ). A stratified quota-sampling scheme was used to randomly select households via postcodes with children aged 2–16 years. Only one child per eligible household was selected, with the survey having a 40 % response rate( 13 ). Data were collected from 4834 children on two separate occasions, the first using a computer-assisted personal interview and the second using a computer-assisted telephone interview. A three-pass 24 h recall method was used to record all foods, beverages and supplements consumed on the day prior to each interview. Both 24 h recalls were designed to represent different days of the week. For children aged 2–8 years, food and beverage intake was obtained from their primary caregiver. Children aged 9 years or older reported their own food and beverage intake( 13 ). Dietary and supplement data were translated into nutrient intake data using the survey-specific nutrient database AUSNUT 2007( 15 ). Data on usual diet type consumption was self-reported in response to a short question.
Supplement data
A search of Zn-containing supplements available on the Australian market was undertaken using a variety of search strategies. These included examining: (i) the AUSNUT supplement database through the Food Standards Australia and New Zealand website( 16 ); (ii) MIMS online( 17 ); (iii) the Therapeutic Goods Administration( 18 ); and (iv) pharmacies and product websites. Information was collected on the amount of elemental Zn per supplement, the presence of other vitamins and minerals, the recommended dosage as stated on the product label, the target market/consumer and the presence of any marketing claims. All Zn-containing supplements were categorised into the following groups: (i) multi-vitamin and mineral (MVM) preparations targeted at children, teens or adults; (ii) Zn-only supplements; and (iii) other Zn-containing supplements including cold and flu, and cold sore preparations (targeted at children or adults).
Statistical analysis
All dietary data collected (computer-assisted personal interview and computer-assisted telephone interview) were included in the present analysis. Total Zn intakes were calculated by summing Zn intakes from diet and supplements per person for each recall day. Total Zn intakes were adjusted for intra-individual variability using the Multiple Source Method (MSM)( Reference Harttig, Haubrock and Knuppel 19 ) for each age and gender subgroup as specified in the nutrient reference values report for Australia and New Zealand( 8 ). MSM is one of several methods used to estimate usual intake distributions and has been found to produce similar results to the Iowa State University and the National Cancer Institute methods( Reference Souverein, Dekkers and Geelen 20 ). MSM uses a Box–Cox transformation to normalise intake data prior to estimating usual intake distribution. The proportions of children meeting their age- and gender-specific EAR and UL were estimated using the cut-point method( 21 ). Supplement users and non-users were analysed separately. Supplement users were identified as those using a Zn supplement on the first, second or both days of the 24 h recalls.
All data were weighted to account for over- and under-sampling to enable representation of the Australian population aged 2–16 years, although sample sizes are reported using unweighted data. Independent t tests (for continuous data) or χ 2 tests (for categorical data) were used to examine differences between Zn supplement users and non-users. ANCOVA was used to examine differences between groups while adjusting for age and gender. Analyses were conducted using the statistical software package IBM SPSS version 19·0. P values <0·05 were considered statistically significant.
Results
Zn supplement use in the 2007 Australian Children’s Nutrition and Physical Activity Survey
Zn supplements were consumed during the 2 d of the survey by 9·4 % of children using seventy-eight different supplements. Zn supplement use varied according to age, gender, BMI and usual type of diet (Table 1). Females were more likely to use a Zn supplement than males, and this was consistent across all age groups. Zn supplement use was higher in the younger age groups and among those who had a lower BMI. Children who consumed a modified diet such as a reduced-fat, gluten-free or low-allergen diet (16·9 %) or those who consumed a vegetarian diet (13·3 %) used Zn supplements to a greater extent than those who consumed a regular diet (8·6 %). Socio-economic indicators such as parental education and income were not associated with use of Zn supplements; neither was geographical location (urban v. rural) or being breast-fed (ever/never). Use of Zn supplements had no influence on Zn intake from food alone, with users and non-users having similar food-only Zn intakes (10·1 (sd 0·13) v. 10·0 (sd 0·04) mg/d, P=0·36 adjusted for age and gender). Vegetarian users and non-users also had similar Zn intakes from food alone (8·7 (sd 0·57) v. 8·5 (sd 0·27) mg/d, P=0·72 adjusted for age and gender), although their total Zn intakes were significantly lower compared with non-vegetarians (8·7 (sd 0·29) v. 10·3 (sd 0·05) mg/d, P<0·001, adjusted for age and gender).
Table 1 Demographic data for zinc supplement users and non-users among Australian children (n 4834) aged 2–16 years; 2007 Australian Children’s Nutrition and Physical Activity Survey
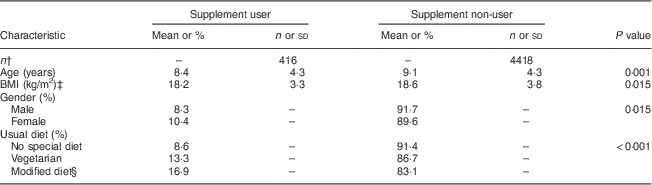
† Unweighted sample size.
‡ Adjusted for age and gender.
§ Weight-reduction, fat modification or diabetic diet.
Supplement users had significantly higher mean intakes of Zn than non-users in all age and gender subgroups (Table 2). Nearly all children aged 2–13 years met the EAR for Zn, regardless of supplemental Zn intake. Boys aged 14–16 years who did not use Zn supplements were at highest risk of inadequate Zn intake (14·6 %).
Table 2 Zinc intakeFootnote † (mg/d) and dietary adequacy by zinc supplement user status among Australian children (n 4834) aged 2–16 years; 2007 Australian Children’s Nutrition and Physical Activity Survey
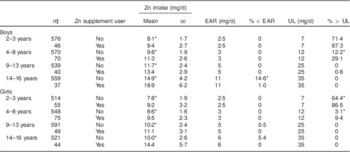
EAR, Estimated Average Requirement; UL, Upper Limit.
*P<0·05 between Zn supplement users and non-users.
† Adjusted using the Multiple Source Method by age and gender subgroup.
‡ Unweighted sample size.
Conversely, children at highest risk of exceeding the UL for Zn were those aged 2–3 years, with 86·5–87·3 % of supplement users and 64·4–71·4 % of non-users consuming amounts above the UL. A significant proportion of children aged 4–8 years also consumed Zn above the UL, in particular those who used Zn supplements (9·4–29·1 %).
The most common Zn supplements used by children in the survey were MVM preparations (92 %), followed by Zn-only supplements (5 %) and other supplements such as cold and flu, and cold sore tablets (3 %). The majority of these supplements contained 1–2 mg Zn. However, eighteen children aged 2–3 years and four children aged 4–8 years consumed a supplement that exceeded the UL for Zn.
Commercially available Zn supplements
We identified sixty-seven types of MVM preparations (twenty-three for children, six for teens and thirty-eight for adults), fourteen varieties of Zn-only supplements and ten other Zn-containing supplements (three for children and seven for adults; see online supplementary material, Supplementary Tables 1–6). The median elemental Zn content of supplements ranged from 2 mg in children’s MVM to 25 mg for Zn-only supplements, and three preparations were found to contain <0·1 mg elemental Zn (Table 3).
Table 3 Elemental zinc content (mg) of zinc-containing commercially available supplements, Australia
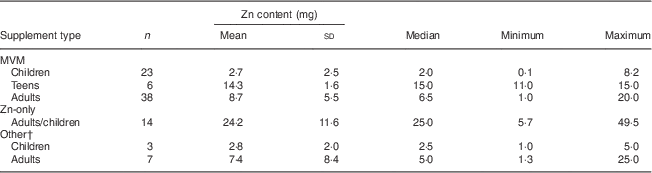
MVM, multi-vitamin and mineral preparations.
† Includes Zn-containing cold and flu supplements and cold sore supplements.
Figure 1 illustrates the contribution of the median amount of supplemental Zn relative to the UL. For children aged 2–3 years, the consumption of any Zn-containing supplement contributes at least 25 % to the UL and consumption of MVM preparations for teens or Zn-only supplements would exceed the UL by 200 % and 350 %, respectively. Similarly, for children aged 4–8 years, the use of MVM preparations for teens or a Zn-only supplement would exceed the UL, as would a Zn-only supplement for children aged 9–13 years.
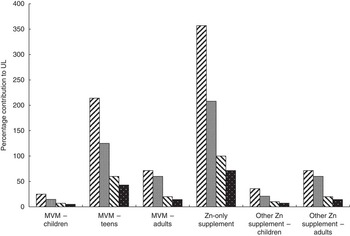
Fig. 1 Contribution of supplemental zinc (median, mg) to the Upper Limit (UL) for zinc by age group (![]() , 2–3 years;
, 2–3 years; ![]() , 4–8 years;
, 4–8 years; ![]() , 9–13 years;
, 9–13 years; ![]() , 14–16 years) among Australian children (n 4834) aged 2–16 years; 2007 Australian Children’s Nutrition and Physical Activity Survey (MVM, multi-vitamin and mineral preparations)
, 14–16 years) among Australian children (n 4834) aged 2–16 years; 2007 Australian Children’s Nutrition and Physical Activity Survey (MVM, multi-vitamin and mineral preparations)
Examination of supplement labels reveals a wide range of marketing claims (Supplementary Tables 1–6). The most common claims used on MVM supplement labels were to promote normal growth, improve health, well-being, and immunity. Zn-only supplements targeted at those >12 years claimed to aid in wound healing, reproductive health and immune function. Frequently stated claims on cold and flu tablets promoted the products for reducing the severity and duration of the common cold and for boosting immune function.
Discussion
The results of the ACNPAS show that use of Zn-containing supplements was relatively infrequent but was dependent on age, gender, BMI and habitual diet. Those being younger, female, of lower BMI and consuming a vegetarian or modified diet were more likely to use a Zn supplement than their counterparts. Supplement users had higher Zn intakes than non-users in all age and gender subgroups. Zn requirements (EAR) were met by most children regardless of supplement usage, with the exception of boys aged 14–16 years who did not use a Zn supplement. In contrast, a high percentage of children aged 2–3 years and 4–8 years exceeded the UL, particularly among supplement users.
Supplement use among children has been examined in recent surveys conducted in the USA, Canada, Australia and European countries, with prevalence rates approximating 25–45 % in children and 15–30 % in adolescents. In general, prevalence rates were higher among younger children than pre-adolescents and adolescents( Reference Sichert-Hellert and Kersting 22 – 29 ). Supplement use among children has been characterised by high parental education and income levels, having better nutrient intakes, lower prevalence of overweight and obesity and having a healthier lifestyle( Reference Sichert-Hellert and Kersting 22 , Reference Dwyer, Nahin and Rogers 26 , Reference Huybrechts, Maes and Vereecken 28 , Reference Reaves, Steffen and Dwyer 30 ). Parental use of supplements has also been associated with supplement use by their children( Reference Bailey, Fulgoni and Keast 25 ). In our study, users had lower BMI but no differences were observed in terms of parental education and income compared with non-users. In addition, users and non-users of Zn supplements had similar Zn intakes from food alone, suggesting that supplemental Zn intake was taken in addition to and not replacing dietary intake. Most studies have reported similar or higher food-only Zn intakes in supplement users compared with non-users( Reference Shakur, Tarasuk and Corey 23 – Reference Bailey, Fulgoni and Keast 25 , Reference Huybrechts, Maes and Vereecken 28 , Reference Bailey, Fulgoni and Keast 31 , Reference Murphy, White and Park 32 ).
MVM supplements are the most commonly used type of dietary supplement( Reference Bates, Lennox and Prentice 27 , 29 , Reference Picciano, Dwyer and Radimer 33 ) and the vast majority of children in our survey who used supplements, took MVM supplements. Zn-only supplements and other dietary Zn supplements were used by less than 10 % of those who took supplements. Users of Zn supplements had higher total Zn intakes than non-users, which is consistent with other large-scale surveys( Reference Shakur, Tarasuk and Corey 23 – Reference Bailey, Fulgoni and Keast 25 , Reference Bates, Lennox and Prentice 27 , Reference Huybrechts, Maes and Vereecken 28 ).
Our results revealed that food alone was sufficient to meet the EAR for all children’s age/gender groups, except for adolescent boys aged 14–16 years. This group has the highest Zn requirement (EAR=11 mg/d) and 15 % of boys were unable to meet this requirement without the use of a Zn supplement. These results are comparable to those of the US National Health and Nutrition Examination Survey (NHANES) 2003–2006, which found 14–18-year-olds who did not use a dietary supplement had the highest prevalence of inadequate Zn intake but results were not specified for males and females( Reference Bailey, Fulgoni and Keast 25 ). In a national Canadian study, adolescent girls were most at risk of inadequate intakes( Reference Shakur, Tarasuk and Corey 23 ) but as recommended Zn requirements( 9 ) are slightly different from those in the USA and Australia, these results are not directly comparable.
Children who followed vegetarian diets had lower Zn intakes but higher supplement use compared with non-vegetarian children. A recent meta-analysis examining Zn intakes found that dietary Zn intakes were significantly lower by 0·9 mg/d in populations that followed habitual vegetarian diets compared with non-vegetarians( Reference Foster, Chu and Petocz 3 ). Although the number of vegetarians in our survey was relatively small, those who used supplements were better able to meet their Zn requirements, especially adolescents.
Supplement use among toddlers and pre-school children has been associated with a higher prevalence of exceeding the UL in other international surveys. Analysis from the NHANES 2003–2006 with 7250 children (2–18 years) found that those who used supplements (23–44 % depending on age/gender group) had a higher prevalence of exceeding the UL for Zn. This was most pronounced in the youngest age groups; 2–8 years (84 % v. 45 %), 9–13 years (32 % v. <1 %), 14–18 years (8 % v. <1 %)( Reference Bailey, Fulgoni and Keast 25 ). The Feeding Infants and Toddlers Study including 3022 US infants and toddlers found that high proportions of users of vitamin/mineral supplements had Zn, as well as vitamin A and folate intakes exceeding the UL( Reference Briefel, Hanson and Fox 24 ). For toddlers aged 12–24 months, 68 % of supplement users and 38 % of non-users had Zn intakes above the UL while less than 1 % of toddlers (users and non-users) had an inadequate EAR for Zn. The authors concluded that in general, healthy infants and toddlers can achieve recommended levels of nutrient intakes from foods alone and care must be taken with supplements to ensure that the doses do not lead to intakes above the UL, especially for nutrients that are widely used as food fortificants( Reference Briefel, Hanson and Fox 24 ).
Even without accounting for supplemental Zn, the prevalence of exceeding the UL for young children is high. The 1994–1996 and 1998 Continuing Survey of Food Intakes by Individuals (CSFII), which examined nutrient intakes of 7474 US children aged less than 6 years, found that the percentage of children exceeding the UL for Zn intake to be 92 % in the 0–6 months age group, 86 % in the 7–12 months age group, 51 % in the 1–3 years age group and 3 % in those aged 4–5 years( Reference Arsenault and Brown 34 ). The high intakes of Zn among children in the USA have been attributed to food fortification( Reference Fulgoni, Keast and Bailey 35 , Reference Sacco, Dodd and Kirkpatrick 36 ).
Adverse effects such as nausea, vomiting, loss of appetite, abdominal cramps, diarrhoea, headaches, decreased Cu absorption, altered Fe function and reduced immune function have been associated with excessive supplementation of Zn( 6 ). These data were obtained mainly from adults and it has been suggested that Zn intakes above the UL in children should not be a cause for concern because the data are limited, and there is little documentation of adverse effects in children( Reference Zlotkin 37 ). In support of this notion, a recent study investigated the effects of Zn supplements on adverse effects among young children( Reference Bertinato, Simpson and Sherrard 38 ). Healthy 6–8-year-old boys consumed Zn supplements ranging from 5 to 15 mg elemental Zn daily in addition to habitual Zn intakes from food that approached or in some instances exceeded the UL. After 4 months of supplementation, biomarkers of Cu status were unaffected by Zn supplementation. The authors of this trial highlight the need for the examination of the current UL for Zn for children( Reference Zlotkin 37 , Reference Bertinato, Simpson and Sherrard 38 ).
While the Zn content of most commercially available Zn supplements is below the UL of Zn intake for most age groups, the recommended number of tablets suggested to be consumed for each age group must also be considered. Consuming one MVM or cold and flu tablet will generally not cause Zn intake to exceed the UL. However, the consumption of multiple doses or Zn-only products in addition to the dietary intake may increase Zn intake to levels above the UL. The Maximum Recommended Daily Dose for Zn set by the Therapeutic Goods Administration in Australia is 50 mg( 39 ), well above the UL for children as well as adults. Similar limits have been set in Canada and the UK has a safe upper limit for supplemental Zn of 25 mg( 40 , 41 ). Lower limits of 10–15 mg Zn maximum per supplement have been proposed by the European Union( 42 ).
There may be some therapeutic advantages of using Zn supplements. The use of Zn supplements for preventing or treating the common cold has been investigated in recent reviews. Zn administered at doses ≥75 mg/d within 24 h of onset of symptoms was found to reduce the duration of common cold symptoms in healthy adults( Reference Singh and Das 43 ). Another systematic review also found beneficial effects of Zn supplementation among adults but not among children( Reference Science, Johnstone and Roth 44 ). Although there is little evidence for a beneficial effect for children in developed countries, Zn supplementation may be of benefit for children in developing countries in lowering rates of acute lower respiratory infections( Reference Roth, Richard and Black 45 ).
The limitations of the present study must be acknowledged. The 2007 ACNPAS used a complex sample design involving stratification, a high degree of clustering and estimation weights. Children residing in very remote areas or in households without a fixed telephone line were not included in the survey. Although survey weights (accounting for age, gender and stratum/location) were applied, prevalence estimates may still be biased and not completely representative of the Australian population. The sample of participants using Zn supplements was relatively small although significant differences were detected between users and non-users. Supplements may be taken irregularly and data collected over a short period of time may not have provided an accurate reflection of usual practices( Reference Dwyer, Picciano and Raiten 46 ) and the type/form of Zn in each supplement was not collected. All Zn supplements were included in the current analysis despite the content of Zn; and information on commercially available supplements such as dosage, claims and consumer targets may have changed since our survey of the market.
Conclusion
In conclusion, analysis of the 2007 ACNPAS shows that the use of Zn supplements resulted in a high prevalence of children who had intakes above the UL. In particular for children aged 2–3 years, supplement use led to excessive intakes of Zn. The vast majority of children met the recommended intake targets by food intake alone with the exception of 14–16-year-old boys. Further investigation of the Zn status of Australian children is needed, including the assessment of Zn biomarkers.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: A.R. and S.S. formulated the research question, A.R. analysed the data, A.J. collated data on commercially available supplements and all authors contributed to the writing of the article. Ethics of human subject participation: Ethics approval for the 2007 Australian Children’s Nutrition and Physical Activity Survey covering ethical, privacy and confidentiality was obtained from the National Health and Medical Research Council-registered Ethics Committees of the Commonwealth Scientific and Industrial Research Organisation and the University of South Australia.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980014000871






