The Stanley Foundation Bipolar Network (SFBN) is a multi-site research programme coordinated at the National Institute of Mental Health (NIMH), with clinical centres in the USA and the Netherlands, as well as newly affiliated sites in Germany. Its mission is to evaluate the long-term effectiveness of conventional and novel pharmacological treatments in a large group of patients with bipolar disorder. Furthermore, the SFBN aims to define longitudinal illness patterns, identify clinical and neurobiological markers of response, and develop new treatment paradigms and algorithms (Reference Leverich, Nolen and RushLeverich et al, 2001; Reference Post, Nolen and KupkaPost et al, 2001, this supplement).
Bipolar disorder is a heterogeneous and highly recurrent disorder, with different mood states of an opposite but related nature (mania, depression and mixed states), and different sub-syndromes (bipolar I and II). There is considerable variability in illness patterns and the frequency of mood episodes. According to DSM-IV (American Psychiatric Association, 1994), rapid cycling is defined as the occurrence of at least four separate solid mood episodes in a year (Reference Bauer and WhybrowBauer & Whybrow, 1996), but many patients with rapid cycling disorders have only brief but nevertheless significant episodes, or a chaotic mood fluctuation of considerable severity (Reference Kramlinger and PostKramlinger & Post, 1996). Moreover, bipolar disorder has a high degree of psychiatric comorbidity (Reference Regier, Farmer and RayRegier et al, 1990; Reference Bebbington and RamanaBebbington & Ramana, 1995; Reference Kessler, Rubinow and HolmesKessler et al, 1997). A detailed description and differentiation of these states and syndromes, as well as longitudinal observation of illness course, are thus important for assessing the efficacy of various treatments and delineating the clinical correlates of treatment response.
Having a heterogeneous but well-defined sample gives a good opportunity to correlate detailed patient and illness characteristics with neurobiological parameters that may have a role in the development of bipolar disorder. This research, which has traditionally relied on in-patient populations, can now be extended over the whole SFBN sample of out-patients. One current focus of our neurobiological studies is on thyroid function and thyroid autoimmunity, and alterations of cell-mediated immunity. The relationship of altered thyroid function and mood disorders has been the subject of many studies (Reference Hendrick, Altshuler and WhybrowHendrick et al, 1998). Some investigators have found a correlation between subclinical or clinical hypothyroidism and rapid cycling (Reference Cowdry, Wehr and ZisCowdry et al, 1983; Reference Bauer, Whybrow and WinokurBauer et al, 1990), although more recent studies of drug-free patients did not (Reference Joffe, Levitt, Joffe and LevittJoffe & Levitt, 1993; Reference Post, Kramlinger and JoffePost et al, 1997). Thyroid autoimmunity (a major cause of hypothyroidism) and changes in cellular immune function have been associated with both unipolar and bipolar mood disorders (Reference Haggerty, Silva and MarquardtHaggerty et al, 1997; Reference Maes, Honig and van PraagMaes, 1997; Reference Kupka, Hillegers and NolenKupka et al, 2000). The SFBN currently studies how these parameters correlate with mood state and treatment outcome.
The main goal of the SFBN is to evaluate potential pharmacological treatments for the various phases of bipolar illness. In the naturalistic follow-up study, as in routine clinical practice, the first step typically is initiating or optimising treatment with a mood stabiliser (lithium, carbamazepine or valproate). After this, the comparative evaluation of different types of combination treatments closely reflects current clinical practice, although it is further informed by systematic longitudinal evaluation. Patients who become symptomatic are treated for their current depressive, manic, hypomanic or mixed episode, with either open treatment as they would receive in the community, or in a more formal, randomised protocol if they wish to participate (see Reference Post, Nolen and KupkaPost et al, 2001).
In this paper, a brief overview of the demographic and illness characteristics of the first group of patients enrolled in the SFBN is given. A detailed description is published elsewhere (Reference McElroy, Suppes and KeckMcElroy et al, 2000; Reference Leverich, Nolen and RushLeverich et al, 2001; Reference Suppes, Leverich and KeckSuppes et al, 2001). We also briefly summarise data on response to add-on open treatment with the atypical antipsychotic agent olanzapine (Reference McElroy, Frye and DenicoffMcElroy et al, 1998) and the anticonvulsants lamotrigine (Reference Suppes, Brown and McElroySuppes et al, 1999), gabapentin (Reference Altshuler, Keck and McElroyAltshuler et al, 1999) and topiramate (Reference McElroy, Suppes and KeckMcElroy et al, 2000).
METHOD
To provide a broad coverage of the bipolar spectrum and its comorbidities, there were few restrictions for patients entering the study. Patients must have a diagnosis of bipolar disorder (I, II, or NOS (not otherwise specified)) according to DSM-IV and be over 18 years old; they may have various psychiatric comorbidities, but not active current substance misuse requiring specific treatment. Although many of the patients had long illness histories and showed various degrees of treatment resistance, newly diagnosed patients and those with a favourable response to standard treatment are also included. All patients enter the naturalistic follow-up study with its longitudinal treatment emphasis; subsequently, they are eligible for further focused study and intervention as emerging symptoms warrant.
Baseline assessment consists of DSM-IV diagnosis on Axis I, using the Structured Clinical Interview for DSM-IV Axis disorders (Reference First, Spitzer and GibbonFirst et al, 1996), and on Axis II (Personality Disorders Questionnaire; Reference HylerHyler, 1994); ratings of depression (Inventory of Depressive Symptomatology, IDS; Reference Rush, Giles and SchlesserRush et al, 1986); mania (Young Mania Rating Scale, YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978); and psychotic symptoms (Positive and Negative Syndrome Scale; Reference Kay, Fiszbein and OplerKay et al, 1987). Overall mood symptomatology over the preceding month is rated on the Clinical Global Impression scale for bipolar disorder (CGI-BP; Reference Spearing, Post and LeverichSpearing et al, 1997). The CGI-BP provides global ratings (separate for manic symptoms, depressive symptoms and overall bipolar disorder) of current severity of mood symptoms and of degree of clinical improvement with any given treatment. Overall symptomatology and functioning are also rated on the Global Assessment of Functioning (GAF) scale (American Psychiatric Association, 1994). Functioning in some specific psychosocial areas is rated on the Life Functioning Questionnaire (further details available from R.K. upon request).
A retrospective life chart of past mood episodes is reconstructed using all available information (Reference Leverich and PostLeverich & Post, 1998). Demographic factors and illness characteristics are assessed by means of both clinician- and patient-completed questionnaires (Reference Suppes, Leverich and KeckSuppes et al, 2001). At follow-up (twice a week), the daily mood ratings, life events and treatment on the patient-rated prospective life chart are reviewed and a clinician-rated prospective life chart is constructed using the NIMH Life Chart Method (NIMH-LCM), together with ratings on the IDS, YMRS, CGI-BP and GAF.
Patients with significant mood symptomatology despite receiving standard treatment can enter specific treatment protocols after giving additional written informed consent. Rating procedures are similar for case series and controlled studies, and largely follow the scheme of the naturalistic follow-up study. In the acute phase of treatment (the first 10 weeks) patients are typically evaluated twice a week, and then monthly in the continuation phase (up to 1 year). This continuity and uniformity permits comparisons between the outcomes of different treatment approaches at a later stage. The availability of clinician-rated prospective life-chart data for all patients also creates the possibility of re-evaluating the CGI-BP-rated treatment responses by a blinded rater.
RESULTS
Demographics and course of illness
As described in detail elsewhere (Reference Suppes, Leverich and KeckSuppes et al, 2001), the first 261 patients enrolled by the SFBN consisted of 145 (56%) women and 116 (44%) men, most of them (83%) aged 30-64 years. The majority had a bipolar I (81%) or bipolar II (16%) diagnosis. The average age of onset of first symptoms was 20 years, but first medication treatment was not received on average until the age of 30 years. Fifty-nine per cent reported a previous history of dysphoric mania/hypomania, and also 59% a history of psychosis; 54% reported a previous history of rapid cycling at some point of their illness, divided into 23% rapid cycling (≥4 episodes a year), 10% ultrarapid cycling (≥4 episodes a month) and 20% ultradian cycling (dramatic mood shifts or cycling within 1 day on 4 or more days a week). Thirteen per cent of all patients reported a history of continuous cycling without a well interval. In addition to this, 54% described a failure to return to euthymia between separate mood episodes. Sixty-two per cent of patients reported that their occupational functioning was significantly limited by their illness; 29% had had five or more hospitalisations; and 29% had made serious suicide attempts.
Comorbidity
Of the first 288 patients in the study, only 101 (35%) had no comorbid diagnosis, while 67 (23%) had one, 52 (18%) had two, and 68 (24%) had three or more additional life-time DSM-IV Axis I diagnoses (Reference McElroy, Altshuler and SuppesMcElroy et al, 2001). Most prevalent were a history of substance misuse (42%) and anxiety disorders (42%), although a variety of other disorders were present (Table 1). By logistic regression analysis, patients with one or more life-time comorbid Axis I disorders had an earlier age of onset (P=0.002) and higher incidence of a positive family history of drug misuse in first-degree relatives (P=0.002). Those with one or more current comorbid diagnosis reported an earlier age of illness onset (P=0.02), more severe episodes over time (P=0.005) and a pattern of cycle acceleration (P=0.017).
Table 1 Age of onset of comorbid Axis 1 disorders occurring before or after onset of bipolar disorder
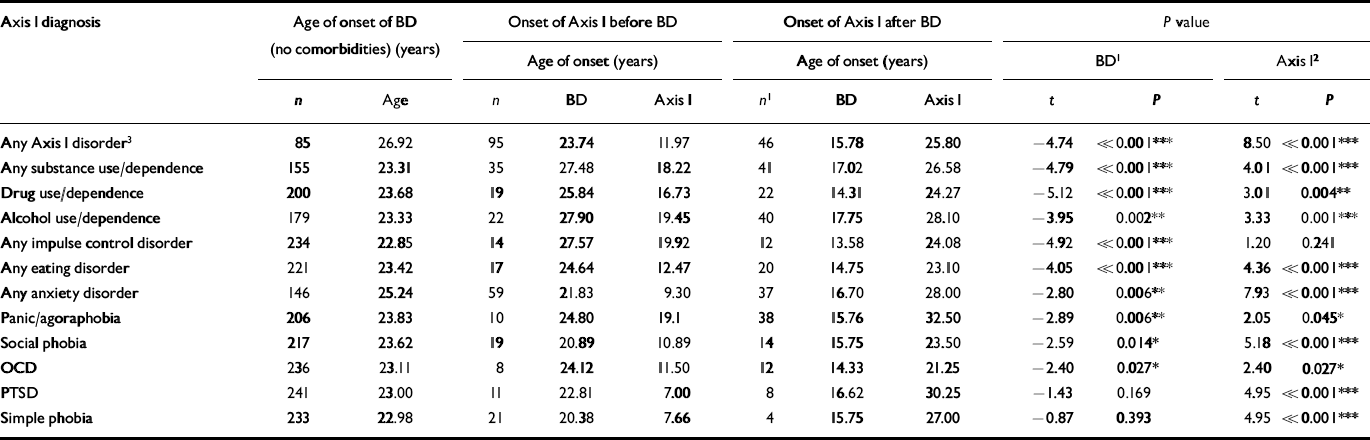
| Axis 1 diagnosis | Age of onset of BD (no comorbidities) (years) | Onset of Axis 1 before BD | Onset of Axis 1 after BD | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset (years) | Age of onset (years) | BD1 | Axis 12 | |||||||||
| n | Age | n | BD | Axis 1 | n 1 | BD | Axis 1 | t | P | t | P | |
| Any Axis 1 disorder3 | 85 | 26.92 | 95 | 23.74 | 11.97 | 46 | 15.78 | 25.80 | -4.74 | <<0.001*** | 8.50 | <<0.001*** |
| Any substance use/dependence | 155 | 23.31 | 35 | 27.48 | 18.22 | 41 | 17.02 | 26.58 | -4.79 | <<0.001*** | 4.01 | <<0.001*** |
| Drug use/dependence | 200 | 23.68 | 19 | 25.84 | 16.73 | 22 | 14.31 | 24.27 | -5.12 | <<0.001*** | 3.01 | 0.004** |
| Alcohol use/dependence | 179 | 23.33 | 22 | 27.90 | 19.45 | 40 | 17.75 | 28.10 | -3.95 | 0.002** | 3.33 | 0.001*** |
| Any impulse control disorder | 234 | 22.85 | 14 | 27.57 | 19.92 | 12 | 13.58 | 24.08 | -4.92 | <<0.001*** | 1.20 | 0.241 |
| Any eating disorder | 221 | 23.42 | 17 | 24.64 | 12.47 | 20 | 14.75 | 23.10 | -4.05 | <<0.001*** | 4.36 | <<0.001*** |
| Any anxiety disorder | 146 | 25.24 | 59 | 21.83 | 9.30 | 37 | 16.70 | 28.00 | -2.80 | 0.006** | 7.93 | <<0.001*** |
| Panic/agoraphobia | 206 | 23.83 | 10 | 24.80 | 19.1 | 38 | 15.76 | 32.50 | -2.89 | 0.006** | 2.05 | 0.045* |
| Social phobia | 217 | 23.62 | 19 | 20.89 | 10.89 | 14 | 15.75 | 23.50 | -2.59 | 0.014* | 5.18 | <<0.001*** |
| OCD | 236 | 23.11 | 8 | 24.12 | 11.50 | 12 | 14.33 | 21.25 | -2.40 | 0.027* | 2.40 | 0.027* |
| PTSD | 241 | 23.00 | 11 | 22.81 | 7.00 | 8 | 16.62 | 30.25 | -1.43 | 0.169 | 4.95 | <<0.001*** |
| Simple phobia | 233 | 22.98 | 21 | 20.38 | 7.66 | 4 | 15.75 | 27.00 | -0.87 | 0.393 | 4.95 | <<0.001*** |
Genetic and environmental factors
Both genetic and environmental or experimental factors could be important to these illness characteristics. In self-reports of family history of psychiatric illness by the first 261 patients, 42% reported a family history of bipolar disease in first-degree relatives (we accepted a rating of ‘probable’ or ‘definite’ by the proband as positive and ‘unlikely’ and ‘no’ as negative), 55% reported unipolar depression and 5% schizophrenia. A family history of suicide or suicide attempts was positive in 19%, alcohol misuse in 38% and drug misuse in 28%. Overall, 66% reported a family history of any mood disorder and 79% any psychiatric illness.
Examination of the concomitants of a positive family history of affective disorder showed that a family history of bipolar disorder was significantly associated with early onset (age 17 years and under), a history of ultrarapid or ultradian cycling, learning disabilities and the experience of being verbally or physically abused. A family history of unipolar depression was significantly associated with female gender, learning disabilities and being verbally or sexually abused.
Specific major psychosocial stressors were categorised by questions about substantial degrees of verbal, physical or sexual abuse, as a child, adolescent or adult. In the first 302 patients (Reference Leverich, McElroy and SuppesLeverich et al, 2000) a report of physical abuse (i.e. ‘occasionally’ or ‘often’, but not ‘rarely’ or ‘never’) or sexual abuse (i.e. ‘rarely’ or more frequent) in childhood was associated with an early onset of bipolar disorder and the pattern of ultradian cycling. Physical abuse was associated with relatively selective increases in the number or severity of manias, and sexual abuse was related to increased frequency of attempted suicide (Reference Leverich, McElroy and SuppesLeverich et al, 2000).
Neurobiological parameters
The prevalence of thyroperoxidase autoantibodies in a subgroup of 226 patients with bipolar disorder was assessed in relation to mood state and long-term course of illness. Thyroid autoantibodies were significantly more prevalent in these patients compared with either an unselected group of recently admitted psychiatric in-patients or normal controls. However, the presence of antithyroid antibodies was not related to gender, sub-diagnosis, mood state or rapid cycling (further details available from R.K. upon request).
Overview of SFBN clinical case series
In addition to the double-blind randomised studies described by Post et al (Reference Post, Nolen and Kupka2001, this supplement), open case series data have been acquired for the atypical neuroleptic agent olanzapine (Reference McElroy, Frye and DenicoffMcElroy et al, 1998) and the newly approved anticonvulsant drugs lamotrigine (Reference Suppes, Brown and McElroySuppes et al, 1999), gabapentin (Reference Altshuler, Keck and McElroyAltshuler et al, 1999) and topiramate (Reference McElroy, Suppes and KeckMcElroy et al, 2000). These drugs were used as ‘add-on’ therapy to ongoing but ineffective treatment with one or more mood stabilisers. These evaluations provided initial preliminary information about dose range, tolerability, side-effects and areas of promising responsivity, each of which helps in the design of subsequent more formal controlled trials (Reference Laska, Klein, Lavori, Prien and RobinsonLaska et al, 1994; Reference Post and LuckenbaughPost & Luckenbaugh, 2001). The results are summarised in Table 1 of Paper 1 (Reference Post, Nolen and KupkaPost et al, 2001, this supplement). Of the 14 patients treated with olanzapine, 12 were treated for a manic, hypomanic or mixed episode, and 2 for depression. Twelve patients met DSM-IV criteria for rapid cycling. It is of interest that patients with manic/mixed forms of the disorder without psychotic features responded as frequently (3 of 6) as those with psychotic features (4 of 6). Olanzapine was generally well tolerated; only one patient discontinued the drug because of side-effects; no patient developed extrapyramidal symptoms or required concomitant anti-Parkinsonian drugs (Reference McElroy, Frye and DenicoffMcElroy et al, 1998).
Of the 17 patients treated with lamotrigine, 6 were treated for a manic, hypomanic or mixed episode and 11 for depression. Six (67%) of the 9 patients with rapid cycling showed significant and sustained improvement in both mood cycling and depressive symptoms. Side-effects were experienced by 7 patients (41%), but were not a reason for discontinuation (Reference Suppes, Brown and McElroySuppes et al, 1999).
Of the 28 patients treated with gabapentin, 18 were treated for a manic, hypomanic or mixed episode and 5 for depression; another 5 patients were treated for persistent rapid cycling while being euthymic at entry. Response rates were high for euphoric mania and hypomania, and also for depression, but low for mixed mania and rapid cycling. Side-effects occurred in 12 patients (46%) and were rated as mild in all cases (Reference Altshuler, Keck and McElroyAltshuler et al, 1999).
Of the 54 patients treated with topiramate, 30 were in a manic, hypomanic, mixed or extremely rapid cycling state, 11 were depressed and 13 were relatively euthymic. The euthymic patients received topiramate for psychotropic drug-induced weight gain and/or binge eating, since this drug has shown anorectic and weight-loss effects in clinical trials in patients with epilepsy. Significant improvement of manic symptomatology was seen in the first subgroup (patients who were initially in a manic, hypomanic, mixed or rapid cycling state), with 63% rated as responders after 10 weeks, while only 27% of the depressed group showed much improvement in their depressive symptoms. The group of euthymic patients showed no significant changes in mood ratings. On the average there was a significant reduction of weight (down 4.9%) and body mass index (down 5.0%) in the whole group of patients. Topiramate was discontinued in 18% of the patients because of side-effects (Reference McElroy, Suppes and KeckMcElroy et al, 2000).
DISCUSSION
This first group of out-patients enrolled in the SFBN showed a considerable severity of past and present illness, as reflected by a history of psychosis, dysphoric mania, rapid cycling, suicide attempts, lack of interepisodic full recovery and multiple hospitalisations. The average of a decadelong delay between the onset of the first symptoms of mood disorder sufficient to meet DSM-IV criteria and the first medication treatment was also seen in a subgroup of members of the National Depressive and Manic-Depressive Association (Reference Lish, Dime-Meenan and WhybrowLish et al, 1994) and in the clinical series of Egeland et al (Reference Egeland, Blumenthal and Nee1987), suggesting a pressing need for earlier recognition and intervention.
The high prevalence of comorbid anxiety disorders and substance misuse is consistent with other community and clinical samples (Reference Regier, Farmer and RayRegier et al, 1990; Reference Bebbington and RamanaBebbington & Ramana, 1995, Reference Kessler, Rubinow and HolmesKessler et al, 1997). In general, the comorbid disorder preceded the onset of bipolar disorder, but this was not the case for panic disorder and alcoholism. The association between a history of substance misuse on the one hand and an early age of onset of bipolar illness, rapid cycling and dysphoric mania on the other, is particularly interesting in relation to stimulants often being described as inducing a euphoric mania-like syndrome on initial use (Reference Post, Weiss, Pert, Raskin and FisherPost et al, 1987) but growing dysphoria on increasing and chronic use (Reference Sonne, Brady and MortonSonne et al, 1994; Reference Brady and SonneBrady & Sonne, 1995). The extent to which substance misuse is driving dysphoric mania, or vice versa, remains to be studied.
Our findings with regard to a family history of psychiatric illness, although based on a proband report, are not widely discrepant from other reported studies, and are consistent with the notion that a distinct subgroup of patients develop vulnerability to bipolar disorder through genetic as well as non-genetic mechanisms. A positive family history of bipolar disorder was associated with a more severe course of illness as reflected by an early onset and the occurrence of rapid cycling. These data were obtained by self-report and are not based on direct interview of the probands and their family members; they thus provide only a rough (but probably conservative) estimate of the true incidence of psychiatric disorders among the first-degree relatives.
Self-reports of verbal, physical and sexual abuse, as examples of major psychosocial stressors, were found to be associated with a more adverse course of illness (Reference Leverich, McElroy and SuppesLeverich et al, 2000). Severe early childhood or adolescent stressors appear to interact in general with the way bipolar illness progresses, in terms of earlier onset and faster cycling patterns. With some specificity, physical abuse was associated with reports of increasing severity of mania, and sexual abuse with increased number of prior suicide attempts. Those with a history of early physical or sexual abuse also reported more adverse life events occurring prior to both illness onset and the most recent affective episodes. Thus, these patients were either sensitised by stress or put at risk of subsequent adverse life events.
Taken together, these associations support the idea that not only is genetic vulnerability contributing to bipolar illness, but medical insults and perinatal and childhood psychosocial stressors could also contribute as well. It appears that both stressors and substance misuse can change gene expression, and possibly add to whatever genetic vulnerability patients may have already inherited (Reference Post, Weiss, Pert, Raskin and FisherPost et al, 1987; Reference PostPost, 1997; Reference Post, Weiss, Joffe and YoungPost & Weiss, 1997). The SFBN aims at assessing how these experiential as well as hereditary factors could affect the unfolding of the illness and response to treatment. Characterising an array of other contributing factors such as family history, comorbidities, lack of social support and course of illness characteristics might help in devising treatment algorithms more specifically tailored to individual patients. Thus, these clinical and demographic variables are interesting in their own right and may also give clues about ultimate treatment response.
With regard to pharmacotherapy, the SFBN is focusing on the best use of existing agents as well as developing new treatment options. Many unimodal antimanic and antidepressant drugs, as well as actual or putative mood stabilisers, are now available. Their efficacy on which symptoms and in which patients is a matter for further systematic study. The SFBN is thus organised to gather both case experience and systematic comparative information as quickly as possible, so that treatments can begin to be sequenced in a more rational manner.
The report suggesting the effectiveness of olanzapine in mania (Reference McElroy, Frye and DenicoffMcElroy et al, 1998) was rapidly followed by double-blind randomised trials conducted by the pharmaceutical industry, and by approval of this agent by the Food and Drug Administration for the treatment of acute mania. Lamotrigine has been found to be effective in about 50% of patients in two double-blind monotherapy trials in refractory mood disorders (Reference Frye, Ketter and KimbrellFrye et al, 2000) and in bipolar depression (Reference Calabrese, Bowden and SachsCalabrese et al, 1999); this agrees with the open add-on case series data reported by the SFBN (Reference Suppes, Brown and McElroySuppes et al, 1999) and in the literature. In contrast, there is a large discrepancy between the results of double-blind studies and open case series involving gabapentin. The 27% blind monotherapy response in the NIMH gabapentin trial (Reference Frye, Ketter and KimbrellFrye et al, 2000) was no better than placebo (23%) and was significantly inferior to lamotrigine (52%). However, in the NIMH trial gabapentin was given blind as monotherapy to people with highly refractory mood disorders admitted to a tertiary referral centre; the response rates in out-patients in open add-on case series in the literature and in the SFBN were much higher (Reference Altshuler, Keck and McElroyAltshuler et al, 1999). Given gabapentin's potential spectrum of efficacy in a variety of conditions that can occur comorbidly with bipolar illness such as social phobia, obsessive-compulsive disorder, Parkinsonian tremor, restless leg syndrome and pain syndromes (Reference Post, Frye and DenicoffPost et al, 2000), it is possible that effects in these areas rather than a primary antimanic effect (Reference PandePande, 1999) accounted for its positive evaluation in adjunctive treatment.
There are only limited data from open studies of topiramate in bipolar disorder, all suggesting that this anticonvulsant agent may be efficacious in mania and rapid cycling. Our case series (Reference McElroy, Suppes and KeckMcElroy et al, 2000) supports this view, but also suggests this drug's limited effectiveness in treating depressive symptoms. Even in the absence of acute antidepressant effects, side-effects of anorexia and weight loss make topiramate an interesting compound, since psychotropic drug-induced weight gain (such as with lithium, valproate, clozapine and olanzapine) is very common in patients with bipolar disorder. Double-blind trials are needed to evaluate the range of efficacy of topiramate in bipolar illness and its effectiveness in preventing or reversing psychotropic drug-induced weight gain. With regard to the latter point, we are planning a randomised comparison of topiramate v. sibutramine to assess the relative merits and liabilities of these two agents.
CONCLUSION
The SFBN aims to improve the understanding and treatment of both the acute and the long-term course of bipolar disorder. Its participants include representative patients with bipolar disorder, with few restrictions, in a naturalistic treatment setting, from several different countries. By using the daily Life Chart Method as the main longitudinal rating instrument, as well as the CGI-BP, neurobiological parameters and treatment outcome can be related to a detailed description of illness state and course. Each formal controlled research protocol or open case series performed in the SFBN uses the same core assessment methodology, thereby enhancing the ability to compare outcomes of different treatment approaches. Open case series give a first impression of the dose, tolerability and spectrum of efficacy of potential new antimanic, antidepressant and mood-stabilising agents, which can then be more formally studied in controlled clinical trials. These open and controlled studies are embedded in a treatment sequence that parallels current clinical practice. In this way, we expect that our findings will help define the optimal treatments for the large group of hitherto inadequately treated patients with bipolar illness.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Bipolar disorder is a recurrent illness with multiple comorbidities in which the long-term outcome is often worse than assumed, despite treatment with conventional agents.
-
▪ There is a considerable degree of treatment resistance and an ongoing need for the development of new treatment options.
-
▪ Open case series with the potential mood-stabilising anticonvulsants lamotrigine, gabapentin and lopiramate show promising results warranting further study.
LIMITATIONS
-
▪ Preliminary treatment outcome is derived from open-label, non-randomised case series, in which the study medication is added to existing, insufficiently effective prophylactic treatment. More detailed controlled trials are now needed to confirm preliminary areas of promise.
-
▪ This brief overview of the initial demographic factors, course of illness, family history and stressful early life events is largely based on patient information and remains to be prospectively validated.
-
▪ Only preliminary data on the first 302 of the 560 patients currently enrolled are presented, and larger studies and more controlled trials will be reported in the future.




eLetters
No eLetters have been published for this article.