1. Introduction
While crickets (Orthoptera, Grylloidea) are commonly used as models in neurobiology (Pollack et al. Reference Pollack, Mason, Popper and Fay2016), bioacoustics (Gerhardt & Huber, Reference Gerhardt and Huber2002) and behavioural ecology (Gwynne & Morris, Reference Gwynne and Morris1983; Choe & Crespi, Reference Choe and Crespi1997), their wide use in evolutionary studies has been prevented by the lack of well-supported phylogenetic analyses, and by a fragmented fossil record. Yet both are necessary to document character ancestral states and transformations, to test evolutionary hypotheses, or to relate clade and character evolution in response to environmental changes. Fossils are then used as calibration points or included in the data matrix as terminals (Jouault et al. Reference Jouault, Legendre, Grandcolas and Nel2021 a, b).
1.a. Cricket phylogeny: a reference under construction
The cricket clade is well-supported within the suborder Ensifera by recent molecular studies (Song et al. Reference Song, Amédégnato, Cigliano, Desutter-Grandcolas, Heads, Huang, Otte and Whiting2015, Reference Song, Béthoux, Shin, Donath, Letsch, Liu, McKenna, Meng, Misof, Podsiadlowski, Zhou, Wipfler and Simon2020) and recognized as the infra-order Gryllidea (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 a, b). At lower taxonomic scale, despite the availability of numerous taxonomic articles, very few references attest the monophyly of the different cricket groups and the inter- and intra- relationships. As a result, the present-day classification of crickets is far from avoiding para- or polyphyletic assemblages (B Warren et al., 2019).
Chintauan-Marquier et al. (2013; Reference Chintauan-Marquier, Legendre, Hugel, Robillard, Grandcolas, Nel, Zuccon and Desutter-Grandcolas2016) proposed the first large-scale study of the infra-order Gryllidea, using 205 terminals and six molecular markers, and supporting the monophyly of two superfamilies (Gryllotalpoidea and Grylloidea), as long proposed for crickets; they recovered five clades within the Grylloidea (i.e. four families Mogoplistidae, Trigonidiidae, Phalangopsidae and Gryllidae, and the subfamily Pteroplistinae incertae sedis), while various classifications through time proposed from one to 12 different families. The upper classification derived from these analyses has since been adopted by the scientific community (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 a).
Other phylogenetic studies of crickets mostly focused either on limited geographical scales, or on limited ingroups, e.g. subfamily Gryllidae Eneopterinae (Vicente et al. Reference Vicente, Kergoat, Dong, Yotoko, Legendre, Nattier and Robillard2017 and references therein) or its tribes (Tan et al. Reference Tan, Malem, Legendre, Dong, Baroga-Barbecho, Yap, Wahad, Japir, Chung and Robillard2021), the Neotropical Phalangopsidae genus Eidmanacris Chopard, Reference Chopard1956 (Campos et al. Reference Campos, Souza-Dias, Desutter-Grandcolas and Nihei2021), the Hawaiian trigonidiine genus Nudilla Gorochov, 1988 (Mendelson & Shaw, Reference Mendelson and Shaw2005, under the name Laupala Otte, Reference Otte1994) or the eneopterine genus Cardiodactylus de Haan, 1844 (Dong et al. Reference Dong, Kergoat, Vicente, Rahmadi, Xu and Robillard2018); He et al. (Reference He, Shen and Wu2020), Shen et al. (Reference Shen, Guo and He2020) and Ding et al. (Reference Ding, Liu, Liao, Shen and He2021) studied the relationships of Chinese species of Trigonidiidae Trigonidiinae and Nemobiinae respectively. More recently, Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022) studied the phylogeny of clade F of Chintauan-Marquier et al. (Reference Chintauan-Marquier, Legendre, Hugel, Robillard, Grandcolas, Nel, Zuccon and Desutter-Grandcolas2016), one of the two clades constitutive of the family Gryllidae, using both morphological and molecular characters in a worldwide perspective: these authors proposed to elevate clade F to family rank, i.e. the Oecanthidae, and propose a revised classification based on well-diagnosed monophyletic entities from family to tribes and genera (Campos et al. Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022): crickets are now distributed into five monophyletic families. Nevertheless, there is a strong deficiency of phylogenetic studies for crickets at a scale allowing the drawing of phylogeny-based classifications along with clade diagnosis (see Tan et al. Reference Tan, Malem, Legendre, Dong, Baroga-Barbecho, Yap, Wahad, Japir, Chung and Robillard2021 and references therein for the Gryllidae Eneopterinae; Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021 for the Trigonidiidae; Campos et al. Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022 for the Oecanthidae).
This situation is made even more complicated by the variations of cricket classification through time and among the authors. For example, the name ‘Gryllidae’ has been used to designate as a family what other authors consider a superfamily (Grylloidea) or even an infra-order (Gryllidea), both of which include a restricted family called Gryllidae. In this general frame, the taxonomic positions of fossils described as ‘Gryllidae’, without precision, are particularly ambiguous.
1.b. The fossil record for crickets
In this frame, the current attributions of fossil crickets should be considered with caution. For example, the Cretaceous Liaonemobius Ren, 1998, which was described as a member of Trigonidiinae (Trigonidiidae) and long-listed in the Gryllidae s. str. (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2021), is actually not a cricket but belongs to the Elcanidae (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021; Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 a, b). The Cretaceous Gryllidium oweni Westwood, 1854, originally considered as ‘Gryllidae’, proved to belong to Phasmatodea (Coram & Jepson, Reference Coram and Jepson2012).
Many cricket fossils have been reanalysed by different authors, and their status reconsidered, but their attribution to a suprageneric category is seldom well-argued in terms of clear synapomorphies. This has been performed for the Trigonidiidae family and its two subfamilies, i.e. the Nemobiinae and Trigonidiinae (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021). In total, among the 12 fossils now listed for the Trigonidiidae, seven could be attributed to the Trigonidiinae (including Birmaninemobius hirsutus Xu et al., Reference Xu, Zhang, Jarzembowski and Fang2020 b as a representative of its stem group, although described as a Nemobiinae), two juveniles could be attributed to the Nemobiinae, and two could not be reasonably considered; Curvospirus huzhengkun Liu et al. Reference Liu, Yu and He2022 clearly belongs to Trigonidiidae, but its morphological features do not allow it to be classified in either the Trigonidiinae, or the Nemobiinae, as it presents characters of both subfamilies in addition to original ones (Liu et al. Reference Liu, Yu and He2022). Apart from a few representatives of morphologically well-characterized clades (such as the mole crickets (Gryllotalpoidae Gryllotalpidae; see Xu et al. Reference Xu, Wang, Fang, Jarzembowski and Zhuo2022), the scaly crickets (Grylloidea Mogoplistidae Mogoplistinae; see Gorochov, Reference Gorochov2010), the prognathous Oecanthidae Oecanthinae (see Yuan et al. Reference Yuan, Zheng, Zheng, Ma and Gu2022), or very recent specimens that belong to modern genera, fossil crickets can prove quite hard to classify in relation to modern taxa, especially if they are isolated wing imprints, or, like many inclusions in Cenozoic amber, juveniles.
Juveniles can be difficult to attribute to a given family, and their classification is always problematic, even for extant specimens. As examples, the two Middle Eocene Nemobiinae described as Baltonemobius fossilis Gorochov, Reference Gorochov2010 (Gorochov, Reference Gorochov2010) and Nemobius sp. (Chopard, Reference Chopard1936) could be included as terminals in a morphological phylogeny, but all adult characters (i.e. wings, male stridulatory apparatus, auditory tympana, female ovipositor) would not be described. These fossils attest however the presence of the subfamily at their period of fossilization.
The interest of specimen imprints, in terms of evolutionary studies, depends on the available characters. Even apparently well-preserved fossils can prove impossible to classify with reasonable certainty. They can often be described as crickets (Grylloidea), but their attributions to familial and infrafamilial categories should be performed according to the observation of given apomorphies, which is rarely the case (e.g. Menatgryllus longixiphus Schubnel et al. Reference Schubnel, Desutter-Grandcolas, Garrouste, Hervet and Nel2020 a, which could belong to the Gryllidae s. str. according to fore tibia apical spurs, but presents an original combination of other characters; Schubnel et al. Reference Schubnel, Desutter-Grandcolas, Garrouste, Hervet and Nel2020 a). Recent fossils can then be compared to extant taxa, but the most ancient fossils are more difficult to classify, even though they can always be described as new genera and species.
Forewing imprints that present the stridulatory apparatus typically found in acoustic crickets can generally be immediately classified as crickets, but more precise attributions to a subgroup may be problematic, because very few cricket groups are currently characterized by wing apomorphies. For instance, the fossil family Baissogryllidae is characterized by the cross-veins in the area between CuPaß and CuA+CuPaα parallel to the basal part of CuPaß; but this character is also present in the Haglidae (Ensifera Tettigoniidea) and the Gryllotalpidae, representing a possible symplesiomorphy or homoplasy (A. Nel, pers. obs.). Other cricket groups have been defined using characters of the stridulum, such as several fossil subfamilies of the Gryllidae (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 a, b), while some extant taxa present unique characters on their wings, such as the Trigonidiidae p.p. (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021), the Phaloriinae (Phalangopsidae) (Desutter-Grandcolas, Reference Desutter-Grandcolas2015), the Pteroplistinae (Chopard, Reference Chopard1936) or some Oecanthinae and Podoscirtinae (Oecanthidae) (Campos et al. Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022). The main problems with wing characters are first that numerous crickets are brachypterous, micropterous or apterous, and secondly that the females are usually devoid of a stridulum, making the stridulatory criteria uninformative. Also, homologies of venation on cricket forewings are still fiercely debated, and no consensus exists today as to the identity of some of the veins (Béthoux, Reference Béthoux2012; Nel, Reference Nel2021), even though the use of microtomography offers solid arguments for vein homologies (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Jacquelin, Hugel, Boistel, Garrouste, Henrotay, Warren, Chintauan-Marquier, Nel, Grandcolas and Nel2017; Schubnel et al. Reference Schubnel, Desutter-Grandcolas, Legendre, Prokop, Mazurier, Garrouste, Grandcolas and Nel2020 b). These difficulties severely limit the use of fossil wings, even for dating the most recent nodes, and call for an extensive study of wing venation in crickets (Josse et al. unpub. data).
Recently, 11 very well-preserved fossil crickets have been discovered and described from early- to mid-Cretaceous amber of France and Myanmar. Thanks to their excellent state of preservation, these specimens have been described as precisely as extant taxa, and the presence of apomorphic characters that define the cricket clades could be checked, even if seven are juveniles (Perrichot et al. Reference Perrichot, Néraudeau, Azar, Menier and Nel2002; Poinar et al. Reference Poinar, Su and Brown2020; Wang et al. Reference Wang, Lei, Zhang, Xu, Fang and Zhang2020; Xu et al. Reference Xu, Fang and He2020 a, b, Reference Xu, Wang, Fang, Jarzembowski and Zhuo2022; Gorochov Reference Gorochov2010; Jiang et al. Reference Jiang, Xu, Jarzembowski and Xiao2022; Liu et al. Reference Liu, Yu and He2022; Yuan et al. Reference Yuan, Zheng, Zheng, Ma and Gu2022).
Here, we describe two additional, particularly well-preserved mid-Cretaceous adult fossils from the amber of Charentes (France), Palaeonemobius occidentalis Laurent and Desutter-Grandcolas, gen. nov., sp. nov. and Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov. These fossils are respectively the oldest representatives of the Nemobiinae (Trigonidiidae) and Podoscirtinae sensu Campos et al. Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022 (Oecanthidae), with which they share the main synapomorphies. Picogryllus Josse and Desutter-Grandcolas, gen. nov. is also the first Mesozoic fossil cricket for which male genitalia can be partly reconstructed and illustrated, and is the smallest adult male specimen (body length 3.3 mm) with a complete stridulatory apparatus ever found in the cricket clade. These fossils complete the small set of well-described fossils available for large-scale evolutionary studies of crickets.
2. Materials and methods
2.a. Geological setting
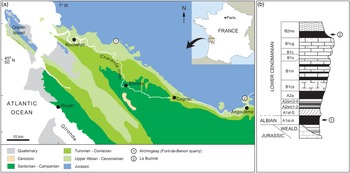
The studied specimens originate from two distinct amber deposits from the Charentes region in SW France (Fig. 1). The specimen MNHN.F.A71375 was found in amber from La Buzinie, a former hamlet now in the town of Champniers, near Angoulême, in the Charente department. The amber piece was collected from a lignitic layer (level B2 in Perrichot et al. Reference Perrichot, Nel and Néraudeau2007, fig. 2; Peyrot et al. Reference Peyrot, Barrón, Polette, Batten and Néraudeau2019, fig. 2; B2ms in Perrichot et al. Reference Perrichot, Néraudeau, Tafforeau and Penney2010, fig. 2) found within sandstones that briefly outcropped during roadworks in 2005. Based on palynomorph evidence, level B2 and hence amber from La Buzinie are considered Early Cenomanian in age, 97–100 Ma (Peyrot et al. Reference Peyrot, Barrón, Polette, Batten and Néraudeau2019).

Fig. 1. Geographical and geological settings of the Cretaceous Charentese amber deposits considered in the present study. (a) Location of deposits. (b) Regional stratigraphic section with indication of amber levels yielding fossil crickets (numbers of sites correlate with (a)). Modified from Perrichot et al. (Reference Perrichot, Nel and Néraudeau2007).
The specimen IGR.ARC-421.1 was found in amber from the Font-de-Benon quarry, between Archingeay and Les Nouillers villages, in the Charente-Maritime department (so-called ‘Archingeay amber’ in a number of previous publications). The amber piece was collected from the lowermost of two amber-bearing strata that outcropped in the quarry (level A1sl2 in Néraudeau et al. Reference Néraudeau, Perrichot, Dejax, Masure, Nel, Philippe, Moreau, Guillocheau and Guyot2002, fig. 2; A1sl-A in Perrichot et al. Reference Perrichot, Néraudeau, Tafforeau and Penney2010, fig. 2; A1 in Peyrot et al. Reference Peyrot, Barrón, Polette, Batten and Néraudeau2019, fig. 2). Based on palynomorph evidence, the A1 series from this quarry cannot be unequivocally dated and has alternatively been considered latest Albian or earliest Cenomanian in age, c. 100 Ma (Néraudeau et al. Reference Néraudeau, Perrichot, Dejax, Masure, Nel, Philippe, Moreau, Guillocheau and Guyot2002; Dejax & Masure, Reference Dejax and Masure2005; Peyrot et al. Reference Peyrot, Jolly and Barrón2005; Polette, Reference Polette2019).
2.b. Specimen imaging
Due to the fully opaque nature of the two amber pieces studied here (see Fig. 2 for an example), their fossil content was revealed by propagation phase-contrast X-ray synchrotron microtomography (PPC-SRµCT) performed at beamline ID19 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) according to the protocol described by Lak et al. (Reference Lak, Néraudeau, Nel, Cleotens, Perrichot and Tafforeau2008 b). The specimen MNHN.F.A71375 was scanned using a multilayer monochromator with an acceleration voltage of the X-ray source of 20.5 keV and a propagation distance of 300 mm between the sample and the detector, an isotropic voxel size of 5.06 µm, and 2000 projections taken over 180° with 0.5 s of exposure time for each projection. The specimen IGR.ARC-421.1 was scanned at 30 keV with a propagation distance of 900 mm, an isotropic voxel size of 20.24 µm and 2500 projections taken over 180° with 0.2 s of exposure time for each projection. The tomographic data were analysed using VG StudioMax (Volume Graphics).

Fig. 2. Piece of opaque amber (size 46 mm) where the male holotype (IGR-ARC-421.1) of Picogryllus carentonensis Josse and Desutter-Grandcolas, gen.nov., sp. nov. (Oecanthidae, Podoscirtinae) was found. Scale 1 cm.
In each piece, the crickets were found among various other inclusions: the Trigonidiidae is preserved along with conifer fragments (one leafy axis and isolated scales) and 19 other arthropods including mites, wasps (Diapriidae (see Lak & Nel, Reference Lak and Nel2009); Platygastroidea), long-legged and moth flies (Dolichopodidae; Psychodidae (Lak et al. Reference Lak, Azar, Nel, Néraudeau and Tafforeau2008 a)), a cockroach (Blattellidae; see Vrsansky, Reference Vrsansky2008) and another, fragmentary Ensifera (Elcanidae); the piece with the Podoscirtinae holds nine other insects including cockroaches, wasps (Platygastroidea), a planthopper (Fulgoromorpha) and a lacewing larva (Neuroptera).
2.c. Cricket classification and morphological descriptions of the fossils
We follow the phylogenetic classification proposed by Chintauan-Marquier et al. (2013; Reference Chintauan-Marquier, Legendre, Hugel, Robillard, Grandcolas, Nel, Zuccon and Desutter-Grandcolas2016), modified by Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022), which recognized five families within the Grylloidea, i.e. Mogoplistidae Costa, 1855, Trigonidiidae Saussure, 1874, Phalangopsidae Blanchard, 1845, Gryllidae Laicharting, 1781 and Oecanthidae Blanchard, 1845, in addition to the subfamily Pteroplistinae Chopard, Reference Chopard1936 incertae sedis within Grylloidea. Chintauan-Marquier et al. (2013; Reference Chintauan-Marquier, Legendre, Hugel, Robillard, Grandcolas, Nel, Zuccon and Desutter-Grandcolas2016) supported a clade ‘Gryllidae’, split into two monophyletic assemblages referred to as ‘clade G’ with present-day Gryllinae, Landrevinae, Eneopterinae and Pentacentrinae, and ‘clade F’ with present-day Euscyrtinae, Oecanthinae (including tafaliscine taxa), Podoscirtinae and Hapithinae (see Chintauan-Marquier et al. Reference Chintauan-Marquier, Legendre, Hugel, Robillard, Grandcolas, Nel, Zuccon and Desutter-Grandcolas2016, figs 5, 6). Using a large dataset of molecular and morphological characters for a large taxonomic sampling, Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022) validate the family status of both clades F and G, as Oecanthidae and Gryllidae respectively; a complete reanalysis of the classification of Oecanthidae on the basis of morphological apomorphies leads to splitting the Oecanthidae into four subfamilies, i.e. Euscyrtinae, Podoscirtinae (Hapithidi + Podoscirtidi), Oecanthinae (Oecanthidi + Diatrypidi) and Tafaliscinae (Paroecanthidi + Tafaliscidi). We use here this resultant five-family classification.
The fossils we describe here belong to the families Trigonidiidae and Oecanthidae respectively. We use the diagnoses proposed by Desutter-Grandcolas et al. (Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021) for Trigonidiidae and its two well-supported and well-characterized subfamilies Trigonidiinae and Nemobiinae, and those proposed by Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022) for Oecanthidae and its subdivisions from subfamilies to tribes. The reference classification of Cigliano et al. (Reference Cigliano, Braun, Eades and Otte2022 b) is now congruent with the phylogenetic classification of Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022).
Morphology is described as for extant cricket taxa, as for example in Hugel & Desutter-Grandcolas (Reference Hugel and Desutter-Grandcolas2021) or Campos et al. (Reference Campos, Souza-Dias, Desutter-Grandcolas and Nihei2021). We separate movable, articulated spurs from immovable outgrowths (spines). Apical spurs (a) are referred to according to their location on the tibia, i.e. on outer or inner side (o, i), and dorsal, median or ventral (d, m, v) on each side. Hind tibial subapical spurs (sa) are counted from TIII apex upwards, on inner and outer margins, in order to follow potential homologies for the spurs. Wing venation is named after Béthoux & Nel (Reference Béthoux and Nel2002), modified by Schubnel et al. (Reference Schubnel, Desutter-Grandcolas, Legendre, Prokop, Mazurier, Garrouste, Grandcolas and Nel2020 b). Male genitalia are described according to Desutter (Reference Desutter1987), modified in Desutter-Grandcolas (Reference Desutter-Grandcolas2003).
2.d. Abbreviations for plates
Morphology: c, cerci; ey, eye; flg, flagellum of antenna; fst, fastigium; fw, forewing; ha, harp of male stridulatory apparatus; hw, hindwing; ia, inner apical spurs (1 to n); l. oc., lateral ocellus; lb. p., labial palpus; max. p., maxillary palpus; md, mandibula; mi, mirror of male stridulatory apparatus; m. oc., median ocellus; oa, outer apical spurs (1 to n); ov, ovipositor; PI, II, III, fore, median and hind leg; pr, pronotum; sa, subapical spur (1 to n); sc, scape; s.gen., subgenital plate; sp, spine; spl, spiracle; tar.III, hind tarsomere; ty, tympanum; vx, vertex.
Male genitalia: l. l., lateral lophi of pseudepiphallus; m. l., median lophi of pseudepiphallus; ps, pseudepiphallic sclerite.
2.e. Repository
IGR, Géosciences Rennes, Université Rennes 1, Rennes, France;
MNHN, Muséum national d’Histoire naturelle, Paris, France.
3. Systematic palaeontology
Order ORTHOPTERA Olivier, 1789
Suborder ENSIFERA Chopard, 1922
Superfamily GRYLLOIDEA Laicharting, 1781
Family TRIGONIDIIDAE Saussure, 1874
Subfamily NEMOBIINAE Saussure, 1877
Genus Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov.
(Fig. 3)

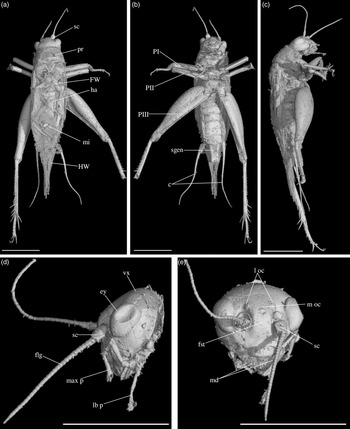
Fig. 3. Palaeonemobius occidentalis gen. nov., sp. nov. (female, holotype, MNHN.F.A71375) in mid-Cretaceous amber from Charentes (France): (a) dorsal view; (b) ventral view; (c) head, frontal view; (d) head, side view; (e) pronotum and forewing, right side view; (f) hind femur, outer side, with outer apical and subapical spurs; (g) hind femur, inner side, with inner apical and subapical spurs; (h) fore tibia with inner auditory tympanum (ty) and apical spurs. Abbreviations: see Section 2. Scales 1 mm (c, d, e, h), 2 mm (a, b, f, g).
urn:lsid:zoobank.org:act:D21831C9-C3D7-4FBF-8AE5-016BD4F1079D
Type species. Palaeonemobius occidentalis Laurent and Desutter-Grandcolas, gen. nov., sp. nov., here designated.
Derivation of name. The genus name is derived from the Greek adjective παλαιός, meaning ‘ancient’, and the usual suffix given to nemobiine crickets (nemobius). Gender masculine.
Diagnosis. Adult female (Fig. 3a). Size small. Head, pronotum and legs with many strong setae. Head wide, with a wide fastigium and scapes wider than long (Fig. 3c); median ocellus at least present (Fig. 3c); maxillary palpi short (Fig. 3c), articles 3 to 5 subequal, article 5 slightly widened toward apex. Second tarsomeres cylindrical, not flattened dorsoventrally; claws simple, not serrated. Fore tibiae with two apical ventral spurs (Fig. 3h). Hind femora with a very wide ventral gutter (Fig. 3g). Hind tibiae (Fig. 3f, g) not serrulated; with five outer and four inner subapical spurs lengthening toward tarsus, the most distal spurs the longest, the most basal spur the shortest; with three inner and three outer apical spurs. Hind basitarsomeres very long (Fig. 3b), more than half hind tibia length; with five outer and two inner spines. A short right forewing present (left one lost: Fig. 3a), not reaching metanotum distal margin; venation hardly visible. Hindwings absent. Ovipositor broken before apex (at least length 4.5 mm), but straight at base.
Differential diagnosis. Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov. belongs to the Grylloidea, as shown by the club-shaped setae on the cerci, three tarsomeres, hind legs adapted to jump, and location of apical and subapical spurs on legs. It has the following characters of Trigonidiidae (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021): size small (body length less than 15 mm); head, pronotum and legs with strong setae; hind tibia not serrulated with apical and subapical spurs. It does not show the apomorphies of Trigonidiinae (i.e. head triangular in front view, fore tibia with only one large apical spur, claw serrated), and does not present either the homoplasies present in this subfamily (second tarsomere flattened dorsoventrally, subapical spurs of hind tibia short, or long but equal in length, pronotum most often narrowed in front).
Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov. displays characters of the Nemobiinae, both apomorphies (wide ventral gutter on hind femora; hind tibia subapical spurs longer toward tibia apex) and homoplasies (two apical spurs on fore tibia; scape small, wider than long; hind femur much longer than hind tibia; cercus long, thin and straight). It seems to have a straight ovipositor, as Nemobiinae, but this cannot be ascertained (apex broken), although the typical shape of ovipositor in Trigonidiinae (curved upwards and flattened laterally) can be excluded.
Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov. could thus represent the oldest representative of the Nemobiinae known today. The only other ascertained fossil Nemobiinae (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021) are two juvenile specimens from the Baltic amber (middle to late Eocene), i.e. Nemobius sp. and Baltonemobius fossilis (Chopard, Reference Chopard1936; Gorochov, Reference Gorochov2010): both differ from Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov. by their hind tibia with three inner and three outer subapical spurs. Palaeonemobius Laurent and Desutter-Grandcolas, gen. nov. is the first evidence of a Cretaceous Nemobiinae, as Birmaninemobius hirsutus Xu et al., Reference Xu, Fang and He2020 a, b, initially described as a Nemobiinae, has been characterized as belonging to the stem group of the Trigonidiinae (Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021), and Curvospirus huzhengkun Liu et al. Reference Liu, Yu and He2022 do not fit either subfamily (Liu et al. Reference Liu, Yu and He2022).
Palaeonemobius occidentalis Laurent and Desutter-Grandcolas, gen. nov., sp. nov.
(Fig. 3)
urn:lsid:zoobank.org:act:ED960F4F-0DAA-463F-A7AD-CB4707FC1F15
Derivation of name. The specific epithet refers to the geographical provenance of the type material, in the west part of France.
Holotype. Specimen MNHN.F.A71375, in a piece of opaque amber with other arthropods and plant fragments (see Section 2 above).
Type locality and stratum. France, Charente, La Buzinie, at Champniers, near Angoulême, lithological subunit B2ms.
Age. Early Cenomanian, Late Cretaceous.
Diagnosis. As for the genus (see above).
Description. Adult female, size very small (body length 10.20 mm). Specimen complete, except for broken ovipositor and antennae (Fig. 3a). Strong setae on the whole body, especially on legs, head and pronotum.
Head. Opisthognathous (Fig. 3c, d), rounded, almost as wide as high in front view (ratio head width / head length 91 %). Eyes relatively small and little protruding (maximal diameter 1.37 mm), separated by a distance of 1.60 mm. Median ocellus present (but head dorsal surface damaged). Fastigium wide, wider than scape, directly prolonging vertex. Scapes small, wider than high; antennae filiform, with more than 42 antennomeres. Maxillary palpi short; articles 1 and 2 very short, articles 3, 4 and 5 subequal (0.92 mm, 0.93 mm and 0.91 mm long respectively); article 5 regularly widened toward truncated apex. Clypeus short, trapezoidal.
Thorax. Pronotum wider than long in dorsal view (2.81 mm long, 3.26 mm wide), globally rounded (Fig. 3a, e). Dorsal disc not flattened; anterior margin slightly sinuated; posterior margin convex; both margins with a row of long and very strong setae. Lateral lobes well-developed; lower margin straight; posterior margin very wide in posterior angle. First (mesothoracic) spiracle not covered by pronotum lateral lobe. Metanotum c. 1.50 mm long, its posterior margin convex.
Wings. Right anterior forewing very short, rounded (Fig. 3a). Left forewing lacking. No visible hindwing.
Legs. Quite short, with strong setae; all femora with a wide ventral gutter; tarsi with three tarsomeres, second tarsomere tubular and not widened, claws long, simple, neither serrated nor bifid. Both fore legs present, but tarsi incomplete; right mid leg absent, left mid leg without tarsus; hind legs complete. Fore and mid femora slightly compressed laterally, longer than tibiae; fore femur 4.07 mm long, 0.39 mm wide; mid femur 3.66 mm long, 0.46 mm wide. Fore tibia 2.95 mm long; with a well-developed, longer than wide tympanum on inner side (Fig. 3h), no outer tympanum; two apical ventral spurs and no dorsal apical spurs (Fig. 3h); basitarsomere long and thin. Mid tibia 3.20 mm long; two ventral apical spurs. Hind femur wide and thick, longer than hind tibia (length 6.42 mm, maximal width 2.83 mm), with one row of dorsal setae and a very wide ventral gutter (Fig. 3f, g). Hind tibia 4.87 mm long; with three inner and three outer apical spurs (Fig. 3f, g), median spur the longest on both sides; with three inner and five outer subapical spurs, increasing in size toward tibia apex, especially on outer side; hind tibia not serrulated above and between subapical spurs, their margins clearly concave between subapical spurs. Hind tarsus: basitarsomere very long (Fig. 3b), more than half hind tibia length (2.10 mm long); with five spines on outer margin and at least two spines on inner margin; second tarsomere very short (about 1/7 basitarsomere length) and cuneiform; third tarsomere about half as long as basitarsomere, thin, with a pair of long, simple claws.
Abdomen. Length 4.50 mm; ovipositor broken before apex; cerci very long (6.20 mm long), relatively thick at base, with club-shaped setae at base on inner side and long filiform setae over whole length. Nine visible tergites and eight sternites; subgenital plate wider than long, posterior margin straight (Fig. 3b). Ovipositor not compressed laterally, almost straight and very slightly upcurved; at least 4.57 mm long; apex lacking.
Family OECANTHIDAE Blanchard, 1845
Subfamily PODOSCIRTINAE Saussure, 1878 sensu Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022)
Genus Picogryllus Josse and Desutter-Grandcolas, gen. nov.
urn:lsid:zoobank.org:act:F43887D6-3D16-4F22-821F-4551770624AE
Type species. Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov., here designated.
Derivation of name. Genus named after its very small size, fully unusual for winged males in crickets, and in Grylloidea in general. Gender masculine.
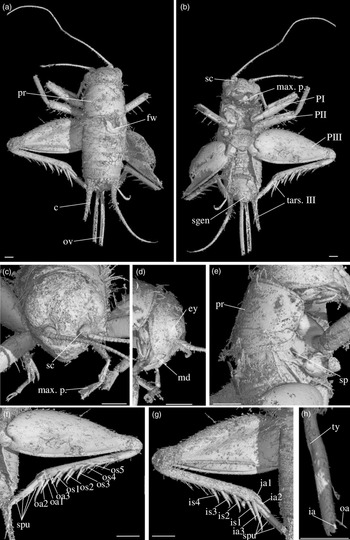
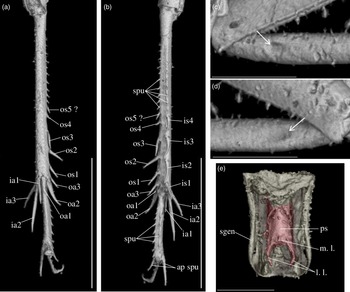
Diagnosis. Size very small. Head wider than long in front view; three ocelli (Fig. 4e); fastigium wider than scape (Fig. 4e); scapes relatively small, slightly wider than long; maxillary palpi (Fig. 4d) short, article 5 longer than article 3, small but regularly widened toward apex. Forewings very long, covering epiproct; hindwings longer than forewings, plicated along body axis and pointed. Stridulatory apparatus complete (file transverse, harp relatively narrow, mirror longer than wide, crossed by one or two veins: Fig. 4a). Fore tibiae with at least three apical spurs, two on inner side and one on outer side; with one inner and one outer tympana subequal in size (Fig. 5c, d), both longer than wide but not slit-shaped. Hind tibiae (Fig. 5a, b) serrulated, with long and thin spines on basal half, above subapical spurs; with four inner and five outer subapical spurs, all equal in length except outer spurs 4 and 5, much shorter; three inner and three outer apical spurs, median apical spurs the longest on both sides, subequal. All tarsi short with three tarsomeres (Fig. 4); hind basitarsomeres flattened dorsally, with two rows of dorsal spines; second tarsomeres dorsoventrally flattened; third tarsomeres long and thin, with a pair of long and thin claws, neither bifid, not serrated. Hind legs much longer than fore and mid legs, with thick femora. Cerci longer than abdomen, bearing club-shaped setae on inner base. Subgenital plate very long, subcarinated ventro-laterally. Male genitalia (Fig. 5e) symmetrical, with two median lophi on distal margin and two latero-distal, wider and hook-shaped lateral lophi; pseudepiphallic sclerite much longer than wide.
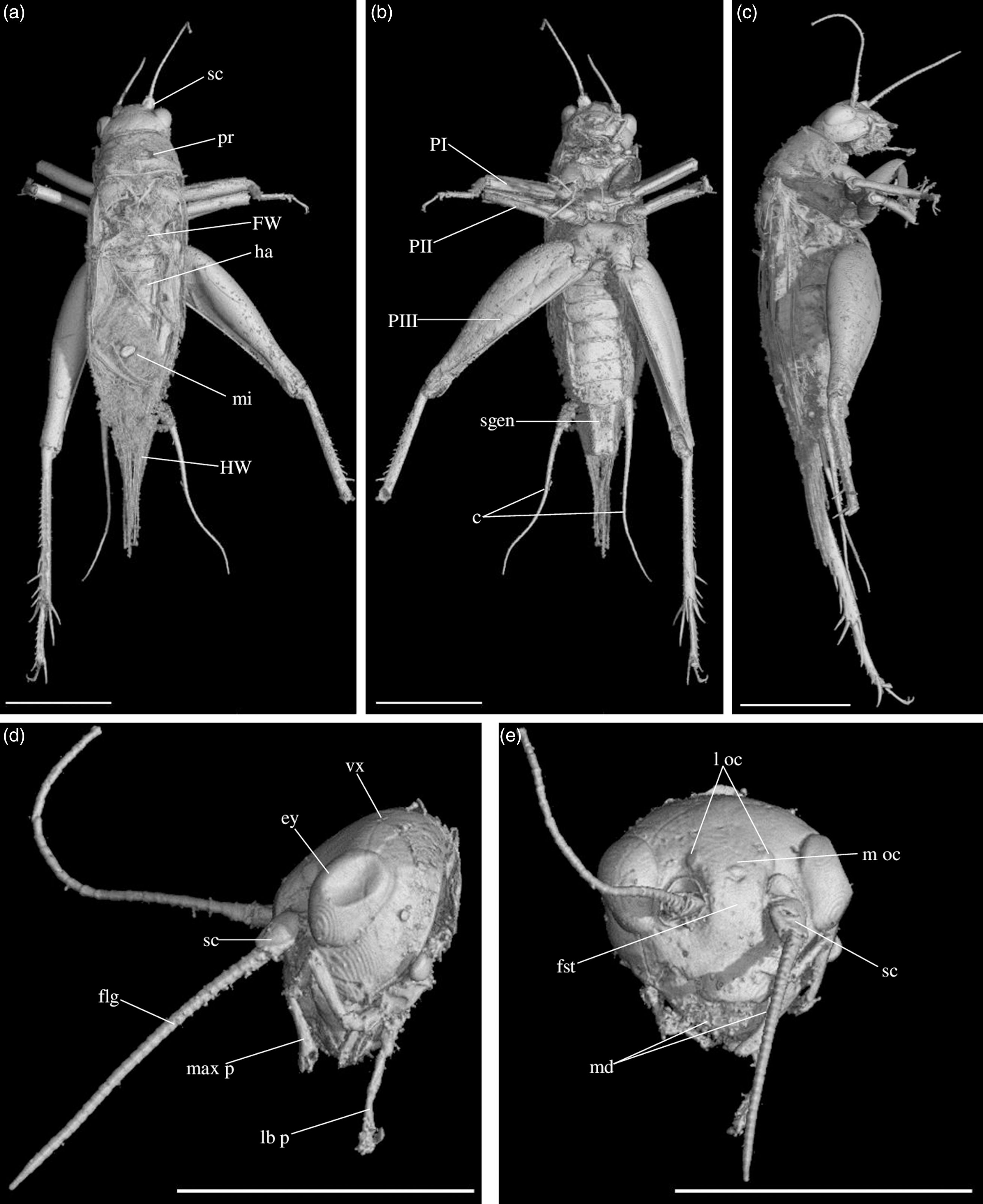
Fig. 4. Picogryllus carentonensis Josse and Desutter-Grandcolas, gen.nov., sp. nov. (male holotype, IGR-ARC-421.1) in mid-Cretaceous amber from Charentes (France): (a–c) male holotype in dorsal (a), ventral (b) and right side (c) views; (d–e) head in left side (d) and dorsal (e) views. Abbreviations: see Section 2. Scales 1 mm.

Fig. 5. Picogryllus carentonensis Josse and Desutter-Grandcolas, gen.nov., sp. nov. (male holotype, IGR-ARC-421.1) in mid-Cretaceous amber from Charentes (France): (a–b), hind tibia in posterior (a) and dorsal (b) views; (c–d), auditory tympana on fore tibia, on outer (c) and inner (d) sides; (e) extremity of male genitalia in dorsal view, in natural position in subgenital plate. Abbreviations: see Section 2. Scales 1 mm.
Differential diagnosis. Picogryllus Josse and Desutter-Grandcolas, gen. nov. can be identified as a cricket by the club-shaped setae on cerci, tarsi with three tarsomeres, hind legs adapted to jump, locations of apical and subapical spurs on legs, and stridulatory apparatus with a file, a harp and a mirror. Among Grylloidea, Picogryllus Josse and Desutter-Grandcolas, gen. nov. is excluded from Trigonidiidae by its serrulated hind tibiae, head shape, lack of strong setae on body, number of apical spurs on fore tibiae, shape of stridulum on male forewings, and male pseudepiphallic sclerite (see Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021). It is also excluded from Phalangopsidae by the number of spurs on fore tibiae, the shape of second tarsomere (not flattened in Phalangopsidae, hardly expanded in some phytophilous Phaloriinae) and the lack of serrulation between hind tibial subapical spurs.
Within the Gryllidae sensu Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022), Picogryllus Josse and Desutter-Grandcolas, gen. nov. is excluded from all subfamilies by the shape of pseudepiphallic median and lateral lophi. It is excluded from the Gryllinae by the presence of serrulation on hind tibiae (lacking in Gryllinae) and head shape (rounded in Gryllinae). It is excluded from the Eneopterinae by the shape of inner tympanum (slit-shaped in most Eneopterinae), the lack of spines between hind tibia subapical spurs (present in Eneopterinae), the fastigium shape (very wide in Eneopterinae) and the size and shape of subapical spurs (inners longer and curved in Eneopterinae). It is excluded from the Pentacentrinae by its general shape (Pentacentrinae are thin, elongate crickets, much resembling Trigonidiinae), complete stridulum (forewings with strong longitudinal veins and at most a file and short harp in Pentacentrinae), hind leg shape (hind basitarsomere of Pentacentrinae much longer and hind tibia much shorter than in Picogryllus Josse and Desutter-Grandcolas, gen. nov.) and head shape (eyes not at all prominent and antennal insertion widely separated from lateral ocelli in Pentacentrinae). It differs from Odontogryllus and related genera by the size and shape of forewing and stridulatory apparatus (forewings short lacking a complete stridulum in these taxa). It can easily be excluded from Itarinae by the shape of its fore wings and stridulum.
Within Oecanthidae sensu Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022), Picogryllus Josse and Desutter-Grandcolas, gen. nov. is excluded from Euscyrtinae by fastigium not flattened dorsally, forewings with developed stridulatory apparatus, hind tibia with at most five subapical spurs (more than six in Euscyrtinae), and inner margin of claws smooth (serrated in Euscyrtinae). It is excluded from Oecanthinae sensu Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022) by having inner ventral apical spur of hind tibiae well-developed (regressed or absent in Oecanthinae), forewing apical field well-developed although slightly shorter than mirror (reduced or absent in Oecanthinae), lateral field of forewings perpendicular to dorsal field in anterior and posterior views (forming an acute angle in Oecanthinae), and tarsal claws simple (bifid in Oecanthinae). It can be excluded from Tafaliscinae as its fastigium is wider than antennal scape in frontal view, its stridulatory file is not sinuous, and spines are absent between subapical spurs of hind tibiae. Picogryllus Josse and Desutter-Grandcolas, gen. nov. can be classified within Podoscirtinae by the shapes of its pseudepiphallic sclerite and lophi in male genitalia, head shape, scape shape, number of subapical spurs of hind tibiae, and stridulum shape.
Only three fossil taxa are currently classified as Podoscirtinae, i.e. Allopterites mutilineatus Cockerel, 1920 (incomplete hindwing; Lutetian, Eocene, England), Stenogryllodes brevipalpis Chopard, Reference Chopard1936 (male juvenile; Eocene Baltic amber) and Madasumma europensis Chopard, Reference Chopard1936 (adult female, dark amber, Lower Oligocene, Germany) (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 b; fossilworks data base at http://fossilworks.org). The position of S. brevipalpis within Podoscirtinae should probably be revised, as its hind tibiae are not serrulated and its fore tibiae have only two apical spurs. It differs anyway from Picogryllus Josse and Desutter-Grandcolas, gen. nov. by its maxillary palpi (article 5 short and wide), the spurs of its fore and middle tibiae (only two apical spurs), and its rounded (not serrulated) hind tibiae with four subapical spurs on each side and only two inner apical spurs.
Allopterites multilineatus is known by only one incomplete hindwing. Thus, it cannot be compared to Picogryllus Josse and Desutter-Grandcolas, gen. nov. in which the hindwings are plicated along the body, but the length of A. multilineatus wing is much too large to fit the new taxon (19 mm against 3.04 mm in Picogryllus Josse and Desutter-Grandcolas, gen. nov.).
Madasumma europensis has been described from a nearly complete adult female, mainly characterized by its small, rounded head, short palpi, little protruding eyes, relatively short legs, hind tibiae (well-serrulated at base, few spines between inner subapical spurs, four pairs of subapical spurs, three pairs of apical spurs), flattened second tarsomeres, hind basitarsomeres elongate with two rows of dorsal spines, setose pronotum and reduced forewing venation (Chopard, Reference Chopard1936; Zeuner, Reference Zeuner1939). It is difficult to compare it to Picogryllus Josse and Desutter-Grandcolas, gen. nov., but size clearly separates the two taxa (body length c. 11 mm in M. europensis).
Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov.
urn:lsid:zoobank.org:act:A9E4F523-329D-4285-8DCD-8A11C448DE2D
Derivation of name. The specific epithet is derived from ‘Carentonia’, the Latin name for the Charente River and Charentes region from which the type specimen originates.
Holotype. Specimen IGR-ARC-421.1, male, in a piece of opaque amber with several other arthropods (see Section 2 above).
Type locality and stratum. France, Charente-Maritime, Archingeay / Les Nouillers, Font-de-Benon quarry, lithological subunit A1sl-A.
Age. Latest Albian or earliest Cenomanian, Cretaceous.
Diagnosis. As for the genus (see above).
Description. Size very small for an adult Podoscirtinae cricket (body length 3.30 mm). Complete adult male, with cerci, but right hind leg broken at level of tibia and antennae broken before c. 30 articles.
Head. Opisthognathous (Fig. 4c, d), small compared to body, wider than high in front view (maximal width 0.70 mm); without clear setae insertion. Eyes not protruding and not very large (eye length 0.30 mm), almost reniform, broadly separated from posterior margin of cheek; separated from one another by a distance greater than 4× apical width of fastigium; minimal interocular distance 0.41 mm. Three large ocelli (Fig. 4e); distance between lateral ocelli twice that between median ocellus and one lateral ocellus; median ocellus subapical on fastigium; lateral ocelli on fastigium basis. Vertex slightly convex between eyes, not separated from fastigium (Fig. 4d). Fastigium wider than scape; not furrowed longitudinally (Fig. 4e). Antennae filiform, inserted between eyes. Antennal pits small, separated by a distance of 0.20 mm. Scapes wider than long, with convex inner margin (Fig. 4b). Maxillary palpi short with five articles (Fig. 4d); articles 1 and 2 very short; articles 3, 4 and 5 respectively 0.20 mm, 0.15 mm and 0.20 mm long; article 5 widened toward apex, with upper margin slightly concave. Labial palpi with five articles (Fig. 4d). Mandibles small and simple.
Thorax. Dorsal disc of pronotum transverse (Fig. 4a); anterior margin almost straight; posterior margin slightly bisinuated; median length 0.40 mm, anterior width 0.70 mm, posterior width 0.90 mm. Lateral lobes (Fig. 4c) quite high; lower margin straight. Metanotum not visible, covered by forewings (presence of glands not checked).
Legs. Short and robust; femora with a wide ventral gutter. All tarsi with three tarsomeres; first (most basal) tarsomere the longest; second tarsomere very short and flattened dorsoventrally; third tarsomere long and thin, with a pair of simple (= neither serrated, nor bifid) claws. Coxae widely separated (Fig. 4b). Fore femora 0.70 mm long; slightly compressed laterally; fore tibiae 0.65 mm long, with at least three apical spurs, with a tympanum on both inner and outer sides (Fig. 5c, d), both longer than wide and not slit-shaped, with two strong setae in ventral side; fore tarsus: length of tarsomeres 1, 2 and 3 respectively 0.05 mm, 0.01 mm and 0.03 mm. Mid legs very similar to fore legs, of same size, without tympanum; tibiae with three apical spurs. Hind femora long and very strong (length 1.80 mm, maximal width 0.50 mm); hind tibiae (Fig. 5a, b) shorter than hind femur (length 1.50 mm), with three inner and three outer apical spurs, inner spurs longer than outer spurs; outer spurs ao2 > ao1> ao3, inner spurs ai2 > ai3 > ai1, ventral spurs ai1 > ao1; in tibia distal half, four inner and five outer subapical spurs, all short and subequal in length, but so4 and so5 distinctly shorter; tibiae serrulated on both inner and outer margins, above subapical spurs; one spine perhaps present between si1 and si2. Hind basitarsomeres very long (length 0.50 mm), flattened dorsally, with two rows of dorsal spines (five inner, eight outer), with two apical spurs, inner spur longer than outer spur but slightly shorter than tarsomeres 3.
Wings. Forewings and hindwings both present and well-developed, covering posterior part of thorax and abdomen beyond epiproct (Fig. 4a, b). Forewings not reaching distal margin of subgenital plate; 2.60 mm long, maximal width 0.90 mm; almost completely overlapping. Stridulatory apparatus complete (Fig. 3a), occupying 75 % of dorsal field length; stridulatory file transverse, slightly oblique; harp relatively narrow; mirror longer than wide, crossed by one (or two?) parallel transverse veins, anterior angle wide. Other characters of venation: innermost chord separated at base from chord 2, diagonal slightly bifurcated, mirror bordered by a very wide distal cell, apical field long with large cells; lateral field wide, with parallel veins perpendicular to forewing outer margin. Hindwings (length 3.04 mm) plicated along body and extending beyond abdomen, between cerci.
Abdomen. Tergites completely hidden by forewings. Nine sternites, sternite 9 forming the subgenital plate. Cerci long and thin, 1.80 mm long, longer than abdomen, complete; some club-shaped setae on basal inner side. Subgenital plate (Figs. 4b, 5e) very long (median length 0.40 mm) and high, rectangular, latero-ventrally subcarinated; posterior margin truncated and sinuated.
Phallic complex. Fig. 5e. Pseudepiphallic sclerite long and narrow; distal margin with two small median process, that could correspond to median lophi, and a pair of lateral hook-shaped structures that could be the lateral lophi.
4. Discussion
Fossils have long been used in evolutionary biology as calibration points, placed a posteriori of phylogenetic analyses on a resultant topology for dating clade emergence and diversification. But today, fossils tend to be introduced in the data matrix as terminals (Edgecombe, Reference Edgecombe2010), as for example in the fossilized birth–death approach (Heath et al. Reference Heath, Huelsenbeck and Stadler2014). The main consequences of this methodological advance have been to revive the phylogenetic study of morphological characters, to link past and extant taxa in the same evolutionary dynamics, and reconcile the taxonomies developed for fossils and for extant taxa (Flores et al. Reference Flores, Bippus, Suárez and Hyvönen2021).
The evolutionary study of Ensifera (Insecta, Orthoptera) primarily considers the emergence of acoustic communication in katydids (Tettigoniidea, Tettigonioidea), crickets s. l. (Gryllidea, Grylloidea and Gryllotalpoidea) and grigs (Tettigoniidea, Hagloidea), to test whether it appeared only once within Ensifera (Bailey, Reference Bailey1991; Otte, Reference Otte1992; Béthoux, Reference Béthoux2012; Chivers et al. Reference Chivers, Béthoux, Sarria-S, Jonsson, Mason and Montealegre-Z2017; Song et al. Reference Song, Béthoux, Shin, Donath, Letsch, Liu, McKenna, Meng, Misof, Podsiadlowski, Zhou, Wipfler and Simon2020), twice independently in katydids+grigs and crickets (Gwynne, Reference Gwynne1995), or multiple times in Ensifera, within a general frame of communication not limited to calling with a wing stridulum (Desutter-Grandcolas, Reference Desutter-Grandcolas2003; Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Jacquelin, Hugel, Boistel, Garrouste, Henrotay, Warren, Chintauan-Marquier, Nel, Grandcolas and Nel2017). The Ensifera is a very ancient clade, with representatives known as early as the Middle Permian or even before that (see reference in Nel, Reference Nel2021). This includes the Tettigonioidea Permotettigonia gallica Nel & Garrouste, 2016, which presents the venational apomorphies of Tettigoniidae, even if Gorochov (Reference Gorochov2021: p. 557) excluded it from this clade without giving any reason and, instead, indicated that it ‘may be a member of the Kamiinae or some other group of possible ecological counterparts of Tettigonioidea in the Oedischiidea’. But Kamia angustovenosa Martynov, 1928 and the Oedischiidea have forewings with numerous branches of CuA+CuPaɑ and of M, unlike Permotettigonia and the Tettigoniidae (see Sharov, Reference Sharov1968). These oldest fossils are isolated wing imprints, which raises the problem, still fiercely debated, of vein homologies (Chivers et al. Reference Chivers, Béthoux, Sarria-S, Jonsson, Mason and Montealegre-Z2017; Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Jacquelin, Hugel, Boistel, Garrouste, Henrotay, Warren, Chintauan-Marquier, Nel, Grandcolas and Nel2017; Schubnel et al. Reference Schubnel, Desutter-Grandcolas, Legendre, Prokop, Mazurier, Garrouste, Grandcolas and Nel2020 b), reconsidered recently thanks to the use of X-ray microtomography (Schubnel et al. 2000). Up to now, ensiferan fossils have been used as calibration points in molecular studies (Song et al. Reference Song, Amédégnato, Cigliano, Desutter-Grandcolas, Heads, Huang, Otte and Whiting2015, Reference Song, Béthoux, Shin, Donath, Letsch, Liu, McKenna, Meng, Misof, Podsiadlowski, Zhou, Wipfler and Simon2020; Chang et al. Reference Chang, Qiu, Yuan, Wang, Li, Sun, Guo, Lu, Feng, Majid and Huang2020) but the choice of the fossils strongly biased the resulting dating, as shown by Nel (Reference Nel2021).
The fossil record currently available for crickets is quite poor and not optimal for total-evidence analyses, including mostly imprints and recent fossils in Cenozoic amber inclusions. The discovery of several fossil crickets in amber-rich deposits from the Early–Late Cretaceous allowed for detailed descriptions, similar to or even better than extant taxa. This state of preservation means that potentially most characters used in a data matrix for extant taxa could be observed and described on these fossils, opening a wide field of characters and character states for phylogenetic studies (Kearney & Clark Reference Kearney and Clark2003). All these Cretaceous amber fossils are of small sizes, and most are juveniles, i.e. Marchandia magnifica Perrichot, Néreaudeau et al., Reference Néraudeau, Perrichot, Dejax, Masure, Nel, Philippe, Moreau, Guillocheau and Guyot2002, Burmagryllotalpa longa Wang et al., Reference Wang, Lei, Zhang, Xu, Fang and Zhang2020, Tresdigitus rectanguli Xu et al., Reference Xu, Fang and He2020 a, T. gracilis Jiang et al., Reference Jiang, Xu, Jarzembowski and Xiao2022, Pherodactylus micromorphus Poinar et al., Reference Poinar, Su and Brown2020 and Protomogoplistes asquamosus Gorochov, Reference Gorochov2010.
The Early Cretaceous diversity of mole crickets (Gryllotalpidae) is attested by M. magnifica, B. longa, T. rectanguli, T. gracilis, Chunxiania fania Xu et al., Reference Xu, Zhang, Jarzembowski and Fang2020 b and the shapes of their head, pronotum and/or fore legs (Perrichot et al. Reference Perrichot, Néraudeau, Azar, Menier and Nel2002; Wang et al. Reference Wang, Lei, Zhang, Xu, Fang and Zhang2020; Xu et al. Reference Xu, Fang and He2020 a, Reference Xu, Wang, Fang, Jarzembowski and Zhuo2022). This confirms previous discoveries of oldest mole cricket representatives from the Aptian Formation of Brazil (125–113 Ma in age; Martins-Neto, Reference Martins-Neto1991, Reference Martins-Neto1995). Based mostly on its head shape, the supposedly Late Cretaceous Protomogoplistes asquamosus has been ascribed to the Mogoplistidae cricket family (see Gorochov, Reference Gorochov2010), a hypothesis supported by the serrulated hind tibia without subapical spurs.
Pherodactylus micromorphus has been described as a juvenile female because of its short ovipositor and undeveloped hindwings, although it presents a clear tympanum on its fore tibia, a character usually observed in adults. It has been designated as the type genus of a ‘Gryllidae’ family, with the following diagnostic characters: ‘head without dorsal bristles, tympana on the outer fore tibia, tarsi three-segmented with 1st tarsomere longer than the other two combined, hind tibia shorter than hind femur; long terminal spurs on hind tibia with spines on proximal portion of hind tibia’ (Poinar et al. Reference Poinar, Su and Brown2020: p. 34). Among these characters, the lack of dorsal bristles on head dorsum is observed in the Gryllidae: Gryllinae. But the presence of three tarsomeres is a plesiomorphy in Gryllidea; a basitarsomere longer than tarsomeres 2+3 and the presence of an outer tympana are observed in nearly all cricket clades; a hind tibia shorter than hind femur is observed in many cricket clades (for example in Nemobiinae, some Phalangopsidae, or many Gryllidae and Oecanthidae); long ‘terminal’ (= apical) spurs on hind tibia are also plesiomorphic in Grylloidea, while the presence of spines on the proximal part of hind tibia is a character largely observed in crickets.
The diagnosis of the genus Pherodactylus Poinar et al., Reference Poinar, Su and Brown2020 itself is as follows: ‘Overall body shape and color typical of the Gryllidae. Body mostly brown, covered with short fine hairs and bearing undeveloped wing pads. Head without prominent bristles. Pronotum longer than wide; middle of pronotal disk with two distinct large dark “eyespots”. Forelegs robust, with three apical spurs arranged on inner side of fore tibia.’ Among these characters, the ‘eyespots’ on pronotum correspond to muscular insertions that exist in all Gryllidea, while wing pads are related to the development stage of the fossil at death. In fact, Pherodactylus certainly belongs to the Gryllidae family, as shown by the three apical spurs on fore tibia, with however a unique specialization (spurs all on inner side of tibia). The lack of setae on head dorsum (apomorphy) and of subapical spurs on hind tibia (apomorphy) are considered characters of the subfamily Gryllinae: they allow the assignment of the remarkable fossil of Pherodactylus micromorphus in this subfamily, as proposed by Poinar et al. (Reference Poinar, Su and Brown2020), but using a different set of characters.
Adult cricket fossils are exceedingly rare in Mesozoic amber. They include Birmaninemobius hirsutus Xu et al. Reference Xu, Zhang, Jarzembowski and Fang2020 b from earliest Cenomanian Burmese amber (98.79 ± 0.62 Ma), which belongs to the Trigonidiinae (Trigonidiidae) as it shares several of the apomorphies used to describe this subfamily (Xu et al. Reference Xu, Zhang, Jarzembowski and Fang2020 b; Desutter-Grandcolas et al. Reference Desutter-Grandcolas, Hugel, Nel, Warren, Souza Dias and Chintauan-Marquier2021); the oecanthine Birmanioecanthus haplostichus Yuan et al., Reference Yuan, Zheng, Zheng, Ma and Gu2022 and Apiculatus cretaceus Yuan et al., Reference Yuan, Zheng, Zheng, Ma and Gu2022, and the two fossils from Albian–Cenomanian French amber described in the present paper, i.e. Palaeonemobius occidentalis Laurent and Desutter-Grandcolas, gen. nov., sp. nov. (Trigonidiidae, Nemobiinae) and Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov. (Oecanthidae, Podoscirtinae sensu Campos et al. Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022). The latter species are the oldest representatives of their respective subfamily, pushing back the presence of Nemobiinae and Podoscirtinae from the Eocene to the mid-Cretaceous, c. 100 Ma. Palaeonemobius occidentalis Laurent and Desutter-Grandcolas, gen. nov., sp. nov., together with B. hirsutus, are congruent with the calibration of the cricket family Trigonidiidae. In the same way, Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov., together with Birmanioecanthus haplostichus and Apiculatus cretaceus, are congruent with the presence of the sister subfamilies Oecanthinae and Podoscirtinae in the early- to mid-Cretaceous, pushing back the diversification of Oecanthidae and Gryllidae deeper in the past as reconstructed by Campos et al. (Reference Campos, Souza-Dias, Audino, Desutter-Grandcolas and Nihei2022) for the first time.
Taken together, these Cretaceous amber taxa include representatives of both cricket superfamilies (Grylloidea, Gryllotalpoidea), and of five of the seven main cricket clades (Gryllotalpidae, Mogoplistidae, Trigonidiidae, Gryllidae and Oecanthidae) acknowledged today on phylogenetic bases. It should be added that the Early Cretaceous occurrence of ant-loving crickets family Myrmecophilidae is also attested by Araripemyrmecophilops gracilis Martins-Neto, Reference Martins-Neto1991. Finally, the only cricket family not yet discovered in the Cretaceous is the Phalangopsidae, documented only by a few recent fossils, from the Middle Eocene (Electrogryllus septentrionalis (Chopard, Reference Chopard1936), Eozacla Gorochov, 2012 in Gorochov & Labandeira (Reference Gorochov and Labandeira2012), Eotrella Gorochov, 2012 in Gorochov & Labandeira (Reference Gorochov and Labandeira2012)) and the Early Miocene (Araneagryllus dylani Heads, Reference Heads2010) (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2022 b). As the sister group of the clade (Gryllidae + Oecanthidae), the Phalangopsidae was already present during the Cretaceous. The Phalangopsidae are most often large crickets that may either escape when partly glued in resin, or get ruined during fossilization in the standing water of ancient lakes in which the Orthoptera have a general tendency to float and decay before being buried. Thanks to the Cretaceous amber deposits, all the main cricket clades proved to be already present and diversified by the Early–Late Cretaceous boundary. These data will help calibrate future phylogenetic analyses of crickets, using a large sample of taxa to take into account their huge morphological diversity.
As said above, amber crickets are actually of very small sizes, and Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov. is the smallest known adult male cricket (3.3 mm long) with a full, functional stridulatory apparatus ever documented in the whole infra-order. It should be noted in this respect that although extremely small for a cricket, Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov. is far bigger than the smallest insects ever found and does not present the morphological reduction currently associated with miniaturized insects (see Polilov, Reference Polilov2015; Minelli & Fusco, Reference Minelli and Fusco2019): on the contrary, it looks like a perfect cricket! Its very small size is anyhow a puzzling trait as far as the functioning of its stridulatory apparatus and its putative acoustic signals are concerned. The smaller the insects, the higher the frequency and the lower the intensity of the acoustic signals: Picogryllus carentonensis Josse and Desutter-Grandcolas, gen. nov., sp. nov. certainly emitted acoustic signals to reproduce, but these signals may have been quite high in frequency, with a poor propagating range in the environment and so little efficiency to attract potential mates (Bennet-Clark, Reference Bennet-Clark1998). Reducing the power of an acoustic signal is a common way to avoid acoustically orienting predators. In katydids (Tettigonioidea), a modern acoustic communication may exist since the Jurassic (Gu et al. Reference Gu, Montealegre-Z, Roberts, Engel, Qian and Ren2012) and predation may have been a burden since the Permian (Garrouste et al. Reference Garrouste, Hugel, Jacquelin, Rostan, Steyer, Desutter-Grandcolas and Nel2016). Crickets, yet more ancient than katydids (Nel, Reference Nel2021), may have faced these biological interactions even deeper in the past and circumvented them with novel communication systems: emitting high-frequency calls in a world without bats may have been one of them.
Acknowledgements
We warmly thank Thierry Lenglet for the donation of the amber piece from La Buzinie, and the Marchand family for permitting access to the Font-de-Benon quarry and collection of amber. We are also grateful to Didier Néraudeau for his efforts and multiple contributions to the collection and study of Charentese amber; Paul Tafforeau and Malvina Lak (ESRF) for their contribution to the synchrotron imaging of the specimens; and two anonymous reviewers who improved our manuscript.
Funding statement
Financial support for field studies, collection, and synchrotron imaging of Charentese amber was provided by the French National Research Agency (ANR, project AMBRACE nº BLAN07-1-184190 to D. Néraudeau). Support for imaging used in the present study was provided by the ESRF through attribution of beamtime on the beamline ID19.
Declaration of interest
The authors of the paper declare no conflict of interest.