Insulin-like growth factor (IGF) axis has a role in the maintenance of normal glucose regulation and it has been suggested to be connected with the development of glucose metabolism disorders( Reference Lewitt, Dent and Hall 1 ). Prospective studies have mostly observed no association between serum total IGF-I concentrations and the risk of type 2 diabetes or impaired glucose tolerance( Reference Lewitt, Hilding and Östenson 2 – Reference Drogan, Schulze and Boeing 5 ) but also positive( Reference Lewitt, Hilding and Brismar 6 ) and inverse( Reference Sandhu, Heald and Gibson 7 ) associations have been published. Serum IGF binding protein-1 (IGFBP-1) concentration has associated inversely with the risk of diabetes( Reference Petersson, Östgren and Brudin 3 , Reference Rajpathak, He and Sun 4 ) but the association has disappeared in some studies after adjustment for different covariates( Reference Lewitt, Hilding and Östenson 2 , Reference Lewitt, Hilding and Brismar 6 ). Serum IGFBP-3 concentration, instead, has related positively with the risk of diabetes( Reference Rajpathak, He and Sun 4 , Reference Drogan, Schulze and Boeing 5 ). At the same time, the IGF system is involved in development and function of visceral adiposity and obesity phenotype( Reference Lewitt 8 ).
It is an open subject whether intakes of macronutrients influence the pathogenesis of type 2 diabetes through the IGF proteins. Clinical trials have suggested that additional dairy products or dairy protein increase serum IGF-I concentration( Reference Heaney, McCarron and Dawson-Hughes 9 , Reference Zhu, Meng and Kerr 10 ). Similarly cross-sectional studies have reported positive associations between total and dairy protein intake and serum IGF-I( Reference Beasley, Wedick and Rajpathak 11 – Reference Holmes, Pollak and Willett 13 ). Data on the relationships between intake of macronutrients and IGFBP is, however, scarce and inconsistent( Reference Beasley, Wedick and Rajpathak 11 – Reference Holmes, Pollak and Willett 13 ).
The aim of this study was to examine in a prospective nested case–control design the associations of serum IGF-I, IGFBP-1, and IGFBP-3 concentrations with the risk of type 2 diabetes among middle-aged male smokers. Another aim was to study whether macronutrient intakes were associated with the serum IGF proteins related to the risk of diabetes.
Methods
Study population
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study was a randomised, double-blind, placebo-controlled primary prevention trial testing whether supplementation with α-tocopherol, β-carotene or both would reduce the incidence of lung cancer and other cancers( 14 ) (ClinicalTrials.gov identifier: NCT00342992). A total of 29 133 Finnish male smokers were recruited between 1985 and 1988 from the total male population aged between 50 and 69 years in southwestern Finland (n 290 406). The ATBC Study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the institutional review boards of the National Public Health Institute of Finland (Helsinki, Finland) and the United States National Cancer Institute (Bethesda, MD, USA). Each participant provided written informed consent at baseline.
Data collection and dietary assessment
At baseline, participants completed a demographic, general medical, physical activity and smoking history questionnaire. Height and weight were measured and fasting venous samples were taken by trained study nurses. Serum was separated and stored in glass tubes at –70°C until analyses.
Diet was assessed at baseline using a self-administered, validated FFQ( Reference Pietinen, Hartman and Haapa 15 ). The questionnaire included 276 food items and mixed dishes. Frequencies of consumption of foods were reported as number of times per day, week or month within the previous 12 months. The questionnaire was used with a picture booklet of 122 photographs of foods, each with three to five different portion sizes, to estimate the usual portion size of foods. The trained study nurses checked the questionnaires with the participants before return.
Nutrient intakes were calculated using the national food composition database and nutrient intake calculation software at the National Institute for Health and Welfare, Finland. Intakes of macronutrients were calculated as a percentage of total energy intake (E%).
Definition of diabetes
Incident diabetes cases were identified from the register of reimbursement for costs of diabetes medication. In Finland, patients needing medical treatment for diabetes are entitled to reimbursement of their medication expenses according to sickness insurance legislation. This necessitates a detailed medical certificate from the attending physician. The certificate is verified to fulfil the diagnostic criteria for diabetes at the Social Insurance Institution (Helsinki, Finland), which maintains a central register of all persons receiving drug reimbursement. The ATBC Study participants were linked to the register through the unique personal identity number assigned to each Finnish citizen.
We excluded from the study the men who at baseline were entitled to reimbursement of diabetes medication, reported using diabetes medication or having physician-diagnosed, diet-treated diabetes or had fasting serum glucose ≥7·0 mmol/l. Since there is usually delay from the appearance of abnormal glucose regulation to diabetes diagnosis and further registration into drug imbursement register, we also excluded cases registered within 2 years after baseline. In addition, both cases and controls had to have fasting (>10 h) serum sample sufficient for IGF protein determinations and adequately filled food frequency questionnaire for calculation of macronutrient intakes.
The follow-up for incident diabetes cases continued for 12 years (up to December 1997). The controls were randomly drawn from the study cohort and were matched by age (±2 years), recruitment day (±60 d) and intervention group to the cases. We first carried out an exploratory analysis of IGF protein concentrations among 132 incident diabetes cases of the placebo group and their controls. Calculations based on these results demonstrated that about 170 incident cases are needed for a study to have 80 % power to detect a significant difference in IGFBP-1 concentration between cases and controls. Accordingly, we decided to double the number of cases to ensure adequate study power. Thus, we selected randomly, in addition to the case–control pairs from the placebo group, sixty-seven incident diabetes cases and matched controls from each three intervention groups (201 pairs altogether) as it has been previously shown that the interventions had no effect on diabetes risk( Reference Kataja-Tuomola, Sundell and Männistö 16 ). However, twenty-three case–control pairs were excluded because of inadequate serum sample or food consumption data. Thus, the final number of subjects was 310 incident diabetes cases and 310 matched controls.
Laboratory assays
Serum IGF-I, IGFBP-1 and IGFBP-3 concentrations were determined in the research laboratory of Women’s Hospital (Helsinki, Finland). IGF-I concentration was measured by enzyme-linked immunosorbent assay kit (Diagnostic Systems Laboratories) with intra-assay CV 4·5–7·1 % and inter-assay CV 4·8–8·8 %, as reported by the manufacturer. IGFBP-1 was determined by immunofluorometric assay using two monoclonal antibodies with intra-assay CV of 3–11 % and inter-assay CV of 4–10 %( Reference Koistinen, Koistinen and Selenius 17 ). IGFBP-3 was measured by monoclonal immunofluorometric assay with intra-assay CV of 3·6–6·2 % and inter-assay CV of 5·4–11 %( Reference Koistinen, Seppälä and Koistinen 18 ).
Serum glucose and insulin measurements were performed in the biochemistry laboratory of the Genomics and Biomarkers Unit at National Institute for Health and Welfare (Helsinki, Finland). The laboratory has been accredited by Finnish Accreditation Service and it fulfils the requirements of the standards SFS-EN ISO/IEC 17025:2005. The methods were hexokinase photometric assay for glucose and microparticle enzyme immuno assay (MEIA, Abbott Laboratories) for insulin. For standardising measurements, the laboratory has taken part in External Quality Assessment Schemes, organised by Labquality, Helsinki, Finland. During the course of the laboratory measurements, the mean CV% of the method was 2·0 % (SD 0·2) for glucose and 4·9 % (SD 1·8) for insulin.
Statistical analysis
Baseline characteristics of cases and controls were compared by using the Wilcoxon signed-rank test. OR and 95 % CI for incident diabetes in quartiles and per one unit (ng/ml) increase of IGF-I, IGFBP-1, and IGFBP-3 were estimated using conditional logistic regression. Model 1 was unadjusted crude model and model 2 was adjusted for BMI; both models were stratified by case–control pairs matched by age, recruitment day and intervention group. Test for trend was conducted using the quartile indicator (1–4) as a continuous variable and testing the significance with the Wald test. As well, significance of OR of the continuous variables was tested using the Wald test.
We also analysed the associations excluding men whose follow-up ended within four years from the baseline to further exclude cases that may have had abnormal glucose regulation at baseline, but the results did not change essentially. In this analysis, 277 cases and 287 controls were included and unconditional logistic regression analysis was used.
Effect modification was tested with likelihood ratio test by including interaction term of IGF variable and the possible effect modifier (BMI and insulin) in the analyses of associations of IGF variables and diabetes risk.
Since IGFBP-1 is dependent on BMI, we also conducted exploratory analysis to evaluate the association of BMI-adjusted IGFBP-1 with incident diabetes (residuals from a linear regression model in which BMI was the independent variable and IGFBP-1 the dependent variable, IGFBP-1 was log transformed to normalise the distribution).
As exploratory analysis we evaluated the associations of macronutrient intakes (carbohydrates, protein, and fat, as E%) and IGFBP-1 (IGFBP-1 log transformed to normalise the distribution) using linear regression models. Since protein intake as a whole group was not associated with IGFBP-1, in contrast to intake of carbohydrates and fat, we explored if this was explained by differences between the associations of protein subgroups (protein from meat, milk, and plant products) with IGFBP-1. Since IGF-I and IGFBP-3 concentrations were not associated with the risk of diabetes, we did not explore the associations between macronutrient intakes and these IGF proteins. All analyses were carried out with R( 19 ).
Results
At baseline, mean age of incident diabetes cases and controls was 56·8 years (Table 1). BMI of cases was higher (mean 29·6 kg/m2) than of controls (mean 26·2 kg/m2). Serum concentration of IGFBP-1 was lower among cases compared with controls. IGF-I or IGFBP-3 did not differ between cases and controls. Serum glucose and insulin of cases were higher than that of controls. Intake of carbohydrates was lower and intake of protein higher among cases, whereas intake of total energy, alcohol, fat or fibre did not differ significantly between cases and controls.
Table 1 Baseline characteristics of incident diabetes cases and controls (Mean values and standard deviations)
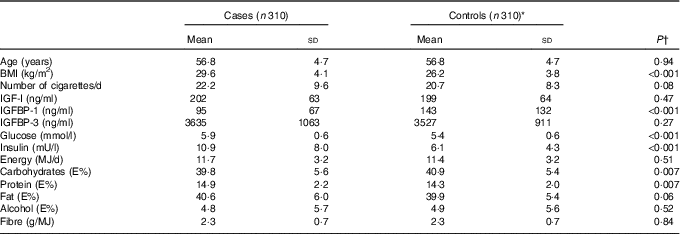
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; E%, percentage of total energy intake.
* Controls were matched to cases by age, recruitment day and intervention group.
† Differences between cases and controls were tested using the Wilcoxon signed-rank test.
IGF-I was not associated with the incidence of diabetes (Table 2). IGFBP-1 was inversely associated with the risk of diabetes in model 1: the OR was 0·25 (95 % CI 0·15, 0·42) in the highest quartile compared with the lowest quartile (P for trend <0·005). The association, however, was not significant when BMI was added to the model 2 (OR 0·76, 95 % CI 0·41, 1·40, P for trend 0·08). BMI-adjusted IGFBP-1 as a continuous variable associated negatively with diabetes risk: per one unit (ng/ml) increase the OR was 0·9967 (95 % CI 0·9936, 0·9998, P value 0·04) in the model adjusted for BMI (Table 3). IGFBP-3 was not associated with diabetes risk.
Table 2 Risk for incident diabetes in quartiles and per one unit (ng/ml) increase of insulin-like growth factor-I (IGF-I), IGF binding protein (IGFBP)-1, and IGFBP-3 using conditional logistic regressionFootnote * (Odds ratios and 95 % confidence intervals)
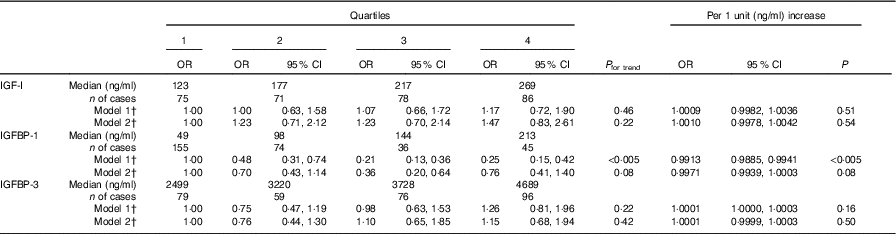
* P values were tested using the Wald test.
† Model 1: unadjusted crude model; model 2: adjusted for BMI; both models were stratified by case–control pairs matched by age, recruitment day and intervention group.
Table 3 Risk for incident diabetes in quartiles and per one unit increase of BMI-adjusted insulin-like growth factor binding protein-1 (IGFBP-1)Footnote * using conditional logistic regressionFootnote † (Odds ratios and 95 % confidence intervals)

* Residuals, IGFBP-1 log-transformed to normalise the distribution.
† P values were tested using the Wald test.
‡ Model 1: unadjusted crude model; model 2: adjusted for BMI; both models were stratified by case–control pairs matched by age, recruitment day and intervention group.
Serum concentration of IGFBP-1 correlated inversely with BMI, glucose and insulin (P<0·005): Pearson correlation coefficients were for BMI –0·45, for glucose –0·28 and for insulin –0·35.
No significant effect modification of BMI or insulin on the associations between IGF variables and diabetes risk was observed (P values ≥0·2).
Higher intake of carbohydrates and lower intake of fat were associated with higher IGFBP-1 in the model 2 adjusted for the matching variables and BMI (Table 4). Higher intake of plant protein and of milk protein associated with higher IGRBP-1, but higher intake of meat protein with lower IGFBP-1. The total protein intake did not associate with IGFBP-1 concentration.
Table 4 Change (%) in insulin-like growth factor binding protein-1 (IGFBP-1) per unit increase in intake of carbohydrates, proteins, and fat (percentage of total energy intake) from linear regression models (n 620)Footnote *
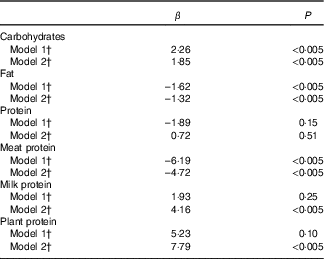
* IGFBP-1 log-transformed to normalise the distribution.
† Model 1: adjusted for age, recruitment day and intervention group (matching variables); model 2: adjusted for age, recruitment day, intervention group, BMI.
Discussion
In this study, a strong inverse association between IGFBP-1 and diabetes risk was observed among male smokers in the unadjusted crude model. However, the association was no more significant after adjustment for BMI. This result is in line with a Swedish case–control prospective study where among men aged 35–56 years low fasting IGFBP-1 concentration predicted the development of type 2 diabetes (n 107) during a 10-year period but after adjustment for waist2:height ratio and proinsulin the association disappeared( Reference Lewitt, Hilding and Östenson 2 ). Among Swedish women aged 35–56 years who developed type 2 diabetes (n 60) during a 8-year follow-up, low baseline IGFBP-1 was also positively associated with the risk of diabetes but the association was attenuated when adding waist2:height ratio to the model( Reference Lewitt, Hilding and Brismar 6 ). Among Swedish women and men aged 40–59 years, IGFBP-1 associated inversely with the risk of diabetes (forty-two incident cases during 17-year observation period) in a multivariate model not adjusted for obesity( Reference Petersson, Östgren and Brudin 3 ). In the prospective Nurses’ Health Study among women aged 30–55 years (742 case–control pairs, median time to diabetes 9 years), an inverse association was apparent in multivariate a priori model including also BMI as a covariate( Reference Rajpathak, He and Sun 4 ).
The IGF system is involved in the development and function of visceral adiposity( Reference Lewitt 8 ). In nondiabetic subjects, it has been shown that IGFBP-1 is inversely correlated with liver fat and is a marker of hepatic insulin sensitivity( Reference Kotronen, Lewitt and Hall 20 ). IGFBP-1 is suppressed in relation to the increase of insulin concentration in obesity( Reference Lewitt, Hilding and Östenson 2 , Reference Lewitt, Hilding and Brismar 6 ). Also in our study, IGFBP-1 correlated inversely with BMI, glucose and insulin. In the progression to type 2 diabetes, IGFBP-1 is, however, less suppressed and is again higher( Reference Lewitt, Hilding and Östenson 2 , Reference Lewitt, Hilding and Brismar 6 ). It is possible that the highest quartile of IGFBP-1 in our study included both non-obese, high insulin sensitive subjects and the subjects who have increased IGFBP-1 along to emerging diabetes progression and this may have attenuated the associations. We also analysed the associations excluding men whose follow-up ended within four years from the baseline, but it had no crucial effect on the association.
To study further the BMI-independent association of IGFBP-1 with diabetes risk, we analysed the association of BMI-adjusted IGFBP-1 with the risk of diabetes. There was a negative association as a continuous variable: one unit (ng/ml) increase of BMI-adjusted IGFBP-1 associated with 0·33 % lower risk of diabetes. The difference of BMI-adjusted IGFBP-1 medians in quartile 1 (51 ng/ml) and quartile 4 (178 ng/ml) was 127 units, thus the potential impact of BMI-adjusted IGFBP-1 on diabetes risk seems meaningful.
In this study, total IGF-I and IGFBP-3 were not associated with the risk of type 2 diabetes. Our result of IGF-I and diabetes risk is in accordance with the results from a former nested case–cohort study of middle-aged women and men (2269 subjects, 776 individuals with incident diabetes, mean follow-up time 7 years) within the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Potsdam( Reference Drogan, Schulze and Boeing 5 ), from the Nurses’ Health Study( Reference Rajpathak, He and Sun 4 ) and from the Swedish studies among men( Reference Lewitt, Hilding and Östenson 2 ) and both genders( Reference Petersson, Östgren and Brudin 3 ) but in contrast to positive association among the Swedish women( Reference Lewitt, Hilding and Brismar 6 ) and to inverse association among women and men in UK (fifty-one cases, 4·5 years of follow-up)( Reference Sandhu, Heald and Gibson 7 ). In contrast to our study, both in the EPIC-Potsdam Study and the Nurses’ Health Study IGFBP-3 was positively associated with diabetes risk( Reference Rajpathak, He and Sun 4 , Reference Drogan, Schulze and Boeing 5 ).
Intake of carbohydrates, plant protein and milk protein were positively associated with IGFBP-1 in this study. Intake of meat protein and fat associated negatively. A former cross-sectional analysis of 1142 men and 3589 women taking part in the EPIC Study did not report corresponding associations( Reference Crowe, Key and Allen 12 ). Instead, among 558 controls enrolled in a nested case–control study within the Nurses’ Health Study of incident type 2 diabetes, carbohydrate and dairy protein intakes were positively associated with IGFBP-1 concentration and fat intake negatively associated in a model adjusted for age and race/ethnicity( Reference Beasley, Wedick and Rajpathak 11 ) but the associations were not evident in further adjusted models.
It is possible that the associations between intakes of macronutrients and IGFBP-1 are indirect. IGFBP-1 is secreted primarily from hepatocytes and the synthesis is inhibited by portal insulin( Reference Lewitt, Dent and Hall 1 ). Consumption of meat has been associated with higher fasting insulin concentrations in a meta-analysis of 50345 Caucasians( Reference Fretts, Follis and Nettleton 21 ). Our result of negative association between meat protein intake and IGFBP-1 concentration is in line with this result.
Observational studies have suggested that intake of dietary fibre is inversely associated with insulin resistance( Reference Lau, Faerch and Glümer 22 , Reference Breneman and Tucker 23 ). In our study population, intakes of carbohydrates and fibre correlated strongly, r 0·77( Reference Larsson, Männistö and Virtanen 24 ). This is a probable explanation for our observation of positive association between carbohydrate intake and IGFBP-1 concentration.
Dietary fibre intake has also been suggested to explain why plant protein, in contrast to animal protein, does not appear to increase insulin resistance, since diets high in plant protein are typically also rich in dietary fibre( Reference Weickert and Pfeiffer 25 ). In our study, both plant protein intake and fibre intake associated positively with IGFBP-1 (P value of the association of fibre intake and IGFBP-1 was 0·02). As well, our result of positive association of milk protein intake and IGFBP-1 is in line with former results on the possible protective effect of dairy products on insulin resistance( Reference Tremblay and Gilbert 26 ). However, insulin concentration or insulin resistance is not the only potential mechanism involved in the associations of macronutrient intakes and IGFBP-1 since also glucagon has been shown to reduce bioactive IGF-I levels, independent of insulin, by increasing IGFBP-1( Reference Sarem, Bumke-Vogt and Mahmoud 27 ).
Since macronutrient intakes from different food sources differ in their associations with IGFBP-1, study of consumption of foods and IGF proteins could be useful. On the other hand, both intakes of macronutrients and consumption of foods share the challenge that different nutrient factors may explain the associations. Our findings on the associations between macronutrient intakes and IGFBP-1 are based on cross-sectional analyses. Controlled clinical studies are needed to demonstrate how foods and nutrients influence serum concentration of IGFBP-1.
A limitation of this study was that our results regard total IGF-I, and we did not separately measure free (unbound) IGF-I suggested to be the bioactive component of total IGF-I. In the Nurses’ Health Study, there was, however, a significant interaction between free IGF-I and insulin; free IGF-I was negatively associated with diabetes risk among subjects with insulin above the median and positively among subjects whose insulin was below the median( Reference Rajpathak, He and Sun 4 ). Another limitation is that the ATBC Study was originally designed to test the associations of risk/protective factors to cancers, so the participants were not screened using oral glucose tolerance test to be free of diabetes at baseline. We, however, were able to exclude the men who at baseline were entitled to reimbursement of diabetes medication, reported using diabetes medication or having physician-diagnosed, diet-treated diabetes and the cases that had fasting serum glucose ≥7·0 mmol/l at baseline. Since substantial proportion of patients with glucose abnormalities do not have elevated fasting blood glucose, we were consequently not able to exclude all the participants with abnormal glucose tolerance at baseline from the analysis and this may have attenuated the associations. Because diabetes cases were identified from the drug reimbursement register, we were not able to include subjects whose diabetes was managed by diet alone. A limitation of BMI as a marker of obesity is that it does not discriminate between fat and muscle mass and is not able to distinguish the distribution of body fat.
We conclude that in our study population of middle-aged male smokers, IGFBP-1 was inversely associated with the risk of type 2 diabetes, but the association was substantially dependent on BMI. IGF-I or IGFBP-3 were not associated with diabetes risk. Intakes of carbohydrates, plant protein and milk protein associated positively with IGFBP-1 whereas intakes of meat protein and fat associated negatively. It is possible that the associations of macronutrient intakes and IGFBP-1 are indirect reflecting influences of intakes of macronutrients or consumption of foods on fasting insulin concentration and insulin resistance.
Acknowledgements
The authors thank Riitta Koistinen and Annikki Löfhjelm for the IGF I, IGFBP-1 and IGFBP-3 measurements.
The ATBC Study was supported by the US Public Health Service contracts (HHSN261201000006C, HHSN261201500005C) from the National Cancer Institute. The present study was supported by the Juho Vainio Foundation and the Finnish Cultural Foundation.
L. M. V., J. V. and M. E. S. contributed to the conception and design of this study, J. P. K. performed the statistical analyses, J. S. was responsible for the glucose and insulin measurements, M. E. S. wrote the manuscript, S. M. is the principal investigator of the ATBC Study. All authors contributed to the interpretation of the results or revision of the manuscript and approved the final manuscript.
The authors have no financial or other relationships that might lead to a conflict of interest.







