The importance of vitamin D in pregnancy has been subject to great attention, and the development of several diseases has been proposed to be linked to vitamin D deficiency during pregnancy( Reference Christesen, Falkenberg and Lamont 1 – Reference Mai, Chen and Camargo 5 ). One of the suspected consequences of vitamin D deficiency in pregnancy is altered fetal growth, which, among other things, may influence the risk of overweight and obesity later in life( Reference Pietilainen, Kaprio and Rasanen 6 ). Several studies have investigated the association between vitamin D status in pregnancy and birth weight, but the conclusion of the results from previous studies is unclear: for example, some studies have shown a direct association between vitamin D status in pregnancy and birth weight( Reference Bowyer, Catling-Paull and Diamond 7 – Reference Watson and McDonald 15 ), one study showed an inverse association( Reference Weiler, Fitzpatrick-Wong and Veitch 16 ), but most studies failed to find any association between them( Reference Akcakus, Koklu and Budak 17 – Reference Yu, Sykes and Sethi 29 ).
Suggestions on mechanisms for how vitamin D in pregnancy may be associated with fetal growth are limited. However, vitamin D has been proposed to improve the development of the placenta( Reference Thorne-Lyman and Fawzi 30 ), and to influence placental function by reducing the risk of placental vascular pathology( Reference Gernand, Simhan and Klebanoff 9 ), and by promoting the transfer of amino acids from the mother to the fetus( Reference Cleal, Barton and Simner 31 ). Vitamin D has also been proposed to be involved in adipocyte differentiation, where vitamin D inhibits the early phase of the differentiation of pre-adipocytes into mature adipocytes( Reference Kong and Li 3 ). Vitamin D could also be related to fetal growth through the effects on Ca metabolism and bone growth( Reference Specker 32 ). Vitamin D deficiency is very common at northern latitudes because vitamin D is only available from a few food sources naturally and vitamin D synthesis in the skin is limited to half of the year when UVB radiation from the sun is sufficiently strong to induce the production of vitamin D( Reference Holick 33 – Reference Jensen, Thorne-Lyman and Vadgård 37 ). For example, in Denmark, vitamin D cannot be synthesised in the skin from October to March( Reference Holick, Shils, Olson, Shike and Ross 38 , Reference Mejborn, Andersen and Bredsdorff 39 ).
Alternative sources of vitamin D are dietary supplements and vitamin D fortification of foods( Reference Holick 33 ). Relying on recommendations on supplementation to increase vitamin D intake may not be practical at a population level as social groups do not follow health recommendations to the same degree( Reference Groth, Christensen and Knudsen 40 ). Conversely, fortification programmes apply to the entire population that consumes the fortified food product( Reference O'Donnell, Cranney and Horsley 41 ). Many countries, such as Finland, Canada and the USA, fortify margarine, milk or flour with vitamin D to increase vitamin D intake in the population( 42 , Reference Piirainen, Laitinen and Isolauri 43 ).
Today, only a few foods fortified with vitamin D are available on the Danish market; however, previously, two vitamin D fortification programmes were undertaken in Denmark. From 1 January 1961 to 1 June 1985, it was mandatory to fortify all margarine with 1·25 μg vitamin D/100 g, after which it was no longer legal, and from 1 January 1972 to 31 December 1976, fortification of low-fat milk with 2·5–3·8 μg vitamin D/1000 g was allowed( Reference Jacobsen, Abrahamsen and Bauerek 44 ). The reasons for initiating and terminating the fortification programmes are not well described. There are no indications that the decisions were evidence based, and they were most probably reflecting the view on fortification in the Danish population at the time of the decisions. It has been estimated that the vitamin D fortification of margarine contributed to approximately 13 % of the total vitamin D intake( 45 ). However, the estimate for the contribution of vitamin D from fortified milk is not available.
The temporality of the fortification programmes offers a unique opportunity to study the impact of additional vitamin D intake from fortified foods on birth weight via information on the date of birth and birth weight from the unique Copenhagen School Health Record Register (CSHRR). In the present study, we aimed to study the impact of the Danish vitamin D fortification programmes on mean birth weight, as well as on the risk of high or low birth weight.
Subjects and methods
Study design
The present study built on the fortification programmes that were applied nationally in Denmark from 1 January 1961 to 31 May 1985. The initiation and termination of margarine and milk fortification serve as cut-off points in time to separate children into groups that differ in prenatal vitamin D exposure. A detailed study protocol has been published previously( Reference Jacobsen, Abrahamsen and Bauerek 44 ).
In total, two birth cohorts were studied for each of the four time points, i.e. initiation and termination of the two fortification programmes. The cohorts included all children selected from the CSHRR who were born during a 2-year period before or subsequent to the initiation and termination of the fortification programmes.
Fig. 1 presents an overview of how the exposure groups were defined. The children born during the fortification periods were defined as exposed, and the children born outside the fortification periods were defined as non-exposed. The groups were formed from children who were either exposed or non-exposed for the full 9 months of pregnancy.
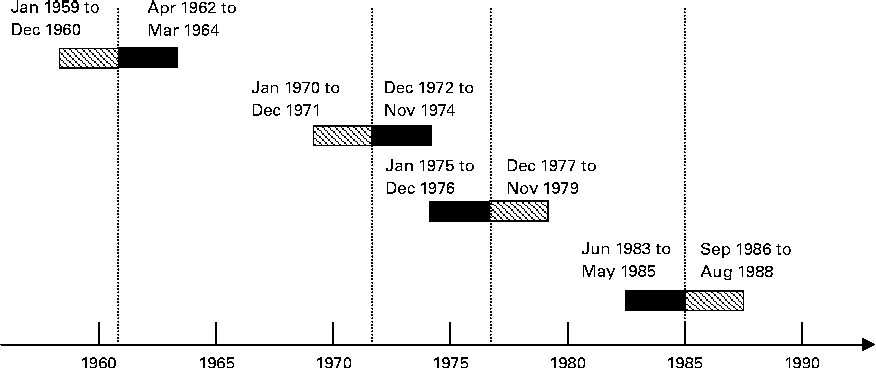
Fig. 1 Definition of the exposure groups. Vertical lines indicate the timing of vitamin D fortification events. Margarine fortification was initiated on 1 January 1961 and terminated on 31 May 1985. Milk fortification was permitted from 1 January 1972 to 1 January 1976. ![]() , Non-exposed; ■, exposed.
, Non-exposed; ■, exposed.
We applied a ‘wash-in’ period of 6 months after the day margarine fortification became mandatory, after which we assumed that all margarine without added vitamin D in stores and households would be replaced with vitamin D-fortified margarine. In the same way, a 6-month ‘wash-out’ period was applied when margarine fortification was terminated. For milk, we applied a wash-out period of 2 months at the beginning and the end of the fortification period.
Study population
The study population was selected from the CSHRR, which includes virtually every school child in Copenhagen born from 1930 to 1989 and comprises in total 372 636 records. For every child, the register contains self-reported information from mothers on birth weight. Extreme values of birth weight ( < 1·5 and >5·5 kg) were excluded as part of the data cleaning of the register. The CSHRR has been described in more detail elsewhere( Reference Baker, Olsen and Andersen 46 ).
Fig. 2 is a flow chart of the study population. After exclusion of children for whom information on birth weight was not available and children born outside the period relevant for studying the effects of the vitamin D fortification programmes (1959–1988), the final study population consisted of 51 883 children (26 553 boys and 25 330 girls).

Fig. 2 Flow chart of the study population. CSHRR, Copenhagen School Health Record Register. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
We defined a low birth weight as below 2500 g and a high birth weight as above 4000 g.
An access and linkage permission was obtained from the Danish Data Protection Agency (J. no. 2012-41-1156). This type of research based on pre-existing routinely collected data does not require ethical permission in Denmark.
Statistical analyses
All statistical analyses were performed separately for each of the four time points, i.e. initiation and termination of margarine fortification and initiation and termination of milk fortification. For each of the time points, the exposed cohort was compared with the non-exposed cohort.
Differences in mean birth weight between the exposed and non-exposed cohorts were estimated by linear regression. OR for high and low birth weight were estimated by logistic regression.
Due to a tendency towards an increase in birth weight across the study period (1959–1988), we modelled the secular trend in birth weight linearly and estimated the expected birth weight for each birth year in order to adjust for the secular trend. In linear regression analyses, we adjusted for the secular trend by calculating residuals (birth weight − expected birth weight) and performed the regression analyses with the residuals as the dependent variable. In logistic regression analyses, we adjusted for the secular trend in the prevalence of high and low birth weight by modelling the trend linearly and estimating the expected odds of high and low birth weight per year, and included the log expected odds as an off-set term in the respective analyses.
Analyses were conducted for each sex separately, and for the sexes combined. We included sex as a covariate in all the combined analyses.
To evaluate whether the timing of exposure to vitamin D fortification in pregnancy was important in the association between prenatal vitamin D exposure and birth weight, we exploited the fact that vitamin D is not synthesised during winter months (October to March)( Reference Holick, Shils, Olson, Shike and Ross 38 , Reference Mejborn, Andersen and Bredsdorff 39 ). The effect of prenatal exposure to vitamin D-fortified foods on birth weight might be more pronounced when vitamin D is not obtained via dermal synthesis. We restricted the analyses to children who experienced their first, second or third trimester of prenatal life during winter, respectively. Children who did not experience an entire trimester during winter were excluded from the secondary analyses, as were children who experienced more than one entire trimester during winter. Children born during July to September were defined as having experienced their first trimester in winter months, those born during April to May as having experienced their second trimester in winter months, and those born during December to February as having experienced their third trimester in winter months. We tested for an interaction between trimester and exposure by likelihood ratio tests.
The level of significance was set at P <0·05. Statistical analyses were performed using Stata Statistical Software (Release 12; StataCorp LP).
Results
Development of birth weight from 1959 to 1988
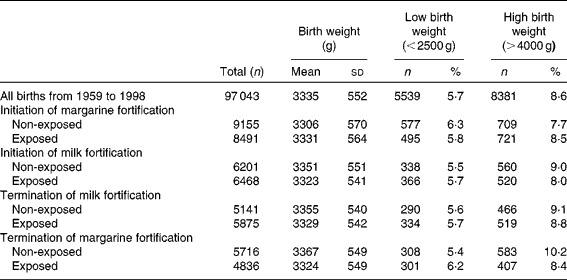
During the study period 1959–1988, the mean birth weight increased significantly (P< 0·02) from 3299 (sd 573) to 3389 (sd 544) g. The prevalence of high birth weight also increased over time (P< 0·01) from 8·0 % in 1959 to 11·2 % in 1988, and the prevalence of low birth weight decreased slightly (P< 0·1) from 6·5 % in 1959 to 4·8 % in 1988 (see Fig. 3 and Table 1).

Fig. 3 Development of birth weight from 1958 to 1989. (a) Annual mean birth weight. (b) Annual prevalence of high birth weight (>4000 g, ![]() ) and low birth weight ( < 2500 g,
) and low birth weight ( < 2500 g, ![]() ).
).
Table 1 Descriptive statistics of the study population (Mean values and standard deviations; number of subjects and percentages)
Differences in mean birth weight between children exposed to vitamin D fortification and non-exposed children
The mean birth weight was significantly different between the exposed and non-exposed children born during the years around all fortification events. There were no notable differences in the association between vitamin D exposure and birth weight between the sexes (initiation of margarine and milk fortification and termination of margarine fortification: P>0·05; termination of milk fortification: P>0·03; see online supplementary Fig. S1), so the analyses were conducted on boys and girls combined and adjusted for sex.
At the initiation of margarine fortification, exposed children were heavier than non-exposed children (27·4 (95 % CI 10·8, 44·0) g; Fig. 4). During the three other fortification periods, exposed children weighed less than non-exposed children (milk initiation − 20·3 (95 % CI − 39·2, − 1·4) g; milk termination − 25·9 (95 % CI − 46·0, − 5·7) g), and the biggest difference was observed for those born around the termination of margarine fortification ( − 45·7 (95 % CI − 66·6, − 24·8) g).

Fig. 4 Mean difference in birth weight between children exposed to vitamin D fortification prenatally and non-exposed children (exposed − non-exposed) who were born during the years around the initiation and termination of margarine and milk fortification. Crude differences (●) and differences adjusted for the secular trend (○) in birth weight.
Stratifying the analyses by trimester in winter months revealed no significant interaction between trimester and exposure in any of the fortification periods (P>0·05), except around the termination of milk fortification (P< 0·001). For margarine fortification, seasonality did not influence the relationship between trimester and exposure (see online supplementary Fig. S2). For milk fortification, there was a tendency towards a decrease in birth weight by vitamin D in the first and third trimesters and the opposite in the second trimester.
OR of high and low birth weight between children exposed to vitamin D fortification and non-exposed children
All analyses showed small and non-significant effects of prenatal vitamin D exposure on high and low birth weight, except for the odds of a high birth weight for children born around the termination of the margarine fortification (OR 0·81 (95 % CI 0·70, 0·92); Figs. 5 and 6, respectively).

Fig. 5 Odds of high birth weight (>4000 g) in children exposed to vitamin D fortification prenatally compared with non-exposed children (as reference). Estimates are presented separately for each fortification event, i.e. initiation and termination of margarine and milk fortification. Crude OR (●) and OR adjusted for the secular trend (○) in the prevalence of high birth weight.

Fig. 6 Odds of low birth weight ( < 2500 g) in children exposed to vitamin D fortification prenatally compared with non-exposed children (as reference). Estimates are presented separately for each fortification event, i.e. initiation and termination of margarine and milk fortification. Crude OR (●) and OR adjusted for the secular trend (○) in the prevalence of low birth weight.
Stratifying the analyses by sex revealed the same pattern in both boys and girls, and restricting the analyses to children who experienced the first, second and third trimesters in winter did not point towards that any of the trimesters were of special importance for the association between exposure to vitamin D-fortified foods and the risk of high or low birth weight (data not shown).
Discussion
The present study investigated the effects of fortifying foods with vitamin D on birth weight by taking advantage of the population-wide societal interventions of mandatory food fortification with vitamin D, implemented in Denmark between 1 January 1961 and 1 June 1985.
Among 51 883 Danish children, we examined prenatal exposure to vitamin D from fortified margarine and milk in relation to birth weight. Children born to mothers who had been exposed to additional vitamin D supplementation around the initiation of the mandatory margarine fortification were heavier than non-exposed children. Subsequently, exposed children weighed less than non-exposed children at birth. However, the clinical relevance of the results is limited because all effects were small (mean difference in birth weight < 60 g), and the direction of the association between exposure to additional vitamin D and birth weight was inconsistent. We did not expect the discrepant findings, showing that exposed children weighed more at birth at the beginning of the first fortification programme but less afterwards. Exposure to additional vitamin D from fortified low-fat milk showed similar effects at both initiation and termination of the fortification programme; however, the low-fat milk fortification programme is the one with the biggest limitation, since we do not have an estimate of how much milk was fortified with vitamin D.
Additional vitamin D from fortified margarine showed opposite effects on fetal growth at initiation and termination of the fortification programme. Food disappearance statistics show that during the period 1961–1985, the intake of margarine decreased( Reference Fagt and Trolle 47 ), which means that the amount of vitamin D obtained from fortified margarine was different at the initiation and termination of the fortification programme. We speculate that the dose of vitamin D may influence the direction of the association. The three later fortification events may have a low dose of vitamin D in common.
The results may also depend on the maternal reserves of vitamin D before pregnancy. Before 1961, there was no vitamin D fortification of any foods and pregnant women from the first analyses (i.e. around the initiation of margarine fortification) presumably had low vitamin D status before conception. Mothers who entered pregnancy after the fortification programmes had been in effect for several years (i.e. three latter fortification events) had higher vitamin D status before conception. We suggest that the association between prenatal vitamin D exposure and fetal growth is modified by preconceptional vitamin D status.
The obesity epidemic is another factor that may modify the association between prenatal vitamin D exposure and fetal growth. In Denmark, there was a steep rise in the prevalence of obesity starting in the early 1970s( Reference Sorensen, Sabroe and Gillman 48 ), and margarine initiation was the only fortification event that happened before this began.
The study population was large, and the possibility of chance findings should be considered. However, the P values of the linear regression analyses of margarine fortification were very small (P< 0·001), and therefore we do not believe that the discrepant findings can be explained by chance.
We mimicked randomisation in the present study design by using a time point, e.g. initiation of margarine fortification to completely separate exposed from non-exposed children. No major or abrupt societal changes happened in Denmark during the years around the initiation or termination of the fortification programmes, and except for the intended difference in prenatal vitamin D exposure, there are no reasons to believe that the exposed and unexposed children are not otherwise fully comparable, which makes the present study design excellent for comparing the impact of additional vitamin D intake from fortified foods on birth weight.
Food disappearance statistics show that during the period 1961–1985, the intake of margarine decreased and the intake of low-fat milk increased( Reference Fagt and Trolle 47 ). It is possible that the change in consumption of fat from dairy products would also influence fetal growth. However, by creating exposure groups separate for each time period and, thereby, only comparing children born in adjacent years, we avoid bias from the trend in food consumption. It is possible that pregnant women have speculated in their consumption of vitamin D-fortified foods during the time period when the fortification status was changed, e.g. some may have increased their intake of margarine when realising it was fortified with vitamin D. However, since all margarine used for production of foods was fortified with vitamin D, the proportion of vitamin D from actual fortified margarine might be small.
We have identified many studies investigating the association between vitamin D status in pregnancy and birth weight; however, none of these has investigated the association between vitamin D from fortified foods and birth weight as we did in the present study. Because of the dissimilarities in study type, design, population, exposure and outcome of the studies, it is difficult to compare the findings.
Previous studies included trials of vitamin D supplementation and observational studies of vitamin D intake and vitamin D status( Reference Bowyer, Catling-Paull and Diamond 7 – Reference Yu, Sykes and Sethi 29 ). The trials are the most comparable to the present study because both study designs include an intervention of additional vitamin D.
Of six trials, two found that birth weight was significantly higher in vitamin D-supplemented groups compared with the controls( Reference Marya, Rathee and Lata 12 , Reference Marya, Rathee and Dua 13 ), which corresponds to what we observed at the initiation of margarine fortification. The difference in birth weight was observed after the administration of either daily vitamin D supplements (1200 IU; 30 μg) during late pregnancy, or two mega doses (60 000 IU; 1500 μg) of vitamin D in late pregnancy. Both these studies were performed in India and included women who most probably had poor nutritional status that might be similar to Danish women giving birth around the initiation of margarine fortification who might have been undernourished in regard to vitamin D.
Another trial investigated the effect of assigning a daily dose of vitamin D (1000 IU; 25 μg) to pregnant women during the last trimester, or a single dose of vitamin D (5 mg) in the 7th month of pregnancy. It concluded that supplementation did not have any effect on birth weight( Reference Mallet, Gugi and Brunelle 24 ). However, the supplemented mothers gave birth to babies who weighed 90 g (daily supplementation) and 250 g (single-dose supplementation) less than the control group. The number of participants in the trial was only seventy-seven, which may be the reason that the difference in birth weight was non-significant. The mothers might have been of good vitamin D nutrition before pregnancy, similar to the mothers giving birth at the later time points in the present study.
The remaining three trials found no effect of vitamin D supplementation in pregnancy on birth weight( Reference Brooke, Brown and Bone 18 , Reference Hollis, Johnson and Hulsey 23 , Reference Yu, Sykes and Sethi 29 ). One trial compared the effect of daily 1000 IU (25 μg) vitamin D supplementation with no supplementation( Reference Brooke, Brown and Bone 18 ), and another compared both daily supplementation of 800 IU (20 μg) vitamin D and single-dose supplementation of 200 000 IU (5000 μg) vitamin D with no supplementation( Reference Yu, Sykes and Sethi 29 ). The third trial investigated the effect of additional vitamin D supplementation by comparing pregnant women receiving either 400, 2000 or 4000 IU (10, 50 or 100 μg) vitamin D3 ( Reference Hollis, Johnson and Hulsey 23 ).
It was estimated that margarine fortification supply on average 13 % of dietary vitamin D intake( 45 ), which may be considered low especially during periods with plenty of UVB exposure from the sun. However, during October to March, where no UVB radiation is available from the sun, even small amounts of additional vitamin D intake might affect vitamin D status. We do not have an estimate of how much vitamin D the milk fortification programme supplied to the habitual dietary intake; however, we suspect that the supply was not trivial because the amount of vitamin D added to milk (2·5–3·8 μg/1000 g) in Denmark was similar or a little lower compared with the amount of vitamin D used by other countries to fortify their milk (Finland 0·5 μg/100 g and USA 1 μg/100 ml)( 42 , Reference Piirainen, Laitinen and Isolauri 43 ). The daily intake of milk in Danish pregnant women was 3·1 (sd 2·0) glasses/d in 1996–2002( Reference Olsen, Halldorsson and Willett 49 ). Hence, daily intake of vitamin D from supplemented milk would have been approximately 1·5–2·3 μg/d. However, there was no registration of how much of the produced milk was fortified with vitamin D, and therefore we do not know how much vitamin D was provided from milk consumption. Fortified low-fat milk might have been more expensive than regular low-fat milk and high-fat milk, and the use of vitamin D-fortified milk might have been restricted to a selected group of people. However, if the use of fortified milk was restricted to a part of the population, this would attenuate the observed effect of fortification.
Recommendations of vitamin D supplementation varied across the study period, and there is no information on to what degree these were followed. Therefore, it was not possible to include vitamin D supplementation in the present analyses.
The CSHRR is a large population-based register, with minimal selection bias from socio-economic status as all children attending Copenhagen public and private schools are included. Birth weight was self-reported by mothers or fathers at the first school health examination, and it is possible that there is a selection because not all parents participated in the first health examination. However, a previous examination of missing information on birth weight revealed no pattern in missing data( Reference Baker, Olsen and Andersen 46 ).
In summary, prenatal exposure to vitamin D from fortified margarine and milk altered birth weight, but the effect on birth weight was small and inconsistent. From a public health perspective, even small differences in birth weight can have a big potential; however, as the direction of the association between prenatal vitamin D exposure and fetal growth was inconsistent, the clinical relevance of the results is limited. More studies on mechanisms relating vitamin D to birth weight are needed to understand how the effects of vitamin D are executed, and why the effect seems to be inconsistent.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001330
Acknowledgements
The present study was funded by the Danish Agency for Science Technology and Innovation, the Ministry of Science, Innovation and Higher Education, under the instruments ‘Strategic Research Projects’ and by a research grant from the Danish PhD School of Molecular Metabolism funded by the Novo Nordisk Foundation.
The supporting bodies had no role in the design, implementation, analysis and interpretation of the data presented herein.
The authors' contributions are as follows: B. L. H. conceived the research idea; C. B. J., T. L. B., M. G., T. I. A. S. and B. L. H. designed the research and wrote the paper; C. B. J. performed the statistical analysis and had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.









