The subthalamic nucleus deep brain stimulation (STN-DBS) is an efficacious treatment for advanced Parkinson’s disease (PD). Interleaving stimulation (ILS) is a DBS programming modality, which is adopted when conventional configurations (e.g. monopolar or bipolar) fail. Although the therapeutic mechanism is not fully understood, ILS aims (i) to reduce side effects and/or (ii) to stimulate different therapeutic sites within STN or nearby areas in order to control specific symptoms.Reference Miocinovic, Khemani and Whiddon1 For instance, through the contemporary stimulation of the STN and of a more dorsal contact, it is possible to target the pallidofugal tract to relieve dyskinesiasReference Aquino, Duffley and Hedges2 or the zona incerta to treat tremor.Reference Kern, Picillo and Thompson3 Each stimulating contact could be programmed with an individual combination of amplitude and pulse width, but with a single frequency value that could not be higher than 125 Hz.Reference Moro, Esselink, Xie, Hommel, Benabid and Pollak4 Hence, ILS has been generally considered less effective on tremor, given that the latter usually improves at higher frequencies.Reference Moro, Esselink, Xie, Hommel, Benabid and Pollak4 Herein, we present a trick to control asymmetric PD tremors overcoming two main ILS limitations, namely, the inability (i) to generate frequencies higher than 125 Hz focused on a cathode and (ii) to program independent frequencies across leads.Reference Amon and Alesch5
A 54-year-old woman with a 12-year history of PD and a modified Hoehn and Yahr score of 3 received a STN-DBS because of motor fluctuations, characterized by sudden and unpredictable offs and disabling peak-dose coreoathethoid dyskinesias, which were no more treatable with oral therapies. Besides, the patient also reported rest tremor of moderate to severe entity, located mainly on her right body side, with a suboptimal response to levodopa. The preoperative levodopa equivalency daily dose (LEDD) was 1154 mg. She received bilateral STN-DBS (Activa-PC, Medtronic), leading to a good control of motor symptoms, except for the right-side tremor (on and off medications). Left STN voltage was increased with improvement of tremor at the cost of side effects (i.e. right facial pulling) on monopolar and bipolar configurations. Hence, keeping left STN voltages below the threshold of side effects, frequencies were raised up to 220 Hz with a complete control of right-side tremor but with the concurrent onset of a facial pulling on the left side. Any attempt to lower the right STN voltage in order to reduce the new stimulation side effect caused a loss of therapeutic benefit. After a further unsuccessful attempt to control tremor stimulating the more dorsal and the more therapeutic contacts with ILS (i.e. zona incerta plus STN stimulation),Reference Amon and Alesch5 a definitive and lasting symptom control was achieved by adopting two interleaved bipolar programs sharing a cathode, pulsing at 250 Hz (as the result of the overlapping 125 Hz fields) in the left lead – i.e., contralateral to the worst trembling body side (Figure 1, Supplementary Table). The LEDD was reduced to 540 mg, with an 18-month follow-up.
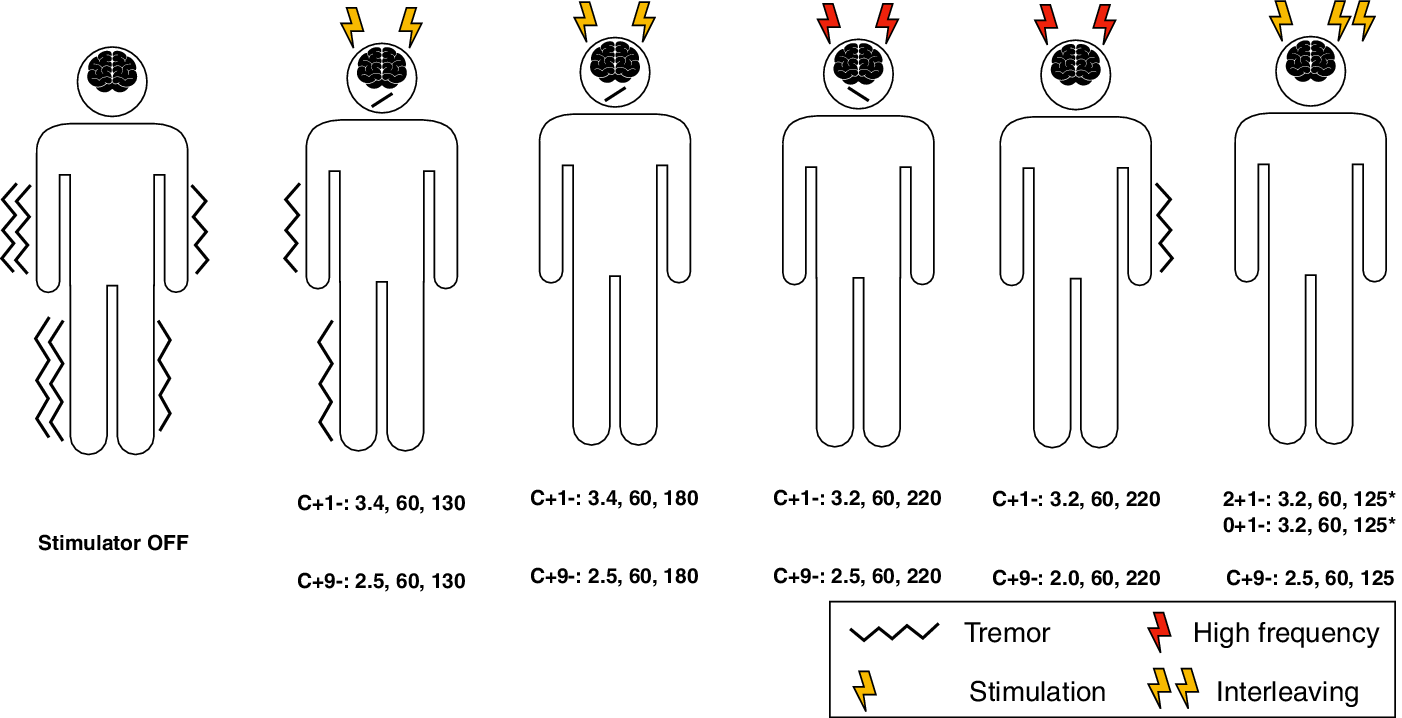
Figure 1: Graphical representation of the patient programming sessions. It was not possible to obtain a full symptomatic control with standard programming techniques (single yellow thunderbolt) due to the presence of stimulation-induced side effects (face oblique line pending to the facial pulling side) on with or without incomplete tremor (wavy line) suppression. Stimulating at higher frequencies (red thunderbolts) was effective on tremor, at constant values of pulse amplitude and width. However, this leads to the appearance of stimulation-induced side effects on the least affected body side. ILS (double-yellow thunderbolt) allowed to adopt different stimulation settings across leads, in particular it was possible to adopt asymmetric frequencies with a full control of tremor on the most affected body side, without adverse effects.
ILS is an advanced programming technique, which is adopted to sculpt the electrical field with the drawback of a quicker battery drain.Reference Miocinovic, Khemani and Whiddon1,Reference Amon and Alesch5 Each electrode could host a couple of virtual leads with a frequency ceiling of 125 Hz, which was imposed by the manufacturer in order to avoid excessive electrical charge density (e.g. occurring over 250 Hz).Reference Miocinovic, Khemani and Whiddon1,Reference Amon and Alesch5 On a clinical point of view, higher frequencies (i.e. >130 Hz) are often required to achieve a better control of tremor.Reference Moro, Esselink, Xie, Hommel, Benabid and Pollak4 Therefore, the possibility to select different frequencies per lead (e.g. higher frequencies contralateral to the trembling body side) would be of help in treating asymmetric frequency-sensitive tremors. This is possible with a unilateral ILS, which is able to increase frequencies of the small region that is included in the overlap area of two close interleaved fields (i.e. running with a phase delay).Reference França, Barbosa, Iglesio, Teixeira and Cury6 However, the proposed ILS setting let the entire left cathode pulsing at a higher frequency than the right one, with a consecutive robust tremor improvement. Of relevance, two interleaved programs could share an entire cathode only if the latter is included into a couple of bipolar configurations (e.g. 2+1− and 0+1−).
The approach we described here would be a further resource in the management of asymmetric PD tremor. Moreover, it could also be speculated that the use of ILS and of higher frequencies would be of help in reducing the exaggerated oscillatory activity pattern in the beta band, a well-known finding in tremor-dominant PD patients.Reference Cagnan, Duff and Brown7
Finally, more studies are warranted to better understand the mechanism behind the ILS effect and how it could help clinicians in “tailoring” DBS therapy for their patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Dr Massimo Marano received speaker honoraria and consultancies by AbbVie and Allergan, research grant by “Alberto Sordi Foundation”; Dr Francesco Motolese reports no disclosures; Dr Daniele Marruzzo reports no disclosures; Dr Riccardo Antonio Ricciuti reports no disclosures; Prof. Vincenzo Di Lazzaro reports no disclosures; Dr Manuela Pilleri consultancies by Boston Scientific, Brainlab, speaker’s honoraria for UCB, AbbVie, Zambon.
Statement of Authorship
MM, FM, DM clinical data collection and drafting of the manuscript; RAR, VDL, and MP drafting the article and revising it critically for important intellectual content, final approval of the version to be submitted.
Ethics Approval and Consent to Participate
The authors declare that ethics approval was not required for this case report that is conducted in accordance with the declaration of Helsinki.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.64.



