The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) defines complementary foods as other solids and liquids introduced into the infant diet apart from breast milk or formula milk( Reference Agostoni, Decsi and Fewtrell 1 ). The introduction of complementary foods into an infant's diet is an important process not only for developmental and growth reasons, but also because of its potential long-term effects on health( Reference Anderson, Guthrie and Alder 2 – Reference Cooke 11 ). Providing a balanced diet while adhering to introduction times recommended by guidelines is pivotal during this period of rapid growth, not only because of the high probability of nutritional deficiencies but also because early complementary feeding has been shown by some studies to increase the risk of overweight, obesity and chronic diseases later in life( Reference Lanigan and Singhal 5 , Reference Singhal and Lucas 6 , Reference Koletzko, Brands and Poston 8 – Reference Wilson, Forsyth and Greene 10 ). Moreover, the transition from milk to solid foods is a crucial point to establish life-long feeding patterns( Reference Cooke 11 – Reference Nicklaus, Boggio and Chabanet 13 ). However, poor adherence to recommendations on the introduction of complementary foods has been reported in the Republic of Ireland (ROI) as well as internationally( Reference Friel, Hanning and Isaak 14 – Reference Caroli, Mele and Tomaselli 18 ).
WHO recommends exclusive breast-feeding during the first 6 months of life of the infant, with the gradual introduction of solids after 6 months( 19 ). The Department of Health and Children in the ROI updated its advice in 2003 to recommend adherence to WHO guidance( 20 ). The new infant feeding guidelines released by the Food Safety Authority of Ireland (FSAI) in November 2012 maintain the recommendation made by ESPGHAN not to introduce complementary foods before 17 weeks and no later than 26 weeks, while also giving the advice to commence the introduction of solids near 6 months of age( Reference Agostoni, Decsi and Fewtrell 1 , 21 ). The FSAI also states that, due to the natural variation in physiological requirements of individual infants, some infants may require the introduction of complementary foods shortly before 6 months of age to support optimal growth and development( 21 ). For this purpose the FSAI provides a number of signs that indicate when an infant is ready to start taking solids( 21 ). However, both ESPGHAN and FSAI coincide in defining the introduction of solids at <17 weeks as early complementary feeding.
Despite these recommendations, Irish studies show rates of exclusive breast-feeding for 6 months of less than 1 %, with 75·0% of infants being introduced to complementary feeding before 17 weeks and 22·6 % of these being weaned prematurely by 12 weeks( Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference McSweeney and Kevany 22 ). However, if parents are to be encouraged to delay the introduction of solids until near 6 months of age, it is important to know their reasons for introducing solids earlier. The identification of predictors of early complementary feeding will enable us to identify those groups in greater need of dietary advice as well as help health professionals to provide this advice effectively( Reference Alder, Williams and Anderson 23 ). To our knowledge, few studies on early complementary feeding determinants have been carried out in ROI. The aim of the present study was therefore to explore the predictors of early introduction to complementary foods by studying cross-sectional patterns in the first wave of the Growing Up in Ireland (GUI) infant cohort.
Methods
Study design and sample
The study sample comprises 11 134 infants aged 9 months who participated in the first wave (2007–2008) of the GUI study. GUI is a nationally representative cohort of 9-month-old infants residing in the ROI. The sampling frame for the project was the Child Benefit Register for the ROI. A random sample of infants born between December 2007 and May 2008 was selected and invited to participate when the child was 9 months of age. Of 16 136 mothers selected from the sampling frame, 11 134 agreed to take part in the study, a response rate of 69·0 %( Reference Quail, Williams and McCrory 24 ).
Questionnaires and measurements
Primary caregivers, defined as the person who spent more time with the child, and secondary caregivers were interviewed at home and asked to complete a main questionnaire and a sensitive questionnaire. Interviews were carried out using a mixture of computer-assisted personal interviewing and computer-assisted self-interviewing. The questionnaires were developed by the GUI Study Team in association with many other groups and advisors involved in the study, such as the Scientific and Policy Advisory Committee which consisted of ten members selected to represent a wide range of disciplines mostly in the areas of large-scale longitudinal studies and children. A two-round Delphi process was carried out in the development of the design and instrumentation where a total of sixty-nine experts provided valuable information on the relative importance of questions in different domains. A panel of experts selected by the GUI Study Team and drawn from a wide range of backgrounds also contributed to the design of the study by providing domains, topics and questions relevant to their particular area( Reference Quail, Williams and McCrory 24 ).
Members of the GUI Study Team also liaised with other stakeholder groups, as well as with other longitudinal child cohort studies, to enable comparison as well as learn from their experiences. Three stages, Pre-pilot, Pilot and Dress Rehearsal, were carried out to test the procedures and instrumentation. In the pre-pilot stage a small convenience sample of families was used, whereas in the pilot and dress rehearsal a sample was selected from the Child Benefit Register. In total the infant cohort questionnaire for wave 1 of GUI consisted of four main questionnaires: the primary caregiver main questionnaire, the sensitive supplement to the primary caregiver questionnaire, the secondary caregiver main questionnaire and secondary caregiver questionnaire sensitive supplement. Some questionnaires are divided into modules of questions according to topic( Reference Quail, Williams and McCrory 24 ).
In addition to the questionnaires, interviewers also recorded the height and weight of both parents as well as the length, weight and head circumference of the infant. A medically approved mechanical SECA 761 weighing scales was used for the adults’ weight and a Leicester measuring stick for their height. The children's length was measured using a SECA 210 measuring mat. Their weight was measured with a SECA 835 weighing scales. The sample was weighted to ensure that it was representative of the population of children aged 9 months( Reference Quail, Williams and McCrory 24 ).
Statistical analysis and dependent variable
The statistical software package IBM SPSS Statistics version 19·0 was used for all statistical analyses. Several independent variables considered as risk factors for early complementary feeding were selected from the literature, as well as other possible predictors and confounding variables available in the database. Data were analysed using cross-tabulations and the χ 2 statistical test, as well as by multivariate binary logistic regression. Independent variables were included in the multivariate analysis if they were significant in the bivariate analysis.
For the purpose of the current paper, ESPGHAN's definition of complementary feeding will be used. The terms ‘complementary feeding’ and ‘introduction of solids’ will be used interchangeably as GUI only considered solids introduced into the infant diet as complementary foods.
The definition of the dependent variable ‘early complementary feeding’ was constructed from a question in the database that asked primary caregivers to indicate when they started to give their infants solid foods at least twice per day for several weeks. Solid foods were defined as baby cereals, puréed fruits, etc. and not milk or drinks. The dependent variable therefore can be defined as established complementary feeding and not as the first time that solids were introduced. Following ESPGHAN's guidelines, a binary dependent variable was created with two categories: <17 weeks for early complementary feeding and ≥17 weeks for acceptable introduction of complementary feeding( Reference Agostoni, Decsi and Fewtrell 1 , Reference Quail, Williams and McCrory 24 ). Statistical significance was taken as a P value of <0·05.
Definition of covariates
Socio-economic status (SES) was assessed using three different indicators: household class, equivalized household income quintiles and household type. Primary and secondary caregivers were asked questions about their current occupation to derive the variable household class. Where the respondent was economically inactive (retired or unemployed) at the time of interview, previous employment was considered. The household class classification adopted was that used by the Central Statistics Office (see Table 3). Income was equivalized to take into account household size and composition using the modified Organisation for Economic Co-operation and Development equivalence scale (first adult, weight = 1; second or higher adults, weight = 0·5; children aged <14 years, weight = 0·3). Household type is a fourfold variable derived from whether the study child is living in a one- or two-parent family as well as the number of children (<18 years) living in the household. This resulted in a classification as follows: one parent, one child; one parent, two or more children; two parents, one child; two parents, two or more children( Reference Quail, Williams and McCrory 24 ).
Maternal level of education was used for analysis as mothers comprised over 99 % of respondents. Maternal education was coded as follows: no formal or primary education, secondary education and tertiary education. Measured parent BMI was classified according to the WHO categories as underweight (<18·5 kg/m2), normal weight (18·5–24·9 kg/m2), overweight (25·0–29·9 kg/m2) and obese (≥30·0 kg/m2). Maternal weight measurements were recorded to the nearest 0·5 kg using a medically approved SECA 761 scales. This is a flat mechanical scale, graduated in 1 kg increments, that has an upper capacity of 150 kg( Reference Quail, Williams and McCrory 24 ). The Centre for Epidemiological Studies Depression Scale (CES-D) was used to calculate depression scores. This scale is a widely used self-report measure that was developed specifically as a screening instrument for depression in the general population, as opposed to being a diagnostic tool that measures the presence of clinical depression. GUI used the eight-item short version of the CES-D( Reference Di Clemente, Wingood and Lang 25 ). A composite score is calculated by summing item responses (range 0–24). Composite scores <7 are categorized as not depressed and ≥7 are categorized as depressed( Reference Quail, Williams and McCrory 26 ). The primary caregiver total depression score was used and recoded into two categories: not depressed and depressed, as previously described.
The question ‘Was baby ever breast-fed?’ refers to breast-feeding initiation regardless of the amount of time the baby was breast-fed; this question includes the colostrum in the first few days after birth. The question ‘Was baby ever exclusively breast-fed?’ refers to the infant receiving only breast milk without any additional food or drink regardless of the length of exclusive breast-feeding. The question about folic acid or folate intake prior to pregnancy was formulated as ‘Did you take folic acid/folate prior to pregnancy?’( Reference Quail, Williams and McCrory 26 ). Therefore, both terms, ‘folic acid’ and ‘folate’, are used interchangeably. The question has been used in our multivariate model to capture health attitudes towards pregnancy without making a distinction on whether the synthetic or natural form of the vitamin was utilized. This question was asked through the computer-assisted personal interview method.
Although infant's gender was captured in the database, in the present study trends of early complementary feeding introduction in the ROI have been studied for boys and girls together, in line with previous studies( Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference McSweeney and Kevany 22 ). Data are also provided for the prevalence of early introduction of solids by gender. Other variables of interest, such as infant birth weight, were analysed in bivariate analysis but only those that were significant statistically in this analysis have been included in the present paper.
Missing data
Some of the independent variables analysed had a large percentage of missing cases: BMI (5·1 %), equivalized household annual income (7·8 %) and formula feeding commencement (5·4 %). This resulted in a large percentage of the sample being missed from the logistic regression analysis. Thus, a ‘not reported’ category was created for categorical variables with >2 % of missing cases for the bivariate and multivariate analysis, so that missing cases could be kept in the analysis and an estimate made of the effect of being missing on that variable.
Results
Characteristics of the study cohort
Table 1 shows the characteristics of the primary caregivers and infants. The primary caregiver was defined as the person who spent most time with the study infant. Of all primary caregivers, 99·6 % were female, and 99·9 % of the females were the biological mothers of the infants. Of all primary caregivers, 46·3 % were either overweight or obese at the time of the interview. Of all mothers, 59·1 % were multiparous and 56·0 % reported having ever breast-fed. The mean birth weight of infants was 3·5 (sd 0·54) kg. Of all infants, 93·3 % were born at >37 weeks of pregnancy and 96·5 % were singleton births.
Table 1 Characteristics of primary caregivers and infants (n 11 134); data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort
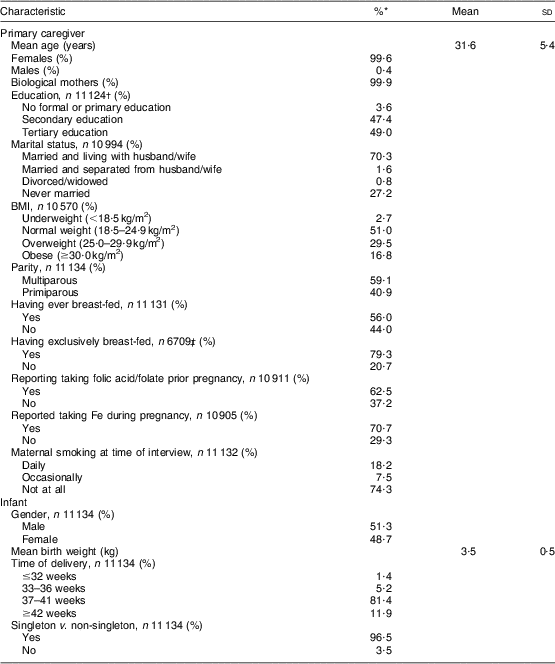
*Percentages provided are valid percentages.
†n provided is the number of primary caregivers who answered each question.
‡Mothers who reported not having ever breast-fed were filtered out.
Timing of established complementary feeding
The mean age of established complementary feeding was 20 (sd 5·4) weeks. Figure 1 shows that 1469 infants, representing 13·5 % of the sample, had been regularly taking solids in the period between 12 and 16 weeks; this percentage increased to 47·0 % of the sample in the period between 16 and 20 weeks. When the sample was segregated into males and females, a higher percentage of males (15·2 %) was introduced to complementary feeding at <17 weeks as opposed to females (13·2 %; P = 0·002). Binary logistic regression adjusting for gender and birth weight showed gender as a significant contributor to the model (P = 0·007) as opposed to birth weight (P = 0·289).
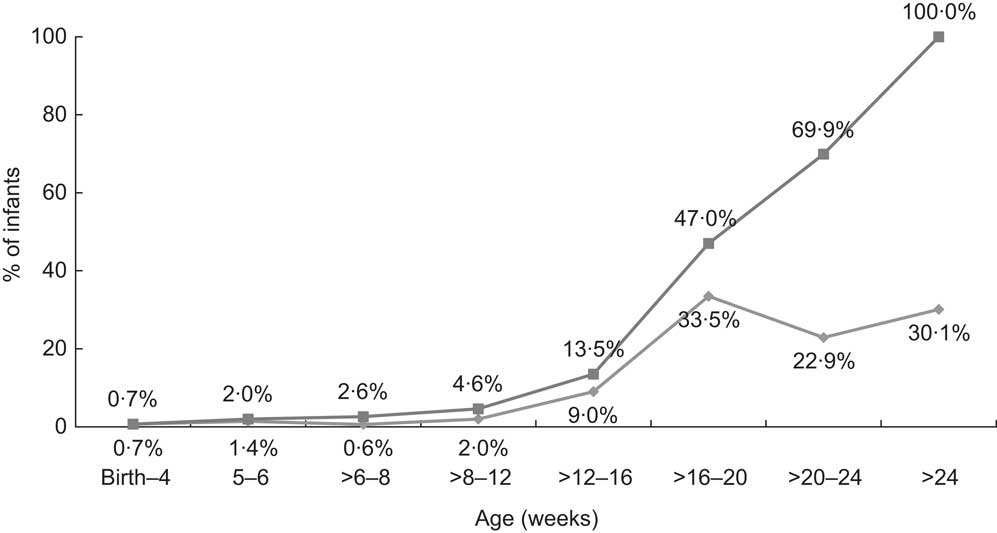
Fig. 1 Timing of established complementary feeding (![]() , percentage fully established on complementary feeding during specific time period;
, percentage fully established on complementary feeding during specific time period;
![]() , cumulative percentage fully established on complementary feeding during specific time period) during the first 6 months; data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort (n 10 868)
, cumulative percentage fully established on complementary feeding during specific time period) during the first 6 months; data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort (n 10 868)
Early complementary feeding predictors resulting as significant in bivariate analysis
Table 2 shows that the independent variables were grouped into five areas: biological, psychosocial, demographic, behavioural, and health and social care determinants. In the biological area, high maternal BMI was a predictor of early complementary feeding introduction (P < 0·001) in bivariate analysis. Demographic factors such as the mother's age, education and SES were strongly linked to early introduction of solids in bivariate analysis (P < 0·001). Breast-feeding initiation and timing of formula feeding commencement were also strong (behavioural) determinants of early introduction of complementary feeding (P < 0·001). Of those mothers who initiated breast-feeding 10·5 % introduced solids prematurely, as opposed to 18·9 % of those who did not initiate breast-feeding (P < 0·001; data not shown). The percentage of infants who had ever been exclusively breast-fed and were introduced early to complementary feeding was 9·2 % (P < 0·001; data not shown).
Table 2 Early complementary feeding predictors resulting as significant after bivariate analysis with the weaning dependent variable <17 weeks v. ≥17 weeks, grouped by area of classification; data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort

SES, socio-economic status; GP, general practitioner.
*Measured as equivalized household annual income (quintiles), household class and household type.
Predictors of early complementary feeding
Bivariate and multivariate analysis of identified predictors
Bivariate analysis showed that demographic, behavioural, biological, psychosocial and health and social care variables were significantly related to early complementary feeding at <17 weeks (Table 2). Table 3 shows that the significant factors that independently predicted the introduction of complementary feeding at <17 weeks, after adjustment, included primary caregiver age, education, BMI at the time of interview, marital status, ethnicity and current smoking status, reporting taking folic acid/folate prior to pregnancy, infant's gender, timing of formula feeding commencement, minder option and number of visits to the general practitioner. Infants from mothers with a tertiary education were 22·3 % less likely to be introduced to solids early compared with infants from mothers with no formal or primary education (OR = 0·777). Household class was also a significant predictor in the adjusted model; unskilled workers were 78·0 % more likely to introduce complementary foods early when compared with professional workers.
Table 3 Characteristics of primary caregivers, their infants and household in the <17 weeks and ≥17 weeks complementary feeding categories, and binary logistic regression of the factors associated with introduction of complementary feeding at <17 weeks; data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort
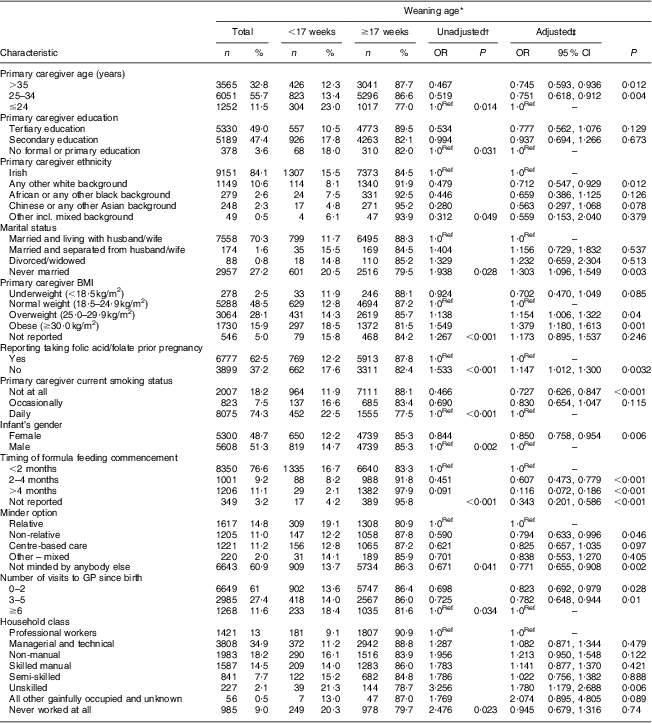
GP, general practitioner.
*Bivariate analysis using χ 2 statistical tests to compare the differences between primary caregivers, infants and households in the <17 weeks and ≥17 weeks groups.
†Values are OR that were obtained from individual bivariate analysis of independent variables when comparing the <17 weeks and ≥17 weeks groups.
‡Values are OR that were obtained from the final binary logistic regression model. The model was adjusted for primary caregiver age, education, BMI, ethnicity, marital status, parity, breast-feeding initiation, folate intake prior to pregnancy, primary caregiver smoking status at interview, being in contact regularly with grandparents, amount of help received from family and friends, type of antenatal care, primary caregiver depression score, infant's gender, use of soother at interview, formula feeding commencement, minder option, number of times baby wakes up at night, number of visits to GP, medical card, private insurance, equivalized household annual income (quintile), household class and household type. 1·0Ref denotes the reference group.
A number of primary caregiver behavioural determinants resulted as significant contributors to the model. Such was the case of current smoking status, with infants of mothers who did not smoke at the time of the interview being 27·3 % less likely to be introduced to solids early compared with infants whose mothers smoked daily (OR = 0·727). Another significant behavioural predictor was reporting taking folic acid/folate prior to pregnancy, with infants of mothers who did not take folic acid or folate prior to pregnancy being 14·7 % more likely to be introduced to complementary feeding early compared with infants whose mothers did.
High maternal BMI was a strong predictor in our model, with overweight and obese women being 15·4 % and 37·9 % more likely, respectively, to introduce solids early compared with normal-weight women.
Another significant predictor of complementary feeding at <17 weeks within the adjusted model was maternal ethnicity, with those babies of mothers coming from a different white background than Irish being 28·8 % less likely to be given complementary foods early (OR = 0·712). The ethnic group least likely to introduce solids early compared with Irish mothers was the Chinese, with their infants being 43·7 % less likely to be introduced to solids early (OR = 0·563).
Infant parity (P = 0·704), breast-feeding initiation (P = 0·848), being in regular contact with grandparents (P = 0·207), amount of help received from family and friends (P = 0·394), type of antenatal care (P = 0·922), primary caregiver depression score (P = 0·203), using a soother at the time of interview (P = 0·447), times the baby wakes up at night time (P = 0·482), being covered by medical card (P = 0·599), having private insurance (P = 0·208), equivalized household annual income (P = 0·448) and household type (P = 0·108) were not predictors of complementary feeding at <17 weeks in the adjusted model (data not shown).
Ethnicity and early weaning
Figure 2 shows the effect of belonging to different ethnicities on the introduction of complementary feeding. Those babies whose mothers come from a different white background than Irish were 28·8 % less likely to be introduced to solids early.
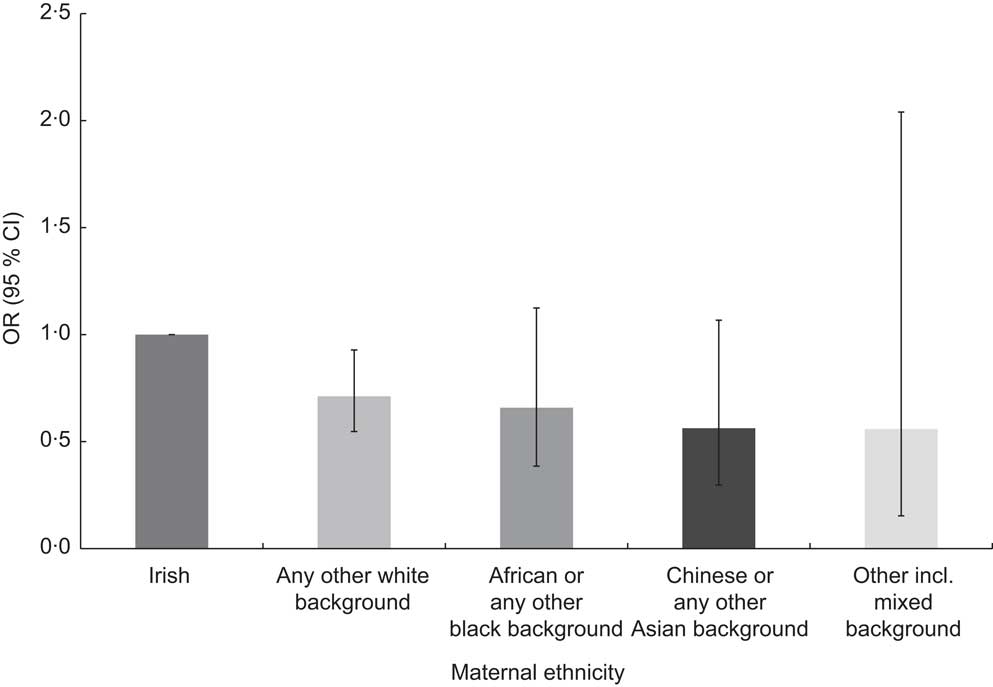
Fig. 2 Adjusted odds ratios of early complementary feeding by maternal ethnicity, with 95 % confidence intervals represented by vertical bars; data derived from the first wave (2007–2008) of the Growing Up in Ireland (GUI) infant cohort (n 10 868)
Discussion
Previous studies on the introduction of complementary feeding in ROI have defined it as the age at which the infant was first offered foods that were not his/her main milk( Reference Tarrant, Younger and Sheridan-Pererira 16 ). In the present study mothers were asked to indicate at which point they started giving their infants solid foods at least twice per day for several weeks. Thus the dependent variable in the present study may be defined as the prevalence of established complementary feeding among mothers of 9-month-old infants in Ireland, rather than the first introduction of non-milk solid or liquid foods.
The percentage of infants who had been taking solids regularly increased to 47·0 % of the sample in the period between 16 and 20 weeks, suggesting that mothers had started the introduction of complementary foods before this period. These figures differ from the prevalence found in previous studies in Ireland, where 22·6 % of babies were introduced to complementary feeding earlier than 12 weeks and 75·0 % at ≤17 weeks( Reference Tarrant, Younger and Sheridan-Pererira 16 ). This is probably due to the difference in definitions used in the study by Tarrant et al. in 2010 and the GUI study.
Thus, the prevalence of infants introduced early to solids in the present study is probably an underestimation of the real situation, with infants captured as having been introduced to complementary feeding at ≥17 weeks probably having commenced taking solids several weeks earlier. Therefore, it can be hypothesized that inappropriate infant feeding practices are taking place in ROI. When the sample was segregated by gender it could be observed that a higher percentage of male infants (15·2 %) were introduced early to solids than female infants (13·2 %). Earlier introduction of male babies to complementary feeding has been reported in other studies. Studies have suggested that this may be due to their larger size and therefore higher energy requirements and appetite( Reference Wright, Parkinson and Drewett 27 ). However, multivariate analysis adjusting for gender and birth weight showed only gender as a significant contributor to the model.
These inappropriate feeding practices have also been observed internationally. A Canadian study from 2010 based on a nationally representative sample of mothers with infants aged 3–12 months found that 83 % of the infants were taking solids at 3 months( Reference Friel, Hanning and Isaak 14 ). Schiess et al. studied complementary feeding in five European countries: Germany, Belgium, Italy, Spain and Poland. The authors found that 54 % of infants had received solids at four completed months. The percentage was higher for formula-fed infants (37 %) compared with breast-fed infants (17 %)( Reference Schiess, Grote and Scaglioni 15 ). A report from 2008 carried out in the Apulia region in Italy showed that 66 % of a cohort of 1824 infants had been introduced to complementary feeding by 5 months of age( Reference Caroli, Mele and Tomaselli 18 ). A study carried out in the USA with a cohort of 1334 mothers who participated in the national longitudinal Infant Feeding Practices Study II found that 40 % of the mothers introduced solids before the age of 4 months( Reference Clayton, Li and Perrine 17 ). Therefore, the tendency at an international level seems to be similar to the situation in the ROI, with most babies being weaned before 6 months and a varying but relevant proportion before 4 months. Early introduction to solids has been associated with an increased risk of being overweight or obese later in life and the development of chronic diseases( Reference Lanigan and Singhal 5 , Reference Morgan, Lucas and Fewtrell 7 , Reference Knip, Virtanen and Akerblom 9 , Reference Eriksson 28 , Reference Fall, Sachdev and Osmond 29 ). When interviewed at 3 years of age, the same cohort as analysed in the present paper was found to have a prevalence of overweight and obesity of 25 %, and those who had been introduced to complementary feeding later were less likely to be overweight or obese( 30 ).
Maternal education, age and SES were significant determinants of early weaning. Younger, less-educated mothers categorized as unskilled workers were more likely to introduce complementary feeding earlier in the life of their infant. This finding corroborates results from previous studies on the determinants of early introduction of solids( Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference Avery, Duckett and Dodgson 31 , Reference Wijndaele, Lakshman and Landsbaugh 32 ). Behavioural aspects such as the primary caregiver's current smoking status and reporting not taking folic acid/folate prior to pregnancy were also significant predictors of early introduction of solids at <17 weeks. These outcomes also correlate with findings from previous studies on determinants for early complementary feeding, suggesting that education for young women on positive health behaviours is required( Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference Di Clemente, Wingood and Lang 25 , Reference Avery, Duckett and Dodgson 31 , Reference Wijndaele, Lakshman and Landsbaugh 32 ). Moreover, the relationship between age at introduction of solids, current smoking status and reporting taking folic acid/folate prior to pregnancy, though attenuated, was still present when analysis was carried out controlling for SES (data not shown), indicating that more education on the consequences of early introduction of solids is needed across social classes.
The introduction of solids process cannot be studied in isolation from the type of milk feeding early in life, as both processes overlap and the feeding method chosen early in life may have an impact on the timing and type of solid foods chosen( Reference Grote, Theurich and Koletzko 33 ). In the adjusted model the timing of formula feeding commencement came out as a strong predictor of early introduction of complementary foods, with those infants fed formula milk at >4 months being 88·4 % more likely to be introduced to solids before 4 months of age. This strong predicting ability of the timing of formula feeding commencement was accentuated when the variable was analysed individually, showing that those babies commenced on formula later than 4 months were 90·9 % less likely to be introduced to complementary foods early. Moreover, a higher percentage of infants who were never breast-fed were introduced early to solids (18·9 %) compared with those whose mothers initiated breast-feeding (10·5 %; P < 0·001). These findings correlate with results from previous studies carried out in the ROI and internationally( Reference Schiess, Grote and Scaglioni 15 , Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference Caroli, Mele and Tomaselli 18 , Reference Avery, Duckett and Dodgson 31 , Reference Wijndaele, Lakshman and Landsbaugh 32 ). Formula feeding has been associated with impairment of appetite self-regulatory mechanisms leading to infants demanding the introduction of solids earlier with no subsequent reduction in milk intake during the complementary feeding period. This interference with self-regulating mechanisms early in life could have long-term health consequences, increasing the risk of overweight and obesity later in life( Reference Grote, Theurich and Koletzko 33 – Reference Mihrshahi, Battistutta and Magarey 36 ).
High maternal BMI was a strong predictor of early introduction of complementary foods in our model. Further analysis of the data showed how the relationship between maternal BMI and early complementary feeding seemed to disappear when formula feeding was introduced at >4 months (data not shown). Therefore, maternal BMI could potentially be a confounder in the relationship between formula feeding commencement and early introduction of solids. The relationship between overweight, obesity and breast-feeding duration has been well studied, suggesting that overweight and obese women are at higher risk of early breast-feeding termination( Reference Baker, Michaelsen and Sorensen 37 – Reference Li, Jewell and Grummer-Strawn 39 ). Biological factors also play a role in the initiation of breast-feeding among overweight and obese women( Reference Baker, Michaelsen and Sorensen 37 – Reference Li, Jewell and Grummer-Strawn 39 ). Biologically, lactation commences when prolactin is secreted and progesterone levels decrease after delivery of the placenta( Reference Neville and Morton 40 ). This process could be impaired in overweight and obese women due to elevated amounts of progesterone produced by excess adipose tissue. Moreover, the physiology of obese women's breasts could impair proper latching of the baby to the breast and impede the commencement of the process of galactopoiesis( Reference Hilson, Rasmussen and Kjolhede 38 – Reference Neville and Morton 40 ). It could thus be postulated that overweight and obese women are a population group to be targeted for breast-feeding education and support during the prenatal and postnatal period. This could result in breaking the cycle of overweight and obese mothers failing to breast-feed and thus introducing complementary feeding earlier, increasing the risk of their infant being overweight or obese later in life.
Ethnicity was a significant predictor of early introduction of solids at <17 weeks in the adjusted model. Belonging to a different ethnicity than Irish was protective against weaning at <17 weeks. The ethnic group least likely to introduce complementary foods early compared with Irish mothers were Chinese mothers, whose infants were 43·7 % less likely to be introduced early to solids. However, when analysis was adjusted for the number of years that non-nationals had been living in the ROI, it was observed that those non-nationals who had been living longer in the ROI tended to introduce solids earlier than those who had resided in the country for a shorter period (data not shown). This result suggests that early complementary feeding is, at least in part, affected by cultural factors and is probably related to low breast-feeding rates in Ireland. A study published in 2010 found that a group of Chinese mothers living in Ireland had a less positive attitude and more misconceptions about breast-feeding than a group of Chinese mothers living in Perth, Australia, suggesting a possible role of ‘acculturation’ and the mothers adapting themselves to the formula feeding culture of Ireland( Reference Zhou, Younger and Kearney 41 ).
Social factors such as the influence of relatives and health-care staff resulted as significant predictors of the early introduction of solids in both bivariate and multivariate analysis. These results have also been reported in previous studies( Reference Tarrant, Younger and Sheridan-Pererira 16 , Reference Di Clemente, Wingood and Lang 25 , Reference Aubel 42 ). Further exploration of these factors is needed as they could have an influence in the planning of education on weaning practices.
Study strengths and limitations
GUI is a large and nationally representative sample. The results of the study can be applied at a population level due to the application of the sampling weights. Parental BMI was measured by trained professionals using validated techniques. Creating a ‘not reported’ category for variables with >2 % of missing cases reduced the amount of cases lost in bivariate and multivariate analysis.
However, there are several limitations to the present study. Comparison with previous studies on early complementary feeding is difficult due to differences in the definition of introduction of complementary foods. The results must also be interpreted with caution as the information was collected retrospectively when the infant was 9 months of age, increasing the possibility of recall bias.
The maternal BMI in the present study is the one at the time of interview which took place when the infants were 9 months old; therefore the present paper postulates that those mothers who were overweight or obese at that point in time belonged to the same BMI category pre-pregnancy. The ‘not reported’ category created for certain variables biases the estimates for the other categories of that variable, as people have moved from their ‘real’ group to the ‘not reported’ group; however, this is preferable to those cases being removed completely from the analysis.
Conclusion
A high prevalence of infants regularly taking complementary foods in the period between 16 and 20 weeks was observed in the present study, suggesting that the introduction of solids had commenced earlier in the life of the infant. Therefore, it can be hypothesized that inappropriate infant feeding practices are taking place in ROI as other studies have previously reported. Direct comparison with previous studies is difficult due to differences in the definition of weaning.
Complementary feeding practices cannot be studied in isolation from early milk feeding methods. Commencing formula feeding at >4 months in the infant's life confers a protective role against early introduction of solids. Therefore breast-feeding to close to 6 months of age should be encouraged. However, those mothers who choose not to breast-feed should be supported to understand their infant's signs of satiety and to reduce the amount of milk intake during the introduction of complementary foods, in order to avoid impairing the infant's self-regulating capacity and overfeeding.
The maintenance of a healthy weight in women during their reproductive years is pivotal to increase the chances of breast-feeding not only from the biological point of view, but also because behavioural pathways of overweight and obese women could lead to early weaning. Education on positive health behaviours is extremely important particularly for those women in the lower SES groups.
Of these findings, the promotion of breast-feeding to near 6 months of age together with education on appropriate bottle-feeding practices for those mothers who choose not to breast-feed would seem to be areas in need of immediate action on the part of health-care staff working with infants and mothers. Overweight and obese mothers and those with less education or in lower SES groups also require more support when making decisions about infant feeding.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflicts of interest: The authors declare no conflict of interest. Authors’ contributions: P.D.C. contributed towards data analysis and interpretation and led the writing; J.K. helped in interpreting the results and providing feedback on drafts of the paper; R.L. helped in interpreting the results and provided critical feedback on the statistical analysis of the data as well as methods used to collect same. All authors approved the final version of the paper.







