Introduction
CHD is the most common birth defect in developed countries, accounting for approximately one-third of all congenital anomalies diagnosed at birth. 1,2 Around 1 in 10,000 babies are born with a severe CHD resulting in a single-ventricle circulation that cannot be repaired into a 2-ventricle circulation. 1,Reference Qu, Liu and Zhuang3 For the vast majority of children born with a single-ventricle circulation, surgical intervention is imperative for survival beyond childhood. Reference Clift and Celermajer4 The Fontan procedure is the repair strategy of choice for most children with a single-ventricle circulation and is the final stage of a series of reconstructive surgical procedures to develop a Fontan circulation. Reference Marshall, D’Udekem and Sholler5,Reference Iyengar, Shann, Cochrane, Brizard and d’Udekem6 With advancements in surgical and cardiac care, the number of children living into adulthood with a Fontan circulation continues to grow; the number of adults now exceeds children. However, this expanding population lives with unique cardiovascular physiology, resulting in complex medical problems extending beyond the cardiovascular system. Reference Marshall, D’Udekem and Sholler5–Reference Rychik, Goldberg and Rand10
People living with a Fontan circulation represent a diverse population from a nutritional perspective; their nutritional needs are not static, and each person across their lifetime will have different requirements depending on their stage of life, nutritional status, and comorbidities. Protein-losing enteropathy, growth deficits, bone mineral loss, and malabsorption are well-recognised nutritional concerns within this population, but increased adiposity, altered glucose metabolism, and skeletal muscle deficiency are also more recently identified issues. Reference Rychik, Atz and Celermajer7–Reference Powell, Wittekind and Alsaied20 The role of nutrition in supporting optimal health outcomes for people living with Fontan circulation is not well established and the evidence is lacking. Many issues remain poorly understood, including the impact of the Fontan circulation on people’s nutritional status, the factors that contribute to their altered body composition, and whether people with a Fontan circulation have different energy and protein requirements than the general population. This narrative review aims to summarise the current literature focused on nutritional considerations in the setting of a Fontan circulation and highlight uncertainties that should be addressed in future research.
A clinical perspective on the Fontan physiology
In a single-ventricle heart, without surgical intervention, functionally only one ventricle pumps blood around the body. Reference Ritmeester, Veger and van der Ven13 Fontan surgery involves restructuring the circulatory system so that the inferior and superior vena cava are connected directly to the pulmonary arteries. Reference Zentner, Celermajer and Gentles11,Reference Amodeo, Galletti and Marianeschi21 The resulting Fontan circulation enables deoxygenated blood to be redirected to the lungs, bypassing the heart, and preventing the mixing of oxygenated and deoxygenated blood. This prevents a large volume load on the single ventricle and alleviates severe cyanosis. Reference Zentner, Celermajer and Gentles11,Reference Ritmeester, Veger and van der Ven13,Reference Amodeo, Galletti and Marianeschi21 Fontan physiology results in a chronic elevation in systemic and portal venous pressure, lymphatic dysfunction, reduced systemic blood flow, and for many, reduced oxygen saturations; these alterations affect almost every system in the body. Reference Rychik, Atz and Celermajer7,Reference Rychik, Goldberg and Rand10,Reference Zentner, Celermajer and Gentles11,Reference Ritmeester, Veger and van der Ven13,Reference Amodeo, Galletti and Marianeschi21
Nutrition and metabolism principles
Energy, protein, and metabolism are integral to survival. A homeostatic state is achieved when the energy consumed is equal to the energy expended from tissues and substrates. Reference Westerterp and Schols22,23 The body expends a minimum amount of energy at rest to support vital functions (known as resting energy expenditure), however, quantifying the energy required is highly individualised and is driven by determinants including age, gender, and body composition (fat mass and fat-free mass). 23 Resting energy expenditure accounts for 60–70% of all total energy expended by an individual. 23 A balance between the synthesis and breakdown of protein is integral to preserving skeletal muscle mass; increasing or decreasing the rate of either synthesis or breakdown can drive growth or loss. Reference Sobotka, Allison, Forbes and Meier24,Reference Fürst25 Reduced skeletal muscle mass has metabolic implications such as the reduced uptake of excess glucose, which is stored as glycogen in skeletal muscle. Reference Sobotka, Allison, Forbes and Meier24–Reference Sinacore and Gulve27 From the perspective of body composition, skeletal muscle mass is a stronger driver of variability in resting energy expenditure in adults and adolescents, whereby weight increases (both for fat-free mass and fat mass) lead to increases in resting energy expenditure. Reference Westerterp28–Reference Westerterp30
Significant stress through illness or injury, as theorised in those with a Fontan circulation, can activate the acute phase response, which is an adaptive response for survival. Reference Cuthbertson31–Reference Barendregt, Soeters, Allison and Sobotka34 This response can lead to alterations in metabolism (i.e., lipid, glucose, amino acids) and neuroendocrine activity (i.e., pro-inflammatory cytokines, growth hormones), consequently increasing energy expenditure and promoting catabolism, and potentially compromising nutritional status and body composition. Reference Westerterp and Schols22,23,Reference Preiser32,Reference Preiser, Ichai, Orban and Groeneveld33,Reference Hammarqvist, Wernerman and Allison35 It is not known whether there are changes in the resting energy expenditure in people living with a Fontan circulation, particularly adolescents or adults. Over their life trajectory, there are multiple stressors (i.e., inflammation, injury, surgical intervention, oxygen supply) that could influence the resting energy expenditure of a person living with a Fontan circulation. Reference Preiser32 For example, under the conditions of cyanosis or hypoxaemia, whereby there is a disruption to the normal supply of oxygen, these stressors induce a metabolic response to decrease resting energy expenditure which is proportionate to the severity of oxygen deficiency. Reference Ritmeester, Veger and van der Ven13,Reference Preiser32,Reference Li, Hafeez and Noorulla36
Fontan physiology influences nutritional status
A wide range of factors are likely to influence the nutritional status of people who have a Fontan circulation across their life continuum at a physiological and biochemical level as shown in Fig. 1.
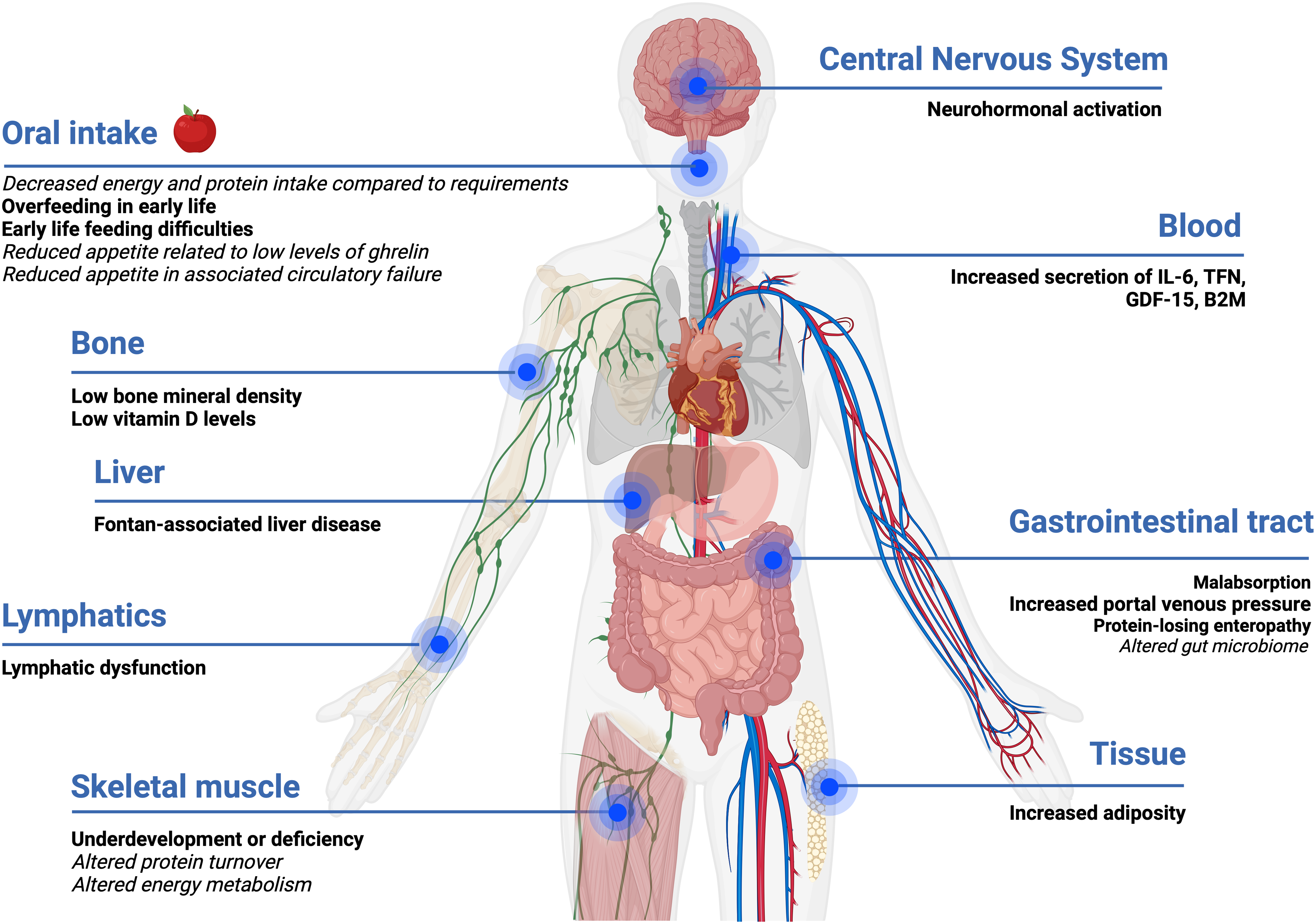
Figure 1. Impact and influence of Fontan physiology on nutrition status. Evidence-based statements are in bold, and hypotheses are in italics. Created with Biorender.com.
Inflammation and neurohormonal activation
The Fontan circulation may lead to a chronic systemic inflammatory state, even in those considered stable, promoting the secretion of pro-inflammatory cytokines (interleukin-6 (IL-6), tumour necrosis factor (TNF-α), growth/differentiation factor-15 (GDF-15), and beta-2 macroglobulin (B2M)). Reference Shiina, Nagao, Shimomiya and Inai37–Reference Shiina, Murakami and Matsumoto39 With worsening of clinical status, this inflammatory state worsens further impacting energy metabolism and body composition. Reference Shiina, Nagao, Shimomiya and Inai37 Neurohormonal activation associated with Fontan physiology is likely to lead to changes in skeletal muscle protein synthesis or breakdown.
Gastrointestinal system, metabolomics, and the gut microbiome
Fontan physiology is characterised by chronically elevated systemic venous pressure, which consequently leads to a passive increase in portal venous pressure and lymphatic dysfunction, which may alter gastrointestinal tract absorption and result in the loss of nutrients (i.e., protein, vitamin D, calcium). Reference Rychik, Atz and Celermajer7,Reference Cordina, O’Meagher and Gould18,Reference RochéRodríguez and DiNardo40 Very little is known about the gut microbiome and metabolomic profile of people living with a Fontan physiology, both of which are likely to be abnormal, particularly if absorption is altered. Research investigating the gut microbiome in people with conditions, such as cystic fibrosis and heart failure, has consistently demonstrated abnormalities compared to the general population. Reference van Dorst, Tam and Ooi41,Reference Linz and Schnabel42 Preliminary research in adults with a Fontan circulation has noted alterations in amino acid metabolism, phospholipid metabolism, and the tricarboxylic cycle. Reference Motoki, Motoki and Utsumi43–Reference Michel, Dubowy and Zlamy45 Together, the gut microbiome and metabolites play an important role in ensuring essential functions are performed including the production of vitamins and amino acids, regulation of the immune system, and maintaining the epithelial barrier of the gastrointestinal system. Reference Ahmed, Roy, Khan, Septer and Umar46,Reference Clemente, Manasson and Scher47 Over the course of a person’s life, environmental factors of diet and medication usage have a role in influencing the composition of the gut microbiome. Reference van Dorst, Tam and Ooi41 Imbalances or disruptions to the gut microbiome at any life stage are known to influence metabolism, immunity, nutrition, and physiology. Reference van Dorst, Tam and Ooi41,Reference Linz and Schnabel42
Abnormal body composition
Body composition is frequently abnormal in the setting of a Fontan physiology and is typically characterised by elevated adiposity and reduced skeletal muscle mass in children and adults. Reference Tran, D’Ambrosio and Verrall8,Reference Cao, Tran and Briody9,Reference Cordina, O’Meagher and Gould18,Reference Powell, Wittekind and Alsaied20 Although body mass index is usually within a “healthy range,” reduced skeletal muscle mass (referred to as Fontan-associated myopenia) is offset by increased adiposity. Reference Tran, D’Ambrosio and Verrall8 In children with a Fontan circulation, above healthy weight range is associated with protection against adverse clinical outcomes. Reference Cao, Tran and Briody9,Reference Payne, Garden and d’Udekem48,Reference Chung, Hong, Patterson, Petit and Ham49 In contrast, elevated adiposity is associated with poor clinical outcomes in adults living with a Fontan circulation including risk for Fontan circulatory failure and ventricular dysfunction. Reference Tran, D’Ambrosio and Verrall8,Reference Cao, Tran and Briody9 Assessment of an individual’s body composition provides a relative indication of the proportion and distribution of body mass compartments—fat mass and fat-free mass. 23 Information derived from body composition assessments is fundamental in evaluating an individual’s nutritional status, assessing relative health risks, and monitoring the effectiveness of nutrition management strategies. 23,Reference Shaw50 No single method (i.e., body mass index, bioelectrical impedance analysis, or dual-energy X-ray absorptiometry) is recognised as the gold standard for body composition assessments in people with a Fontan circulation. Reference Powell, Wittekind and Alsaied20,23,Reference Shaw50 Body mass index (kg/m2), although widely used for simplicity, is particularly limiting as there is no distinction between fat-free mass and fat mass. 23,Reference Shaw50 Other factors that influence body mass index include ethnicity, age, sex, and body composition (i.e., high muscle mass). 23,Reference Shaw50
Fontan-associated myopenia
While reductions in skeletal muscle (also known as fat-free mass) are prevalent in those with complex CHD, limited data have suggested that the deficiencies are more pronounced in the setting of a Fontan circulation, particularly in the lower half of the body. Reference Tran, D’Ambrosio and Verrall8,Reference Cao, Tran and Briody9,Reference Shiina, Murakami and Matsumoto39,Reference Sandberg, Johansson and Christersson51–Reference Hansson, Sandberg and Ohlund53 A study by Powell and colleagues observed a 14% increase in adiposity and a 15% decrease in lean muscle mass in adolescents with a Fontan circulation when compared to those with complex CHD. Reference Powell, Wittekind and Alsaied20 In the setting of Fontan physiology, this is especially concerning because the skeletal muscle in the lower limbs acts as a peripheral muscle pump contributing to venous return and cardiac filling. Reference Ritmeester, Veger and van der Ven13,Reference Cordina, O’Meagher and Gould18 Fat-free mass loss is more severe in people who experience long-term complications associated with their Fontan circulation such as plastic bronchitis, protein-losing enteropathy, and circulatory failure. Reference Powell, Wittekind and Alsaied20 The reduction in skeletal muscle mass is more accurately described by the term myopenia (Fontan-associated myopenia) rather than sarcopenia. Reference Tran, D’Ambrosio and Verrall8,Reference Cao, Tran and Briody9 . Although their diagnostic criteria are similar, sarcopenia is characterised by a progressive muscle loss associated with ageing, in contrast to myopenia that may, at least in part, be developmental or “intrinsic.” Reference Tran, D’Ambrosio and Verrall8,Reference Cao, Tran and Briody9,23,Reference Shiina, Nagao, Shimomiya and Inai37,Reference Shaw50,Reference Sandberg, Johansson and Christersson51,Reference Maurer and Tutarel54–Reference Cruz-Jentoft, Baeyens and Bauer57
Fontan-associated myopenia is likely due to a myriad of factors, which are poorly characterised. These factors include, but are not limited to, physical inactivity, increased protein turnover in the myocardium and skeletal muscle, increased energy requirements of the peripheral pump with exercise, metabolic alteration of substrates (i.e., glucose, lipids, amino acids) secondary to body composition changes, inadequate dietary intake compared to nutritional requirements, malabsorption, nutrition-impact symptoms, feeding difficulties during childhood, and reduced appetite secondary to low levels of the appetite hormone ghrelin. Reference Ritmeester, Veger and van der Ven13,Reference Ohuchi, Miyamoto and Yamamoto15,Reference Ohuchi, Negishi and Hayama16,Reference Cordina, O’Meagher and Gould18,Reference Powell, Wittekind and Alsaied20,Reference Shiina, Murakami and Matsumoto39,Reference Michel, Dubowy and Entenmann44,Reference Michel, Dubowy and Zlamy45,Reference Cordina and d’Udekem52,Reference Michel, Zlamy and Entenmann58–Reference O’Connell, Logsdon, Mitscher and Payne62 Potential mitigators of suboptimal body composition include amino acid supplementation, nutritional education, and exercise training, however, there is a lack of evidence on the therapeutic effectiveness except for isolated resistance training. Reference Shiina, Nagao, Shimomiya and Inai37,Reference Cordina and d’Udekem52,Reference Collamati, Marzetti and Calvani63 In addition to reduced mass, the skeletal muscle may also be dysfunctional. Unsurprisingly, reduced muscle mass is associated with reduced strength. Reference Tran, D’Ambrosio and Verrall8 There is also abnormal skeletal muscle oxygen uptake and autonomic dysfunction in the setting of Fontan physiology. Reference Brassard, Bedard, Jobin, Rodes-Cabau and Poirier64,Reference Brassard, Poirier and Martin65 A study assessing skeletal muscle metabolism (phosphocreatine recovery) using magnetic resonance spectroscopy before, during, and after exercise in adults with a Fontan circulation demonstrated impairments in the aerobic capacity of skeletal muscle compared to controls. Reference Cordina, O’Meagher and Gould18,Reference Bogdanis66
Increased adiposity
In general, body mass index underestimates adiposity in people with a Fontan circulation as the increased fat mass is masked by reduced fat-free mass. Reference Tran, D’Ambrosio and Verrall8,Reference Powell, Wittekind and Alsaied20,Reference Chung, Hong, Patterson, Petit and Ham49,Reference Martinez, Byku and Novak67–Reference Freud, Webster and Costello69 As health outcomes and survival continue to improve beyond the Fontan procedure, so does the prevalence of people with a Fontan circulation who are above a healthy weight range. Reference Tran, D’Ambrosio and Verrall8,Reference Powell, Wittekind and Alsaied20,Reference Chung, Hong, Patterson, Petit and Ham49,Reference Martinez, Byku and Novak67–Reference Freud, Webster and Costello69 Research investigating the weight trajectories of people with a Fontan circulation has demonstrated that adolescence is often the period when weight gain occurs. Reference Payne, Garden and d’Udekem48 Concerningly, adiposity tends to accumulate in the trunk or abdominal area. Reference Cao, Tran and Briody9,Reference Hansson, Sandberg and Ohlund53,Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70 Given this distribution tendency, there may be merit in measuring waist circumference across all ages to aid in monitoring for excess adiposity although this measurement will be confounded in the presence of ascites. 23,Reference Shaw50,Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70–72 Ascites is likely to develop in 17% of those with a Fontan circulation as a subsequent complication of Fontan-associated liver disease. Reference Wu, Kogon and Earing73 In broader populations, there is no consensus on the use of waist circumference or waist-to-height ratio within international guidelines. Reference Lau, Douketis and Morrison71,72,74–Reference Hampl, Hassink and Skinner76 A limitation of this measurement is the absence of evidence determining an appropriate threshold associated with morbidity risk in children. Reference Lau, Douketis and Morrison71,72,74–Reference Hampl, Hassink and Skinner76
Nutritional considerations prior to Fontan completion
The nutritional management of children prior to the completion of Fontan surgery has been described in detail elsewhere. Reference Salvatori, De Rose and Massolo14,Reference Baldini, Librandi, D’Eusebio and Lezo77,Reference Herridge, Tedesco-Bruce, Gray and Floh78 The pre-Fontan circulation stage is recognised to overlap a sensitive period for growth and development, whereby disturbances in nutrition can significantly impact growth trajectories later in life and the risk of adverse outcomes (i.e., length of stay, quality of life, mortality, wound healing, morbidity). Reference Shine, Foyle, Gentles, Ward and McMahon12,Reference Salvatori, De Rose and Massolo14,23,Reference Nydegger and Bines79–Reference Martini, Beghetti and Annunziata81 Abnormalities often originate in the prenatal phase and manifest as fetal growth restriction (previously known as intrauterine growth restriction). Reference Salvatori, De Rose and Massolo14,Reference Courtney, Troja and Owens80,Reference Ghanchi, Derridj and Bonnet82–Reference Story, Pasupathy, Sankaran, Sharland and Kyle87 Approximately 20% of all CHD are diagnosed with fetal growth restriction, while in non-syndromal babies, fetal growth restriction has been shown to be associated with certain types of CHD (i.e., Tetralogy of Fallot). Reference Salvatori, De Rose and Massolo14,Reference Courtney, Troja and Owens80,Reference Ghanchi, Derridj and Bonnet82–Reference Story, Pasupathy, Sankaran, Sharland and Kyle87 Newborn weight for those with single-ventricle physiology is reported to be within the normal range for their gestational age, but comparatively lower than the general population matched for age. Reference Shine, Foyle, Gentles, Ward and McMahon12,Reference Baldini, Librandi, D’Eusebio and Lezo77,Reference Courtney, Troja and Owens80,Reference Martini, Beghetti and Annunziata81,Reference Story, Pasupathy, Sankaran, Sharland and Kyle87,Reference Burch, Gerstenberger and Ravishankar88 However, a child’s birth weight is not a predictor of their weight gain trajectory; children who are born with low birth weight are more likely to demonstrate greater gains in weight than those who were born at higher birth weight. Reference Burch, Gerstenberger and Ravishankar88 The nutritional efforts (i.e., oral intake, enteral nutrition) for growth catch-up, particularly for weight, may influence body composition leading to an increased accumulation of adiposity rather than lean muscle mass later in life. Reference Payne, Garden and d’Udekem48,Reference Burch, Gerstenberger and Ravishankar88
Paediatric growth and age-related development
Children with a Fontan circulation may have growth and age-related development concerns (i.e., pubertal delays), both of which are known to potentially impact body composition. Reference Powell, Wittekind and Alsaied20,Reference Payne, Garden and d’Udekem48,Reference Shaw50,Reference Hansson, Sandberg and Ohlund53,Reference Freud, Webster and Costello69,Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70,Reference McCrindle, Williams and Mital89 Over the last decade, the growth deficits experienced by children with a Fontan circulation compared to their peers have been increasingly well characterised. Reference Freud, Webster and Costello69,Reference Baldini, Librandi, D’Eusebio and Lezo77,Reference Mancilla, Zielonka and Roizen90–Reference Cohen, Zak and Atz95 Terms frequently interchangeably utilised to describe growth deficits include growth failure, malnutrition, poor growth, faltering growth, and undernutrition. It is important to recognise these terms do not necessarily share the same definition or diagnostic criteria. 23,Reference Shaw50 Research consistently demonstrates that children with a Fontan circulation are at a higher risk of growth deficits or delays, both for height and/or weight, which have been shown to persist for several years following their Fontan surgery. Reference Freud, Webster and Costello69,Reference Mancilla, Zielonka and Roizen90–Reference Cohen, Zak and Atz95 Studies have also demonstrated that those children who experience clinical complications, such as protein-losing enteropathy, are more likely to experience further significant deficits in their height. Reference Rychik, Goldberg and Rand10,Reference Mancilla, Zielonka and Roizen90 It is also recognised that the delayed onset of puberty, initiated by the hypothalamic-pituitary-gonadal axis, could also contribute towards growth deficits in children with a Fontan circulation. 23 Children with a Fontan circulation frequently experience delayed puberty; one study found that, on average, 50% of children aged 8 to 16 years were delayed by one Tanner stage parameter and noted late pubertal physiological changes. Reference Menon, Al-Dulaimi and McCrindle91,Reference Avitabile, Goldberg and Zemel96,Reference Avitabile, Leonard and Zemel97 When comparing the pubertal development to the general population for age using the Tanner stage, those with a Fontan circulation were shown to be 2 years delayed in more than one parameter. Reference Menon, Al-Dulaimi and McCrindle91 The aetiology of growth deficits and pubertal delays is not fully understood, although are likely related to a host of factors such as increased nutritional requirements, chronic hypoxaemia, inadequate dietary intake, malabsorption, low cardiac output, altered eating behaviours, neurohormonal activation, as well as the impact of Fontan physiology on other organ systems. Reference Ritmeester, Veger and van der Ven13,23,Reference Shaw50,Reference Mancilla, Zielonka and Roizen90,Reference Cohen, Zak and Atz95,Reference Payne, Wilson and Haghighi98,Reference Khairy, Fernandes and Mayer99 Delays in pubertal development may also impact adult height; limited studies have demonstrated that adult men and women with a Fontan circulation (>21 years) are shorter compared to the general population. Reference Lambert, McCrindle and Pemberton92,Reference Sandberg, Rinnstrom and Dellborg100
Overfeeding and growth catch-up nutrition strategies
There are no specific nutritional guidelines that include recommendations for optimising growth catch-up in children with a Fontan circulation. General advice based on other populations suggests that optimal catch-up growth in children should promote a balanced accumulation of approximately 73% lean muscle mass and 27% fat mass to achieve weight and height gains, through dietary supplementation of energy and protein, which is appropriate for the child’s nutritional requirements for their age, sex, weight, and clinical condition. 23,Reference Shaw50 Without a nutritional target to promote catch-up growth, it is imperative that dietary intakes and anthropometric measurements are routinely monitored to ensure nutritional adequacy is achieved. Reference Rychik, Goldberg and Rand10 However, this approach remains problematic because, without clear targets on the energy and protein to achieve optimal catch-up growth, it raises concerns about whether current nutrition provision strategies are fostering overfeeding. Overfeeding is shown to be associated with rapid weight gains, contributing to increased adiposity, and weights above their healthy range later in life. 23,Reference Shaw50,Reference Zheng, Lamb and Grimes101,Reference Ong, Kennedy and Castañeda-Gutiérrez102 Unfortunately, there is no evidence available to compare the dietary intake of children with a Fontan circulation to their energy requirements. In children with complex CHD, nutrition provision is often higher in energy and frequency in comparison to healthy controls matched for age, although still not adequate for estimated requirements for age-related growth. Reference Hansson, Öhlund, Lind, Stecksén-Blicks and Rydberg103
Nutrition and dietary intake in people with a Fontan circulation
Research on the dietary intake and eating patterns of people living with CHD is limited. Hansson et al. conducted a follow-up study in Swedish children with complex CHD, including those corrected by a Fontan circulation, compared to healthy controls matched for age at 1 year, 4 years, and 9 years of age; there was a higher intake of fats and a lower intake of cardiovascular protective foods from the food groups fruits, vegetables, dairy, and whole grains in children with complex CHD. Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70 A Japanese study demonstrated that the dietary patterns of people with CHD, including those with a Fontan circulation, were cardio-protective with a higher intake of vegetables, fruits, fish, and dairy products compared to the general population. Reference Shiina, Matsumoto and Okamura56 Similar findings were reported by Jackson et al. in American adolescents and adults with CHD in terms of dietary fat intake. Reference Jackson, Tierney, Daniels and Vannatta104 Additionally, it was found that individuals with lower dietary fat intake had a greater understanding of general (i.e., identify protective or risk-reducing actions) and risk knowledge (i.e., identify the risk of cardiac-related complications) about their CHD. Reference Jackson, Tierney, Daniels and Vannatta104 It has been postulated that the dietary profile in children with complex CHD could reflect nutritional advice to promote growth catch-up earlier in life that recommended consuming foods with higher energy contributions such as discretionary foods. 23,Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70 Not surprisingly, adherence to this dietary profile beyond growth stabilisation could contribute to increased metabolic risk and the adverse body composition trends reported in adolescents and adults with a Fontan circulation. 23,Reference Hansson, Lind, Öhlund, Wiklund and Rydberg70
Energy requirements
Research on resting energy expenditure in people with a Fontan circulation across all ages is extremely limited and is focused on the early post-operative period. Reference Mehta, Costello and Bechard105 In the setting of Fontan physiology, energy requirements may be altered secondary to malabsorptive losses, the presence of inflammation, surgical intervention, and cyanosis. Reference Rychik, Atz and Celermajer7,Reference Ritmeester, Veger and van der Ven13,Reference Nydegger and Bines79,Reference Nydegger, Walsh, Penny, Henning and Bines106 Theories regarding the energy expenditure in people with a Fontan circulation are largely underpinned by evidence in children related to growth trajectories, catch-up growth, and body composition changes but of course, other factors are also likely to influence energy expenditure including gender, age, sex, age-appropriate growth, body weight, body composition, exercise, nutritional intake (including nutrition support), medical therapies, stress, pain, injury, trauma, infection, inflammation, illness severity, organ failure, and sympathetic nervous system activity. 23,Reference Westerterp30,Reference Preiser32,Reference Preiser, Ichai, Orban and Groeneveld33 There is contention within the literature regarding whether children with a Fontan circulation have higher energy requirements than their peers matched for age and sex or compared to those with CHD. There is no data on the estimated energy requirements or energy expenditure for adolescents or adults living with a Fontan circulation.
Comorbidities and additional considerations
Malnutrition
Malnutrition is defined by the European Society for Clinical Nutrition and Metabolism consensus as a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased muscle mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcomes from disease. Reference Cederholm, Barazzoni and Austin107 Unintentional loss of weight, muscle mass, and adiposity are common characteristics of malnutrition for all, in addition to potential growth and development deficits in children. 23,Reference Cederholm, Barazzoni and Austin107 No specific malnutritional screening or assessment tools are validated in people living with CHD. Malnutrition is considered a significant health issue in Australia and internationally; up to 50% of hospitalised adult patients on admission and anywhere from 14 to 55% of children hospitalised in developed countries are found to experience malnutrition, but there remains uncertainty as to whether these figures are a true representation. Reference Cass and Charlton108,Reference White, Dennis and Ramsey109 Limited studies exist on children with complex CHD; it is estimated that 33–52% of children experience malnutrition, particularly during infancy (1–2 years) when rates of surgical intervention are high. Reference Herridge, Tedesco-Bruce, Gray and Floh78
People with a Fontan circulation should be considered at high risk of malnutrition at any life stage. A retrospective study evaluating the prevalence of malnutrition in children who underwent Fontan surgery identified rates ranging from 6 to 16%, of moderate-severe malnutrition defined based on Z-scores of weight, height, and body mass index for age. Reference Sekhon, Foshaug and Kantor110 More surprisingly, moderate-severe malnutrition was identified in 38% of participants at any point in the 10 years following their Fontan procedure. Reference Sekhon, Foshaug and Kantor110 Several evidence-based nutrition guidelines are available to inform the management of malnutrition in population groups including cystic fibrosis and cancer. Reference Saxby, Paintern and Kench111–Reference Muscaritoli, Arends and Bachmann113 Nutritional advice to manage malnutrition is generally consistent, focusing on nutrition interventions (i.e., nutrient-rich foods, oral nutrition support) and nutrition education. Reference Saxby, Paintern and Kench111–Reference Muscaritoli, Arends and Bachmann113 Those with malnutrition are encouraged to eat small frequent meals (i.e., three main meals and three snacks) of nutrient-rich foods that are high in energy and protein. It is strongly emphasised that foods are chosen to optimise enjoyment and taste to encourage better oral intake. Reference Saxby, Paintern and Kench111,Reference Kiss, Loeliger and Findlay112 Modifications are recommended in instances where a person is unable to meet their estimated energy and protein requirements. Additional nutrition provision is encouraged by 1) high-energy high-protein fortification to increase energy and protein intake without having to consume more food; or 2) oral nutrition supplements. Reference Saxby, Paintern and Kench111–Reference Muscaritoli, Arends and Bachmann113 Given that children around the time of their Fontan surgery experience malnutrition and issues impacting their nutrition status, a pragmatic approach would be to supplement suboptimal intakes to mitigate nutritional risk deficits and their impact. Reference Sekhon, Foshaug and Kantor110 The prevalence of, and contributors to, malnutrition amongst adolescents and adults with a Fontan circulation are not known.
Altered glucose metabolism
It is unclear whether increased abdominal adiposity in the setting of Fontan physiology increases the risk of metabolic syndrome, as established in the general population. Reference Hansson, Sandberg and Ohlund53 Metabolic abnormalities of impaired glucose tolerance, insulin resistance, and diabetes mellitus are increasingly recognised as comorbidities associated with Fontan physiology. Reference Ritmeester, Veger and van der Ven13,Reference Ohuchi, Miyamoto and Yamamoto15,Reference Ohuchi, Negishi and Hayama16 Although the pathophysiologic mechanisms explaining abnormalities in glucose metabolism are not fully understood, it has been postulated that it may be related to Fontan-associated liver disease or altered body composition and exacerbated by physical inactivity. Reference Ritmeester, Veger and van der Ven13,Reference Ohuchi, Negishi and Hayama16 Decreased adiponectin, an insulin sensitivity biomarker, has been associated with increased adiposity in adults with a Fontan circulation and may reflect insulin resistance. Reference Powell, Wittekind and Alsaied20,Reference Hivert, Sullivan and Fox114 The role of skeletal muscle mass in the development of pre-diabetes mellitus has been observed among groups with altered body composition (i.e., sarcopenia, elderly) who are shown to have an increased risk of insulin resistance or impaired glucose tolerance, and this phenomenon may occur in those with Fontan physiology. Reference Srikanthan and Karlamangla26,Reference Sinacore and Gulve27,Reference Moon115 Limited studies have demonstrated that adults with a Fontan circulation have altered glucose metabolism with a reported average prevalence of up to 40% for impaired glucose tolerance and up to 5% for diabetes mellitus. Reference Ohuchi, Miyamoto and Yamamoto15,Reference Ohuchi, Negishi and Hayama16 Although the prevalence of impaired glucose tolerance is relatively high in comparison to those without a Fontan circulation, interestingly the prevalence of diabetes mellitus is relatively low given it is estimated that 70% of people with pre-diabetes during their life will progress to type 2 diabetes. Reference Ohuchi, Miyamoto and Yamamoto15,Reference Ohuchi, Negishi and Hayama16,Reference Tabák, Herder, Rathmann, Brunner and Kivimäki116,Reference Nathan, Davidson and DeFronzo117 More longitudinal studies are needed to determine the incidence of impaired glucose tolerance and diabetes mellitus within the ageing Fontan population. Reference Ritmeester, Veger and van der Ven13,Reference Ohuchi, Negishi and Hayama16 The role of modifiable factors (i.e., dietary patterns, weight, physical activity) in altered glucose metabolism among people living with a Fontan circulation is not known but are likely contributors due to their well-recognised role in metabolic pathways.
Reduced bone mineral density
Bone mineral density is frequently reduced in people with a Fontan circulation compared to the general population across all age groups but appears to be more common later in life. Reference D’Ambrosio, Tran and Verrall19,Reference Avitabile, Goldberg and Zemel96,Reference Goldberg, Dodds and Avitabile118–Reference Diab, Godang and Müller122 The pathophysiology of these bone alterations remains undetermined but is likely multifactorial. Probable contributors include reduced physical inactivity, malabsorption, vitamin D deficiency, medications such as warfarin, skeletal muscle deficits, neurohormonal activation, protein-losing enteropathy, liver dysfunction, and haemodynamic alterations inherent to Fontan physiology such as high venous pressure, reduced blood flow, and cyanosis. Reference Rychik, Atz and Celermajer7,Reference Ritmeester, Veger and van der Ven13,Reference D’Ambrosio, Tran and Verrall19,Reference Avitabile, Goldberg and Zemel96,Reference Diab, Godang and Müller122 In the general population, warfarin usage has been associated with reduced bone mineral density, although the dietary patterns of people using warfarin (i.e., low vitamin K intake) may also contribute. Reference Signorelli, Scuto and Marino123,Reference Fawzy and Lip124 . Bone mineral density in children with a Fontan circulation has been observed to be lower in those on warfarin compared to aspirin, while in adults, the association is less clear. Reference Attard, Monagle and d’Udekem17,Reference D’Ambrosio, Tran and Verrall19,Reference Bendaly, DiMeglio, Fadel and Hurwitz120 Skeletal muscle is also recognised as another important determinant of bone health outcomes during childhood, whereby increases in skeletal muscle mass from growth or physical activity have been shown to induce adaptive changes in bones in dimension and strength gains. 23,Reference Shaw50,Reference Avitabile, Goldberg and Zemel96 While bone mineral density deficits in children and adults with a Fontan circulation are reported, no studies to date have diagnosed osteoporosis in children, while conversely, one small cohort study reported osteoporosis in around 5% of younger adults (19–33 years). Reference D’Ambrosio, Tran and Verrall19,Reference Avitabile, Goldberg and Zemel96,Reference Goldberg, Dodds and Avitabile118,Reference Bendaly, DiMeglio, Fadel and Hurwitz120,Reference Diab, Godang and Müller122 There is a paucity of evidence to inform dietary interventions and management strategies for bone health in people living with a Fontan circulation. In the wider population, it is recognised that weight-bearing exercise and nutrition are modifiable factors that can improve bone outcomes (i.e., bone mass development and maintenance) and prevent osteopenia and osteoporosis; addressing micronutrient deficiencies, particularly calcium and vitamin D, to support good bone health is warranted. Reference Rychik, Goldberg and Rand10,23,Reference Berger, Shenkin and Schweinlin125
Vitamin D deficiency
Vitamin D deficiency and secondary hyperparathyroidism are common in children and adults with a Fontan circulation and probably impact bone density. Reference D’Ambrosio, Tran and Verrall19,Reference Avitabile, Goldberg and Zemel96,Reference Diab, Godang and Müller122 It is unclear why people with a Fontan circulation may be more susceptible to vitamin D deficiency. The prevalence of vitamin D deficiency (<50 nmol/L) among children with a Fontan circulation is estimated to be 20–25%. Reference Hansson, Sandberg and Ohlund53,Reference Avitabile, Goldberg and Zemel96,Reference Avitabile, Leonard and Zemel97,Reference Sarafoglou, Petryk and Mishra119,Reference Diab, Godang and Müller122 Dietary data on vitamin D intake and levels in adults are lacking. One small cohort study in Australian adults (>16 years) with a Fontan circulation reported an average serum vitamin level of 66 nmol, with 24% of the cohort classified as deficient (<50 nmol/L). Reference D’Ambrosio, Tran and Verrall19 Other Fontan-associated factors that warrant further investigation include the impact of Fontan-associated liver disease on vitamin D metabolism, altered absorption from the gut with increased portal pressure and lymphatic dysfunction, and microvascular fibrosis that may impact the conversion pathways in the skin. Individuals are encouraged to include foods that are high sources of calcium and vitamin D in their daily intake, with supplementation of these micronutrients if they are unable to meet their recommended dietary intake. Reference Saxby, Paintern and Kench111,Reference Berger, Shenkin and Schweinlin125–129 In the general population, vitamin D supplementation in combination with calcium has been shown to be more effective for the promotion of bone mineral density than vitamin D alone. Reference Saxby, Paintern and Kench111,Reference Berger, Shenkin and Schweinlin125–129 For those who are diagnosed with osteopenia or osteoporosis, the goals of nutrition interventions are to 1) achieve optimal growth in children and maintain weight within a healthy weight range for all ages which preserves muscle mass; 2) achieve the recommended dietary intake of calcium and vitamin D through supplementation; 3) engage in regular exercise which is appropriate to a person’s life stage and health needs; and 4) aim for serum vitamin D above >50 nmol/L. Reference Saxby, Paintern and Kench111,Reference Berger, Shenkin and Schweinlin125–129
Fontan-associated liver disease
The pathophysiology and nutritional management of Fontan-associated liver disease have been described in detail elsewhere. Reference Gordon-Walker, Bove and Veldtman130,Reference Hilscher, Wells, Venkatesh, Cetta and Kamath131 Evidence is lagging to inform dietary management strategies for more advanced Fontan-associated liver disease. Reference Rychik, Atz and Celermajer7,Reference Zentner, Celermajer and Gentles11,Reference Gordon-Walker, Bove and Veldtman130 Nutritional advice is generally based on recommendations from other liver diseases and suggests limiting hepatotoxins including alcohol and maintaining a healthy weight range through a balanced nutritious diet. Reference Rychik, Atz and Celermajer7,Reference Zentner, Celermajer and Gentles11,Reference Gordon-Walker, Bove and Veldtman130 In general, cirrhosis has important implications for an individual’s nutrition status because energy and protein requirements are increased; secondary malnutrition is common and should be screened for routinely in nutrition assessments to ensure timely intervention to optimise body composition. Reference Bischoff, Bernal and Dasarathy132 Evidence-based guidelines derived from general liver disease in adults recommend that individuals with cirrhosis eat small frequent meals, aiming for a meal pattern of 3–5 main meals per day and 1 carbohydrate snack late in the evening to minimise the overnight fasting period. Food choices should be high in energy and protein and low in salt aiming for an energy intake of 126–147 kJ/kg/day (30–35 kcal/kg/day) and a protein intake of 1.2–1.5 g/kg/day. Reference Bischoff, Bernal and Dasarathy132 Modifications are recommended in instances of micronutrient deficiencies, ascites, obesity, or disease severity. Nutritional support via enteral or parenteral nutrition is only recommended when the individuals cannot tolerate food orally or cannot meet their nutritional targets from food alone. Reference Bischoff, Bernal and Dasarathy132 Dietary advice for children is dependent on their clinical status, symptomology, and degree of liver dysfunction. Reference Nightingale and Ng133
Protein-losing enteropathy
Previous reviews have described the pathophysiology and nutritional management of protein-losing enteropathy in Fontan physiology. Reference Alsaied, Lubert and Goldberg134,Reference Barracano, Merola, Fusco, Scognamiglio and Sarubbi135 A collaborative individualised treatment approach is recommended, one first-step approach being dietary intervention, as no single treatment is recognised as the most effective in resolving protein-losing enteropathy. Reference Zentner, Celermajer and Gentles11,Reference Alsaied, Lubert and Goldberg134 There is limited evidence from high-quality clinical trials evaluating the impact of dietary treatment of protein-losing enteropathy, therefore, nutrition targets for protein and fat within the literature are found to vary. Recommendations are to aim for less than 25 per cent of dietary fat energy (sourced from medium-chain triglycerides) and more than 2 g/kg of protein per day in both adults and children. Reference Rychik, Goldberg and Rand10,Reference Zentner, Celermajer and Gentles11,23,Reference Shaw50,Reference Alsaied, Lubert and Goldberg134,Reference Barracano, Merola, Fusco, Scognamiglio and Sarubbi135 Nutrition targets don’t differ between children, adolescents, and adults as they are calculated based on individuals’ body weight or percentage of total energy consumption. Additional dietary support should be considered for those experiencing chronic diarrhoea with electrolytes and micronutrients including folate, iron, and fat-soluble vitamins. Reference Zentner, Celermajer and Gentles11,23,Reference Alsaied, Lubert and Goldberg134,Reference Barracano, Merola, Fusco, Scognamiglio and Sarubbi135 This dietary approach is not long-term and is only recommended during periods of protein-losing enteropathy flares. Reference Rychik, Goldberg and Rand10 In those whose protein-losing enteropathy is managed by diuretic therapy, particularly potassium-sparing diuretics, ensuring serum potassium levels are optimised, considering dietary potassium is important. Reference Lamb, Kennedy and Raine136 In critical cases, where dietary intervention is unsuccessful, parenteral nutrition may be indicated. Reference Shaw50
Nutritional management
In general, nutritional management for children with a Fontan circulation can be divided into three distinct stages (see Fig. 2)—stage 3: the early post-operative phase (includes children aged 2–5 years pre- and post-Fontan surgery), stage 4: childhood beyond the early post-operative phase until adolescence (<12 years), stage 5: adolescence (12–18 years), followed by stage 6: adulthood (>18 years). 23,Reference Shaw50 Each of these distinct paediatric stages, even without the presence of an acute or chronic medical condition, has specific nutritional requirements for adequate growth and development. 23,Reference Shaw50 Further research is needed to assess the nutritional management goals and strategies for each of the three stages for children with a Fontan circulation.

Figure 2. Growth and body composition changes throughout life stages for people with a Fontan circulation. Evidence-based statements are in bold, and hypotheses are in italics. Created with Biorender.com.
In the setting of the Fontan physiology, the focus of nutrition interventions should be to 1) optimise nutritional status in children pre- and post-Fontan surgery to minimise growth deficits, or 2) maintain adolescents’ and adults’ weight within their healthy weight range through exercise, diet and limiting discretionary foods. Reference Rychik, Atz and Celermajer7,Reference Rychik, Goldberg and Rand10,Reference Zentner, Celermajer and Gentles11 There is a growing recognition of the need for multidisciplinary care, including nutrition and dietetics, to be embedded within Fontan clinical services to allow for adequately tailored appropriate interventions to achieve optimal long-term care outcomes. Reference Rychik, Goldberg and Rand10,Reference d’Udekem, Iyengar and Cochrane137 Current care models should ultimately incorporate nutrition-related concerns specific to people living with a Fontan circulation to be appropriately addressed and actioned with evidence-based recommendations. Reference Rychik, Goldberg and Rand10,Reference d’Udekem, Iyengar and Cochrane137 Guidance on the nutrition assessment of people living with a Fontan circulation is broad and generalised. A framework for clinicians to assess nutrition status and referral pathway to dietetic services is outlined in Figs. 3 and 4. Clinicians are recommended to use the framework to consider important indices in assessing nutrition status to identify Fontan-associated comorbidities and considerations warranting referral to dietetics services.
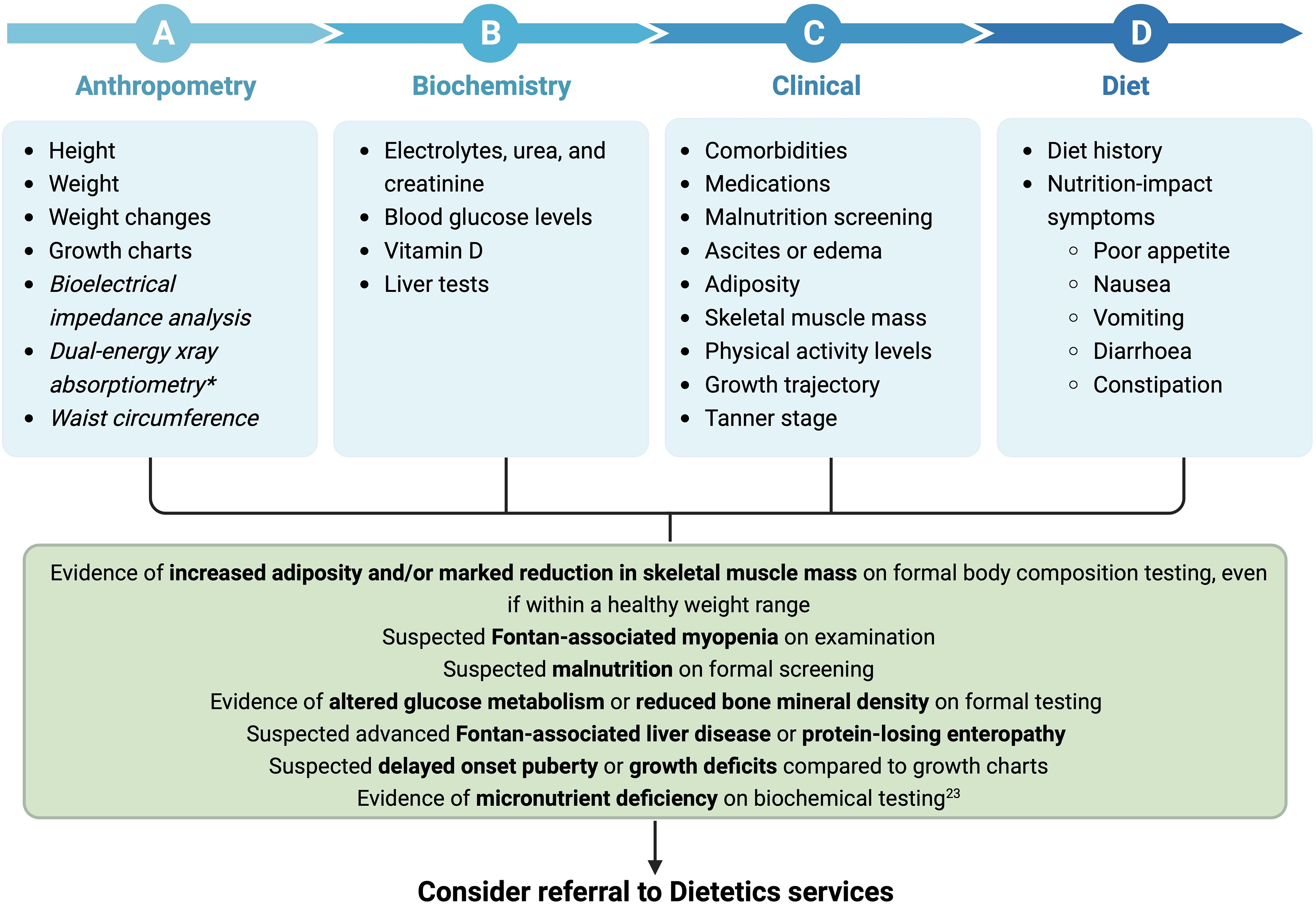
Figure 3. Clinical framework for nutrition assessment of paediatrics with a Fontan circulation. Unestablished tests are in italics. *Dual-energy X-ray absorptiometry provides small radiation exposure. Created with Biorender.com.
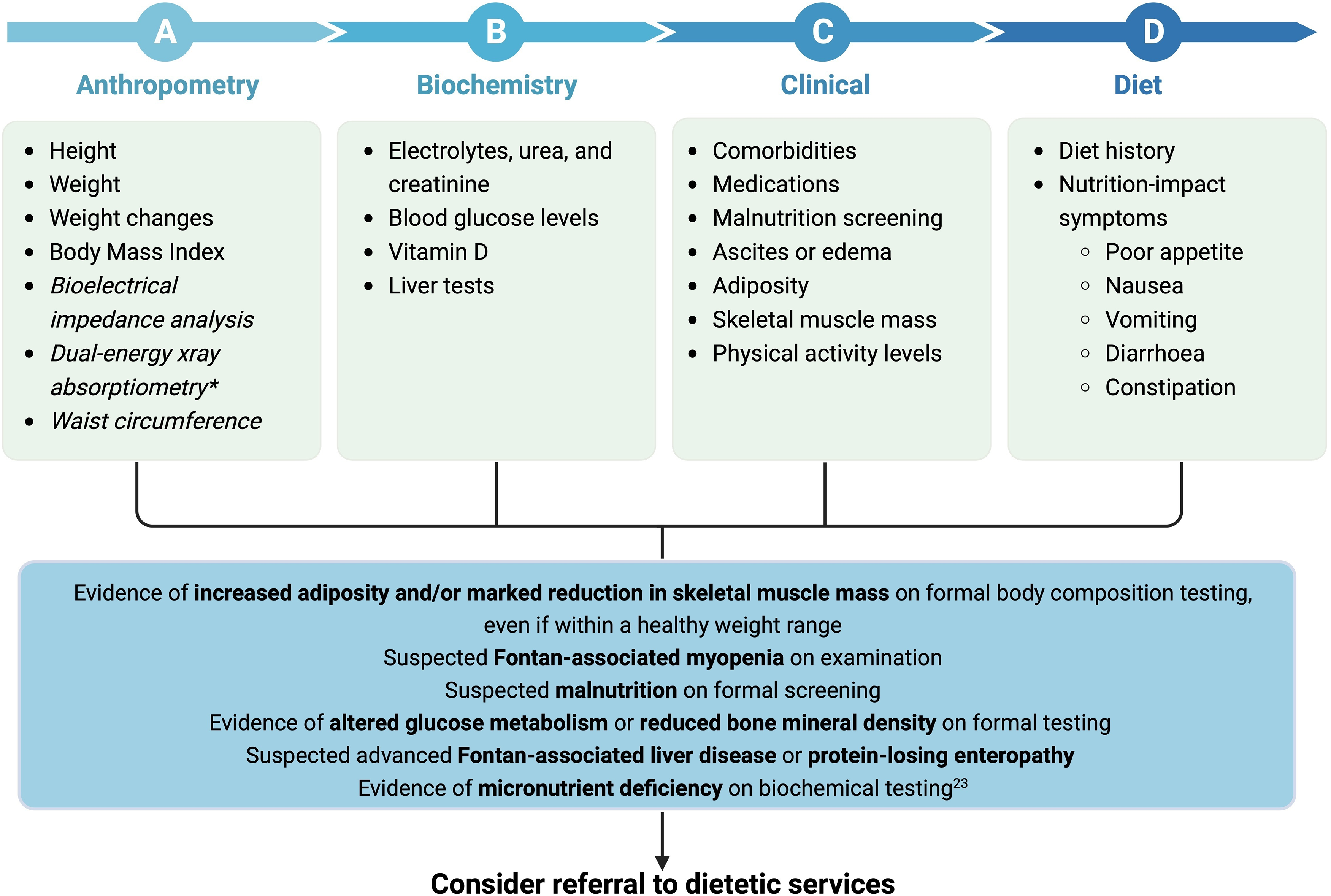
Figure 4. Clinical framework for nutrition assessment of adults with a Fontan circulation. Unestablished tests are in italics. *Dual-energy X-ray absorptiometry provides small radiation exposure. Created with Biorender.com.
General nutritional management in children living with a Fontan circulation
Paediatric nutritional guidelines are available to assist in informing the management of children with CHD broadly, however, the guidelines do not differentiate the type of cardiac surgery or severity of CHD. Reference Mehta, Skillman and Irving138,Reference Tume, Valla and Joosten139 International guidelines recommend providing 35–55 kcals/kg/day (146–230 kJ/kg/day) of energy and 1.5 g/kg/day of protein post-operatively. Reference Mehta, Skillman and Irving138,Reference Tume, Valla and Joosten139 While these nutritional recommendations are a broad target to ensure nutritional requirements are achieved, they do not reflect the variability in the nutritional needs of children with a Fontan circulation. Reference Baldini, Librandi, D’Eusebio and Lezo77 At present, the goal for children with a Fontan circulation is to optimise nutrition to minimise the risk of malnutrition and meet increased energy requirements for age-related growth and development, catch-up growth, and metabolic response to surgery. 23,Reference Preiser, Ichai, Orban and Groeneveld33 Energy requirements are usually calculated using predictive equations or general population values, which are adjusted based on factors including growth, body composition, physical activity levels, and condition-related stress/injury factors. 23,Reference Baldini, Librandi, D’Eusebio and Lezo77 Nutritional assessments theoretically should prioritise measuring children’s energy expenditure using indirect calorimetry, if available, in combination with dietary intake assessments (i.e., 3-day food record or 7-day diet history) to ensure optimal nutrition is achieved until further research can establish nutritional targets. Reference Rychik, Goldberg and Rand10,Reference Baldini, Librandi, D’Eusebio and Lezo77 Monitoring and assessments of growth, in terms of body composition and body mass index, are useful indicators to gauge if nutritional interventions are appropriate. Reference Rychik, Goldberg and Rand10 Most children living with a Fontan circulation are encouraged to adopt healthy eating principles from national guidelines, including those of the World Health Organisation, with modifications made for any nutritional deficiencies or nutrition-related issues. 140
General nutritional management for adults living with a Fontan circulation
The paucity of nutrition research means no evidence exists to develop specific dietary recommendations for people living with a Fontan circulation. Current nutritional advice for people living with Fontan circulation, without any comorbidities requiring nutrition management, is aligned with international and country-specific national health guidelines for general health promotion and chronic disease prevention. Reference Rychik, Goldberg and Rand10 Malnutrition screening and nutritional assessments (including anthropometrics, biochemistry, clinic information, and dietary intake) are fundamental to inform nutritional management strategies, by identifying nutrition-related considerations and complications that can affect an individual’s nutritional status. 23,Reference Shaw50
Conclusion
Since the first Fontan procedure was performed over 50 years ago, there has been an exponential growth in our understanding of the late health consequences and experiences of people living with a Fontan circulation. A wide range of nutrition-related issues associated with the Fontan physiology exists across the lifespan but are poorly characterised. The Fontan model of care is continually evolving and shifting towards a multidisciplinary approach, however, nutritional care is suboptimal and is primarily targeted towards those with critical needs and overt health complications rather than providing adequate strategies and education to optimise general health and well-being throughout the life trajectory. More evidence is needed to characterise the nutritional status of this cohort and the nutrition-related outcomes to identify how dietary and lifestyle interventions can assist in optimising health outcomes.
Acknowledgement
None.
Financial support
Melanie Clode and Derek Tran were supported by the University of Sydney, and a grant from Additional Ventures (1048066), the National Heart Foundation of Australia Vanguard Grant (102277), the Medical Research Future Fund—Cardiovascular Health Mission—Congenital Heart Disease Grant (ARGCHDG000016), and the NSW Health Cardiovascular Research Capacity Program—Early-Mid Career Researcher Grant (H21/174585).
Competing interests
None.







