Introduction
The round sardinella, Sardinella aurita Valenciennes, 1847, is a coastal small pelagic fish widely distributed in the Atlantic Ocean, including the eastern coastal waters from the Gulf of Biscay to South Africa, with higher abundances in the three West African upwelling areas (the highest, off Mauritania), the Mediterranean and Black Seas. In the western Atlantic Ocean, it occurs from Cape Cod in the USA to Argentina (Froese & Pauly, Reference Froese and Pauly2021). Round sardinella is a schooling, strongly migratory and warm-water species, with preferred temperatures between 18–25°C (Bianchi et al., Reference Bianchi, Carpenter, Roux, Molloy, Boyer and Boyer1999). Its resilience and plasticity to adapt itself to new environments (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019) is leading the expansion of S. aurita, driven by global warming (Sabatés et al., Reference Sabatés, Martín, Lloret and Raya2006; Tsikliras, Reference Tsikliras2008; Zeeberg et al., Reference Zeeberg, Corten, Tjoe-Awie, Coca and Hamady2008).
There are noticeable differences in the commercial relevance of round sardinella among countries, being highly targeted by some fisheries (such as in Mauritanian waters, where round sardinella also constitutes a primary source for the fishmeal industry (Corten et al., Reference Corten, Braham and Sadegh2017)), retained bycatch, or even discarded in others (FAO, in press).
The Canary Islands (located in the central-eastern Atlantic Ocean between 27–29°N and 13–18°W, Figure 1) is a Macaronesian archipelago characterized by abrupt bathymetry, with profiles that rise sharply from depths of over 2000 m to narrow island shelves. There are deep inter-island channels and the islands act as obstacles to the south-westwards flow of the Canary Current and the trade winds (Barton et al., Reference Barton, Arístegui, Tett, Cantón, García-Braun, Hernández-León, Nykjaer, Almeida, Almunia, Ballesteros, Basterretxea, Escánez, García-Weill, Hernández-Guerra, López-Laatzen, Molina, Montero, Navarro-Pérez, Rodríguez, van Lenning, Vélez and Wild1998; Arístegui et al., Reference Arístegui, Álvarez-Salgado, Barton, Figueiras, Hernández-León, Roy, Santos, Robinson and Brink2006). In contrast to the nearby NW African coast, one of the richest fishery regions worldwide, the Canary Islands are characterized by oligotrophic waters, leading to low rates of primary production and limited fishing resources (Braun, Reference Braun1980). Among others, these characteristics make the region rather low productive, where pelagic species produce the main fishery yields in biomass. In this context, after tuna, small pelagic species are the main fishing resource in the archipelago, round sardinella being one of the four species targeted by the artisanal purse-seine fleet (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, González-Lorenzo, Jiménez, Sotillo, Acosta and Santamaría2019). The monitoring of this fishery and the assessment of the targeted species have been included in the framework of the Fishery Committee for the Eastern Central Atlantic (CECAF) since 2015 (FAO, 2016). For statistical purposes, S. aurita is grouped with S. maderensis (Lowe, 1838) as Sardinella spp. due to known taxonomic misidentification in the official reporting process (FAO, 2020). Based on the short time series (considered reliable from 2013 onwards), the importance of Sardinella spp. in landings has strongly decreased in the region since 2018 (from 400 mean tons/year during 2013–2017 to 94 mean tons/year during 2018–2020). A similar trend is shown by the European sardine, Sardina pilchardus (Walbaum, 1792) (FAO, in press), which has almost disappeared in the Canary Islands waters since 2019. These two species constitute one of the species groups (clupeids) defined in the region, with higher landings during the warmer season; whereas the other group, conformed by medium-sized pelagic species (i.e. Scomber colias Gmelin, 1789 and Trachurus spp.) shows higher landings during the cooler season (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, González-Lorenzo, Jiménez, Sotillo, Acosta and Santamaría2019).

Fig. 1. Study area allocation and name of the eight islands which constitute the Canary Islands archipelago.
Despite the importance of the species for the local market, little is known about the life history traits of the species inhabiting Canary Islands waters. In addition, aside from the technical regulations imposed on the fleet, any specific consideration is applied to S. aurita at the national level, not even a size limitation. The short time series available and the limited knowledge of the biology of the species in the area make it a typical data-poor stock whose assessment has not been feasible so far (Quinzán & Jurado-Ruzafa, Reference Quinzán and Jurado-Ruzafain press).
Based on the high variability in the reproductive traits of this strongly adaptable species (Whitehead, Reference Whitehead, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984, Reference Whitehead1985), the aim of the present study was to describe the main reproductive traits of the round sardinella inhabiting the Canary Islands waters, in order to provide updated information and useful knowledge for the assessors and managers of a critically decreasing species.
Materials and methods
In January 2013, a long-term monitoring system was launched for the artisanal purse-seine fleet in the Canary Islands, through the European Data Collection Framework (EU, 2017). When available, 100 randomly selected individuals were measured on a monthly basis for their total length (TL, precision 0.1 cm) and weighed (TW, precision 0.1 g). Sex and maturity are assigned macroscopically, based on a general 5-stages key (Holden & Raitt, Reference Holden and Raitt1975). Virgins and recovering individuals were considered immature, and the maturing, spawning and post-spawning individuals were considered mature. Finally, gonad weight (GW, precision 0.1 g) was recorded. In addition to the biological analyses, length samplings of commercial landings of the metier are performed in both fish markets and onboard. During these samplings, length classes (L i, to the lower cm) and weight of the total fish sampled were registered. Based on all the length data (including biological analyses, length samplings in fish markets and onboard), the total and annual length frequencies were obtained. Length and biological samplings of round sardinellas commercial landings are carried out in Tenerife, where more than 70% of the total landings of small pelagic species in the archipelago are performed (FAO, 2020). During 2020, commercial catches of S. aurita were not available for sampling due to several reasons (including the pandemic situation). As a consequence, a total of 17,320 individuals caught between 2013 and 2019 and covering length classes from 9–32 cm were measured from commercial landings in the Canary Islands.
Length-weight relationships (LWR) were calculated using the log-transformation of the power formula TW = a × TLb (where a is the condition factor and b is the allometry coefficient). A Student t-test was applied to compare LWRs between sexes. Growth pattern (allometry) was tested using the Student's t-test modified by Pauly (Reference Pauly1984).
Sex ratio was studied for the whole sample, by quarters and by L i. The χ2 test was applied to detect significant differences in the hypothesized 1:1 relationship (Zar, Reference Zar1996). Spawning behaviour was followed based on the seasonal changes in gonad development, including both sexes, by correlating two approaches: quantitative (the quarterly evolution of the mean gonadosomatic index (GSI = GW × 1000/TW)) and qualitative (the monthly variation of the percentage of mature stages vs the immature ones) (Jennings et al., Reference Jennings, Kaiser and Reynolds2001; King, Reference King2007).
The length at first maturity (LFM, length at which 50% of individuals are in sexually mature stage) was estimated from the curves of maturity by length class (L i). Proportions of mature specimens (p i; mature sexuality was considered for individuals in stage III, IV and V) were estimated for each L i including the whole year, as spawning individuals are found all the year round. Maturity ogives were then estimated using the non-linear regression of the Gompertz model (Pope et al., Reference Pope, Margetts, Hamley and Akyüz1983). The equation for the model is: pi = exp[ − β × exp(β1 × L i)], pi being the proportion of mature individuals for each L i (p’ for moving average was used = (p i−1 + p i + p i+1)/3), and β and β1 were the function parameters.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 15.0 and Microsoft Office Excel 2019.
Results
A summary of descriptive statistics for the 7363 round sardinellas sampled in the laboratory is presented in Table 1, jointly to the LWR estimations. In all the cases, the power formula fitted well to the observed data (R 2 > 0.96) and no statistically significant differences were found between sexes (Student's t = 1.056; P > 0.05). Growth pattern should be taken with caution. No differences are evident between sexes, therefore the growth pattern assumed by the authors is the isometric model obtained for the whole sample of round sardinellas analysed, which includes the widest length range.
Table 1. Descriptive statistics of the round sardinella biologically analysed in the Canary Islands from commercial landings (time period 2013–2019) and LWR estimated for the total and by sex

N, number of individuals; TL, total length; TW, total weight; Min–Max, minimum–maximum; SD, standard deviation. For the length-weight relationships (LWR): a and b are the estimated parameters of the power function, R 2 the correlation factor, GP is the growth pattern (* P < 0.05, based on the Student t-test modified by Pauly (Reference Pauly1984)), being isometric growth (I) when b = 3, or allometric positive or negative (A + or A−, respectively) if b ≠ 3.
The analysis of the whole sample (Figure 2) resulted in a bi-modal length structure, with the main mode representing adult individuals of 20–23 cm (accounting for more than 50% of the fish measured) and the smaller mode accounting for the 4% of individuals of 12–13 cm, probably juveniles. Although the sampling design has been maintained throughout the study period, when observing the annual length frequencies, no clear pattern was observed. One main mode is clear around 20 cm, but with a noticeable exception in 2016 when a tri-modal distribution was obtained with an averaged TL lower than for the other years of the period. The low number of individuals measured in 2019 was mainly due to the obvious decrease in landings which made the availability of the species difficult.
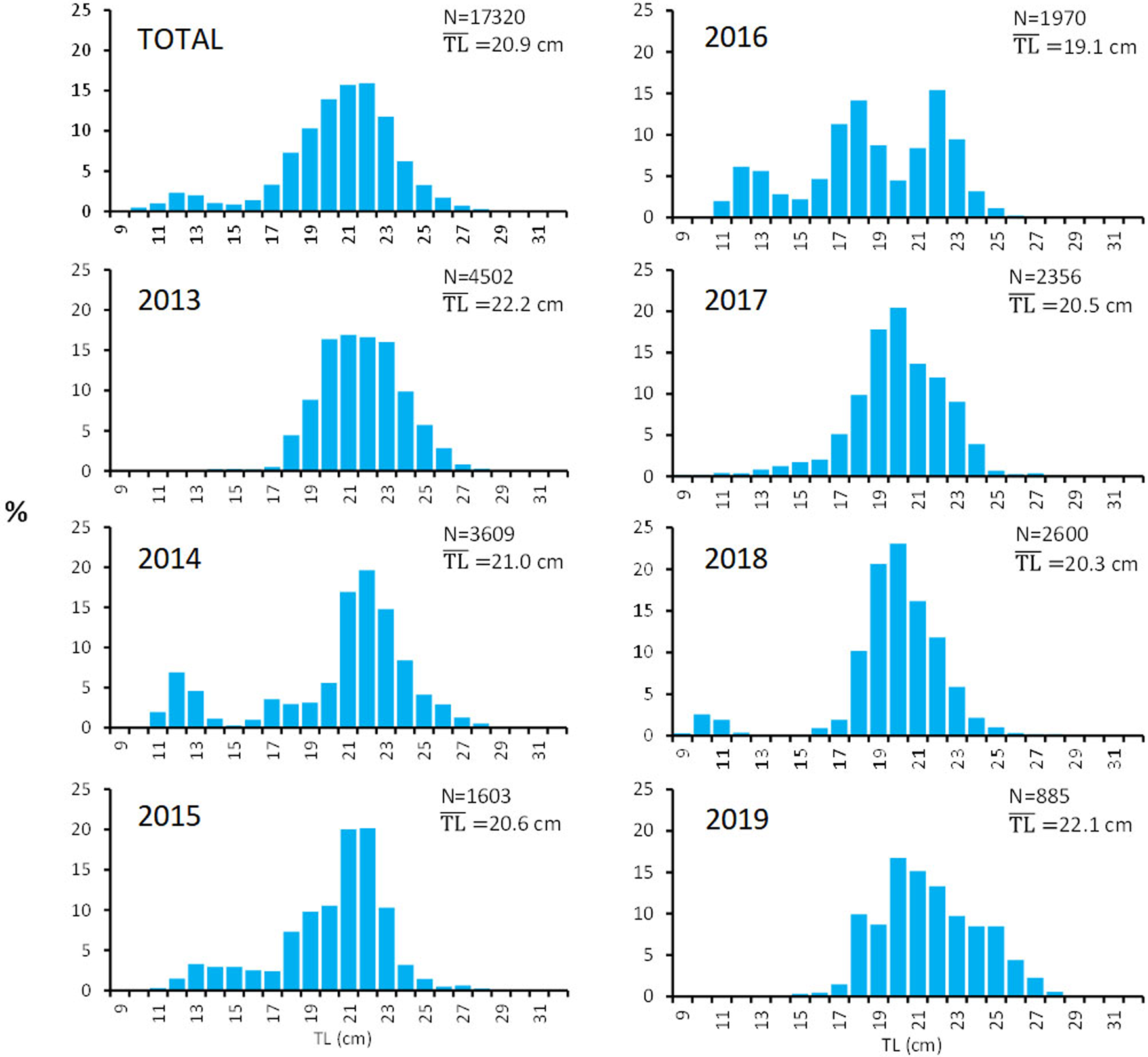
Fig. 2. Total and annual length frequencies (%) of the S. aurita landings analysed in the Canary Islands. N, number of fish measured; $\overline {{\rm TL}}$![]() , average total length.
, average total length.
Considering the overall analyses, males significantly outnumbered females in a proportion 1:0.92 (χ2 = 11.043, P = 0.001). On a monthly basis, different sex ratios were found with balanced proportions or a major presence of males, with the exception of January, when females outnumbered males when the whole study period was considered (Figure 3). However, a noticeable variability was found in the sex ratio among length classes (L i), mainly among the largest sizes of the range (>19 cm), with males being predominant in the length classes 19–21, a balanced sex ratio occurred in L i = 22–23 cm, and females being predominant from 24 cm onwards (Figure 4).

Fig. 3. Monthly sex ratios of the S. aurita landings analysed in the Canary Islands (time period: 2013–2019). (*) indicates χ2 values with P > 0.05, meaning balanced sex ratios.

Fig. 4. Sex ratios by length class of the S. aurita landings analysed in the Canary Islands (time period: 2013–2019). (*) indicates χ2 values with P > 0.05, meaning balanced sex ratios.
The quarterly evolution of the GSI did not allow identification of clear seasonal spawning peaks, but the lowest mean GSIs occurred in the fourth quarter (Figure 5). Most of the spawning activity happened during the first and/or second quarters except in 2015, when the highest GSI occurred during the third quarter. On the other hand, no clear recruitment seasons were observed in the time period studied, based on the quarterly averaged TL of the landings analysed. Nevertheless, the monthly proportion of mature/immature individuals found in landings (Figure 6) seems to indicate a noticeable spawning peak from January to February, when more than 90% of the analysed individuals were sexually mature. Additionally, a short time period with high proportion of immatures between October and November indicates a period of recovery process.

Fig. 5. Quarterly evolution of the mean values of the gonadosomatic index (GSI) and the total length (TL) of the round sardinellas landed in the Canary Islands.

Fig. 6. Monthly proportions of mature and immature sexual stages of the round sardinellas landed in the Canary Islands (time period: 2013–2019).
Results of the maturity ogive fitting the Gompertz model are presented in Figure 7 (β = −678.5 SE = 0.325; β1 = −0.379 SE = 0.015), and the LFM estimated for p i = 0.5 was 18.2 cm.

Fig. 7. Maturity ogive obtained for the round sardinellas landed in the Canary Islands (time period: 2013–2019).
The p i is the mature proportion by length class (L i, cm). R 2 is the coefficient of determination when fitting the p i observed by L i to the Gompertz model (resulting formula presented in the graph).
Discussion
The annual landings series show a drastic decrease in the landings of clupeids in the Canary Islands waters since 2019 (FAO, 2020). Santamaría et al. (Reference Santamaría, González, Barrera, López Abellán, Quintero, Balguerías, Fréon, Barange and Arístegui2008) suggested a replacement of the European sardine by round sardinella in the archipelago. However, Jurado-Ruzafa et al. (Reference Jurado-Ruzafa, González-Lorenzo, Jiménez, Sotillo, Acosta and Santamaría2019) detected significant seasonal patterns in landings related to the environmental variations, grouping clupeids (Sardinella spp. and S. pilchardus) on one hand, and medium pelagic on the other. Both sardinellas and European sardines are experiencing a decreasing trend in the latest years. In this context, the almost collapse of S. pilchardus in Atlantic Iberian waters observed until 2019 (when a total ban was imposed in Portuguese waters), as well as the stopping of the purse-seiners activity in Spanish Iberian waters during 2019 and 2020 (ICES, 2021) should have alerted managers in the Canary region to avoid the probable consequences in the archipelago, where these species represent residual landings by the artisanal purse-seiners since 2019.
The annual length frequencies of round sardinella landings in the Canary Islands between 2013 and 2019 did not show any clear pattern. However, length frequencies clearly differed from the demographic structures found in NW African countries, including noticeably larger individuals (up to ~40 cm sized individuals, with modes in ~20–22 cm, ~26 and ~30 cm) (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019; Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020). The growth pattern observed in the present study should be considered as an informative punctual-trait, to be updated ad hoc if necessary in future studies, because (a) statistically significant differences between sexes were rejected, and (b) previous works in the area showed different results as well as significant seasonal variations (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Bartolomé, Carrasco and Duque-Nogal2016).
The overall sex ratio obtained for the round sardinella in the Canary Islands resulted in a significant unbalance towards males, in contrast with NW African waters where balanced sex ratios have been observed off Mauritania (Wagué & M'Bodj, Reference Wagué and M'Bodj2002; Chesheva, Reference Chesheva2006), or where females predominated in landings off South Morocco (Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017), Mauritania (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020) and Senegal (Boëly, Reference Boëly1982; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019). Unbalance to females is also reported in the Mediterranean Sea (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006; Mustac & Sinovcic, Reference Mustac and Sinovcic2012). Regarding the sex ratio variation by length range, the predominance of females in the largest sizes, as well as a balance for the middle length classes, had been observed for both the Atlantic (Wagué & M'Bodj, Reference Wagué and M'Bodj2002; Baali et al., Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018; Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020) and the Mediterranean round sardinellas (Tsikliras & Antonopoulou, Reference Tsikliras and Antonopoulou2006).
Sardinella aurita spawns year-round in NW Africa with a maximum period from June to September–December (depending on the area included) (Boëly et al., Reference Boëly, Fréon and Stequert1982; Chesheva, Reference Chesheva2006; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018; Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019; Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020). Conversely the spawning peak observed in the Canary Islands occurs in winter, matching with the breeding periods found in the other small pelagic species caught by the same fleet, i.e. S. colias (Lorenzo & Pajuelo, Reference Lorenzo and Pajuelo1996), Trachurus picturatus (Bowdich, 1825) (Jurado-Ruzafa & Santamaría, Reference Jurado-Ruzafa and Santamaría2013) and S. pilchardus (Méndez-Villamil et al., Reference Méndez-Villamil, Lorenzo, González and Soto1997). The coldest period (i.e. winter) also matches with the main annual upwelling event in the archipelago (De León & Braun, Reference De León and Braun1973; Barton et al., Reference Barton, Arístegui, Tett, Cantón, García-Braun, Hernández-León, Nykjaer, Almeida, Almunia, Ballesteros, Basterretxea, Escánez, García-Weill, Hernández-Guerra, López-Laatzen, Molina, Montero, Navarro-Pérez, Rodríguez, van Lenning, Vélez and Wild1998; Moyano & Hernández-León, Reference Moyano, Hernández-León, Clemmesen, Malzahn, Peck and Schnack2009; Valdés & Déniz-González, Reference Valdés and Déniz-González2015). The coincidence of the spawning peaks with upwelling processes is in line with previous observations on breeding of round sardinellas. It seems synchronized with the major upwelling events in the nearby areas off South Morocco (Amenzoui & Baali, Reference Amenzoui and Baali2018), Mauritania (Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020), or with the period when the sea surface temperature reaches the annual minimum values (Baldé et al., Reference Baldé, Sow, Ba, Ekau, Brehmer, Kantoussan, Fall and Diouf2019). Furthermore, the coincidence of the spawning peaks in the main target species of the fleet (artisanal purse-seines) should be considered as an advantage by the fishery managers who face the huge challenge of managing mixed fisheries (Penas Lado, Reference Penas Lado and Penas Lado2019), as the one addressed in the present study, and even more for a species in expansion in the current climate change scenario (Baudron et al., Reference Baudron, Brunel, Blanchet, Hidalgo, Chust, Brown, Kleisner, Millar, Mackenzie, Nikolioudakis, Fernandes and Fernandes2020). In fact, expansion of species leads to transboundary geographic distributions, which in the case of S. aurita is promoting several projects whose aim is to facilitate collaboration between countries involved to share stocks to achieve the coordinated management programmes in NW Africa, such as the ‘Shared sardinella’ project (FAO, 2022).
The length at first maturity estimated for S. aurita inhabiting the Canary Islands waters (18 cm) is notably smaller than the LFMs estimated in NW African waters (ter Hofstede et al., Reference ter Hofstede, Dickey-Collas, Mantingh and Wague2007; Baali et al., Reference Baali, Yahyaoui, Amenzoui, Manchih and Abderrazik2015, Reference Baali, Bourassi, Falah, Abderrazik, Manchih, Amenzoui and Yahyaoui2017; Amenzoui & Baali, Reference Amenzoui and Baali2018; Ndiaye et al., Reference Ndiaye, Sarr, Faye, Thiaw, Diouf, Ba, Ndiaye, Lazar and Thiaw2018; Jurado-Ruzafa et al., Reference Jurado-Ruzafa, Hernández, Duque-Nogal, Pascual-Alayón, Carrasco, Sancho and Santamaría2020, among others). This is a common result in other small pelagic species, probably due to the more favourable oceanographic conditions in the continental NW African coasts, characterized by the influence of one of the four major upwelling systems of the world (Barton et al., Reference Barton, Arístegui, Tett, Cantón, García-Braun, Hernández-León, Nykjaer, Almeida, Almunia, Ballesteros, Basterretxea, Escánez, García-Weill, Hernández-Guerra, López-Laatzen, Molina, Montero, Navarro-Pérez, Rodríguez, van Lenning, Vélez and Wild1998; Cury et al., Reference Cury, Bakun, Crawford, Jarre, Quiñones, Shannon and Verheye2020). Going deeper into the landings' length frequencies, we can observe the presence of high proportions of juveniles (<18 cm, as in 2016), which could be explained by the absence of legal size limitations for this species, which makes it a perfect bycatch when other more valued and size-limited small pelagics are not found.
Several studies suggest a potential African larvae inflow into Canary Islands waters based on the presence of clupeid larvae in cooler filaments reaching the archipelago and originating in African upwelling areas (Rodríguez et al., Reference Rodríguez, Hernández-León and Barton1999; Moyano, Reference Moyano2009; Moyano & Hernández-León, Reference Moyano, Hernández-León, Clemmesen, Malzahn, Peck and Schnack2009). In the CECAF region, latitudinal differences in seasonal patterns of the demographic composition and reproductive traits in S. aurita have been related to different temperature regimes and food availability (Amenzoui & Baali, Reference Amenzoui and Baali2018). The present study supports the reproductive behaviour complexity of S. aurita described by Whitehead (Reference Whitehead1985), also showing longitudinal differences in the demographic composition and the reproductive biology of S. aurita when compared with the species caught in NW African coasts. In this context, there are several studies discussing the population structure of the species in the CE Atlantic (e.g. Chesheva & Zimin, Reference Chesheva and Zimin2004; Riveiro et al., Reference Riveiro, Guisande, Iglesias, Basilone, Cuttitta, Giráldez, Patti, Mazzola, Bonanno, Vergara and Maneiro2011; Bacha et al., Reference Bacha, Jeyid, Jaafour, Yahyaoui, Diop and Amara2016), but the use of genetic markers indicates low genetic variability for the Atlantic S. aurita (Kinsey et al., Reference Kinsey, Orsoy, Bert and Mahmoudi1994; Chikhi et al., Reference Chikhi, Bonhomme and Agnèse1998; De Donato et al., Reference De Donato, Mimbela de Loroño, Ramírez and Marín2005; Takyi, Reference Takyi2019). Therefore, although the results obtained in the present study strengthen the consideration of the Canary S. aurita as a different management unit than the NW African stock assumed by the CECAF experts group (FAO, 2020), further efforts are needed to describe the actual population structure of the round sardinella, including the whole geographic distribution of the species in the west Atlantic Ocean.
The round sardinella is experiencing an expansion process through latitudes where temperatures are getting warmer due to global warming (Navarro, Reference Navarro1932; Zeeberg et al., Reference Zeeberg, Corten, Tjoe-Awie, Coca and Hamady2008; Amenzoui & Baali, Reference Amenzoui and Baali2018). In the NW African context, where it is considered a valuable protein source (Ba et al., Reference Ba, Schmidt, Dème, Lancker, Chaboud, Cury, Thiao, Diouf and Brehmer2017), from a precautionary approach and based on the limited data available, the assumed one-stock is considered overexploited (FAO, 2020), which should alert the Spanish managers to pay more attention to the Canary sardinellas. The knowledge of the biology of species is crucial to achieving reliable assessment and sound management measures focused on the conservation of the natural resources along with sustainability of the fishing activity. In the critical scenario presented for S. aurita, the updated biological knowledge for this little-known species in the Canary Islands provides the base to address its status assessment and evaluate the management options.
Author contributions
Alba Jurado-Ruzafa: conceived and carried out the study, performed biological sampling and data collation, analysed the data, interpreted the findings and wrote the article. Begoña Sotillo de Olano, Zoraida Santana Arocha, Bertín García Mañé, Clara Estil-las García, Eva Hernández: contributed to samples' acquisition, biological sampling and data collation. All authors discussed the results and commented to the final manuscript.
Financial support
This project was partially funded by the EU through the European Maritime and Fisheries Fund (EMFF) within the Spanish National Programme of collection, management and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy.
Conflict of interest
The authors declare no conflict of interests.
Ethical standards
The research did not involve animal experimentation or harm.
Data availability
Data available on request from the authors.











