Introduction
Non-tuberculous mycobacteria (NTM) are a major cause of opportunistic infection in immunocompromised hosts. NTM include over 125 different species of mycobacteria ubiquitous in the environment [Reference Tortoli1] and cause morbidity worldwide [Reference Prevots and Marras2]. NTM species implicated in human disease can produce non-specific symptoms ranging from asymptomatic to destructive or even fatal disease [Reference Tortoli1]. In cases where symptoms and signs occur, they are often indistinguishable clinically and radiographically from those caused by Mycobacterium tuberculosis (TB) [Reference Al Jarad3]. Consequently, many NTM-infected patients in TB endemic countries, such as Mali, receive unnecessary long treatment with anti-TB drugs that are not only expensive but also toxic [Reference Maiga4]. In Mali, during a study conducted at SEREFO laboratory in Bamako, we found that 18% of TB treatment failure patients had NTM disease [Reference Maiga4]. In TB endemic countries, NTM disease was considered to be negligible until more recent reports indicated the impact they may have in the management of patients with TB-like symptoms [Reference Maiga4–Reference Verweij7]. In addition, many recent reports revealed that NTM is increasing worldwide [Reference Nishiuchi, Iwamoto and Maruyama8]. Reasons for this increase is unclear but environmental changes, availability of better detection tools and greater disease awareness are likely contributing factors [Reference Nishiuchi, Iwamoto and Maruyama8, Reference Hermansen9].
In high-income countries, the prevalence of NTM is estimated to be between 1 and 1.8 cases per 100 000 [Reference Mazza-Stalder, Jaton-Ogay and Nicod10] with infections by Mycobacterium avium complex (MAC) as the most common [Reference Johnson and Odell11]. In the US, the Center for Disease Control and Prevention reported that MAC is the cause of 75% of NTM-related respiratory infections [Reference Griffith5, Reference MacDonell and Glassroth12], and MAC was followed by Mycobacterium fortuitum and Mycobacterium kansasii [Reference Griffith5]. In Denmark, the annual incidence rate of NTM disease is 1.20/105 with a majority of infections also due to MAC [Reference Hermansen9]. In Cambodia, a low-middle income country, the prevalence of NTM disease was considered high at 10.8% of patients with presumptive TB in a recent study [Reference Bonnet13].
Mali, a West African country, with an estimated population of 17 million, had a prevalence of TB of 90/100 000 and general HIV seroprevalence of 1.1% in 2014 [14–16]. Sputum smear microscopy is the only tool available to diagnose TB in most areas and physicians are unable to differentiate between TB and NTM pulmonary disease. The extent to which NTM contributes to TB treatment failure and development of drug resistance is uncertain. Thus, we sought to evaluate the epidemiology of NTM disease in Mali by examining the prevalence of NTM disease and its associated factors in patients with suspected pulmonary TB at the Pneumology Department of the Point-G University Teaching Hospital of Bamako (PGUTH).
Methods
Study type and population
We performed a cross-sectional study conducted from January 2006 to December 2013 in which we included patients referred for presumptive TB at the PGUTH. We enrolled all patients suspected of having TB who were 14 years or older with persistent cough for over 15 days, fever, night sweats and/or weight loss. The city of Bamako is administratively divided into six municipalities with each having a local health referral center capable of diagnosing and treating TB patients. These centers refer all TB treatment failure patients to the PGUTH where our study took place. The study participants were referred from the six health referral centers of Bamako. The PGUTH receives 50–100 treatment failure cases each year.
Laboratory testing
We performed laboratory testing at the SEREFO Biosafety Level-3 Laboratory (BSL-3) facility affiliated with the University of Sciences, Techniques, and Technologies of Bamako (USTTB) and the US National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID). We performed sputum smear stained with Auramine Rhodamine and sputum culture (MGIT™ and 7H11) before molecular identification of mycobacterial species as previously described [Reference Maiga4]. In brief, nucleic acid probes (AccuProbe, Gen-Probe, San Diego CA, USA) for M. tuberculosis complex (MTBc), MAC, Mycobacterium gordonae and M. kansasii were used for initial identification and sequencing was performed as needed using published protocols [Reference Woo17, Reference Zelazny18].
For HIV status, we performed a rapid diagnostic test by Determine® (Determine® HIV-1/2, Abbott Laboratories, Matsudo Shi, Chiba, Japan), followed by Genscreen™ ULTRA HIV Ag-Ab (Genscreen, HIV-1/2 Version 2 Assay, Bio-Rad Laboratories, Marnes – La Coquette, France). Western blot (New Lav Blot I and II, Bio-Rad Laboratories, Marnes – La Coquette, France) was used for confirmatory testing of all positives.
Clinical examination
We conducted a standardised interview, performed physical examination and collected three consecutive early morning sputum samples. Blood samples were also collected for HIV testing and haematological parameters. We completed a case report form for each participant to capture socio-demographic data (profession, sex, age, address, level of education, HIV status and others), clinical symptoms, laboratory results and medications.
Data analysis
Fisher's test or χ 2 test was used for the comparison of frequencies and t test for comparison of means. Multivariate logistic regression was performed with NTM (yes/no) as the dependent variable. In univariate and multivariate models, we assessed the following risk factors for NTM: gender, age, weight loss, fever, heart rate, rales crackling, night sweat, cough, leucocytes and haemoglobin. We considered P value <0.05 of comparisons as statistically significant and significant factors in univariate analyses were included in multivariate models. Multivariate models also included age and sex regardless of P value since they were known risk factors for NTM disease. EPI-info version 7.0.9 and SAS version-9 were utilised to conduct these statistical analyses.
Ethical considerations
The study protocol was approved by the Ethics Committee of the University of Bamako and the Institutional Review Board (IRB) of the US National Institutes of Health. All participants provided informed consent and signed a study-specific consent form prior to enrolment.
Results
Study population and infections’ prevalence
A total of 439 patients were included (Fig. 1) in our analysis. Of those included, 77% (336/439) were male, mean age was 35.8 years (14–83) and 87.24% (383/439) resided in the Bamako District with 132 participants (30.07%) from the Commune VI part of this district. There were no significant differences in educational level between the groups.

Fig. 1. Descriptive groups of the study.
After sputum analysis, we found that 9.34% (41/439) were NTM, 66.29% (291/439) were M. tuberculosis complex and 24.37% (107/439) were sputum culture negative (Fig. 1). Among those with sputum culture positive, NTM was identified in 12.34% (41/332) and M. tuberculosis complex in 87.66% (291/332). The 41 NTM were identified morphologically and by fluorescent acid-fast microscopy with solid medium cultures, to be mycobacteria that are not M. tuberculosis (negative for MTBc Gen-Probe). Twenty of the 41 NTM were further formally specified (the type of NTM) using other available probes or 16S sequencing (Fig. 2). Of these NTM cases, 41.46% (17/41) were new TB suspects and 58.54% (24/41) were identified as failing TB treatment. Male sex was more common in both the M. tuberculosis complex group with 76.29% (222/291) and the NTM group with 80.49% (33/41) (Table 1). The most affected age category was 14–30 year olds with 46.74% (136/291) in M. tuberculosis complex group and 30–45 year olds with 46.34% (19/41) in the NTM group. Of note, when both TB and NTM were identified in the same sputum sample, we categorised the patient as having TB disease with NTM colonisation as per American Thoracic Society (ATS) definition [Reference Griffith5].
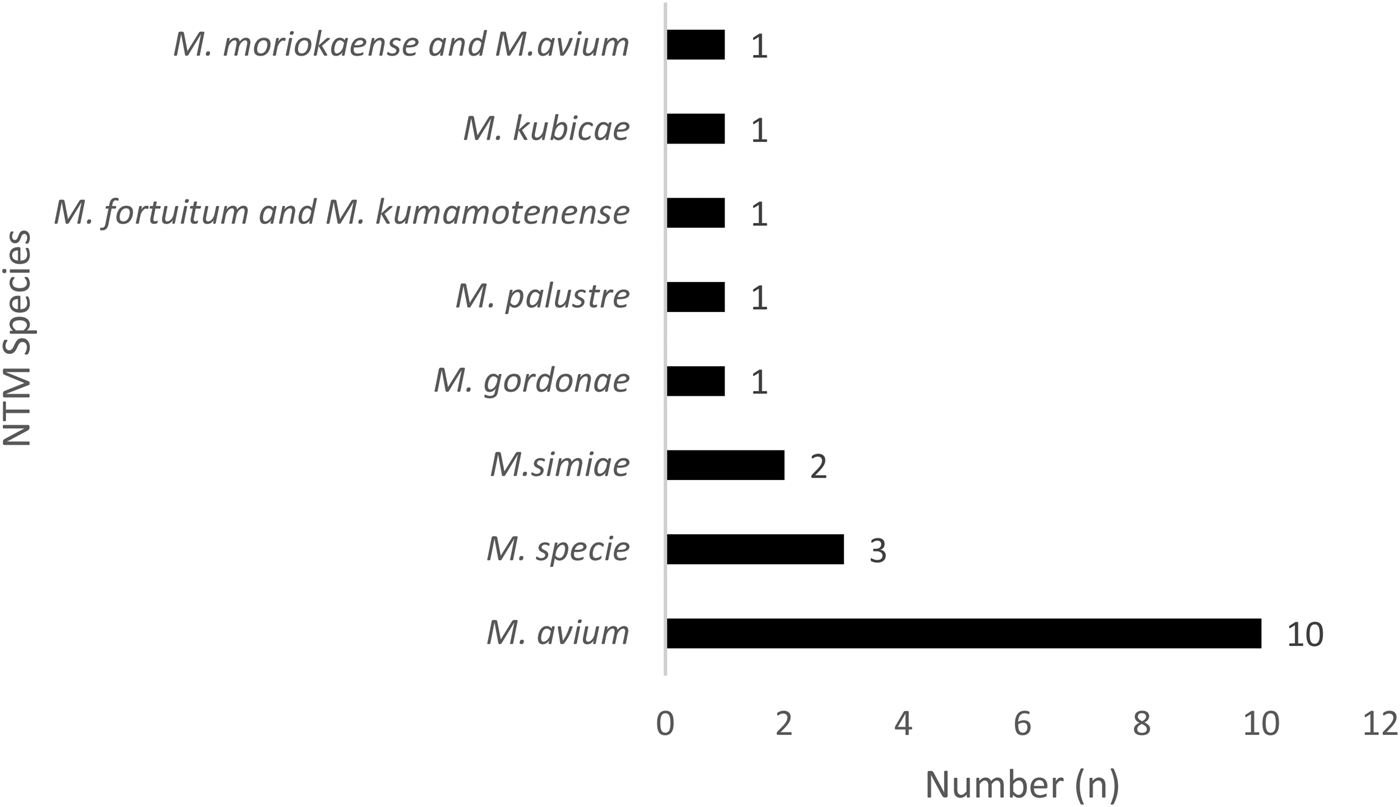
Fig. 2. Distribution of the 20 non-tuberculosis mycobacteria species in Bamako, Mali.
Table 1. Socio-demographic and clinical characteristics of tuberculous and non-tuberculous mycobacteria (NTM)-infected patients, from 2006 to 2013 in Bamako, Mali
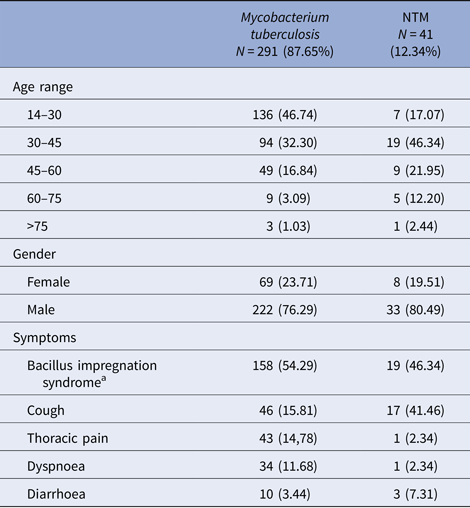
a Bacillus impregnation syndrome: group of symptoms made of asthenia, anorexia fever, night sweats and weight loss.
Symptoms associated with infections
Among those who were culture positive for M. tuberculosis complex, we observed symptoms of bacillus impregnation in 54.29% (158/291), thoracic pain in 14.78% (43/291) and cough in 15.81% (46/291) of the participants (Table 1). However, in the NTM disease group, we observed symptoms of bacillus impregnation in 46.34% (19/41), thoracic pain in 2.34% (1/41) and cough in 12.19% (17/41). The bacillus impregnation syndrome is a group of symptoms made of asthenia, anorexia fever, night sweats and weight loss [19]. Overall, body weight more than 55 kg at time of referral to the study was less likely to be associated with pulmonary NTM disease (OR = 0.53).
There were no statistically significant differences in haematological parameters between those with TB and NTM disease (data not shown).
NTM disease
At inclusion, the mean weight of those with NTM disease was 51.05 ± 8.7 kg and the mean heart rate was 96.53 ± 17.63 beats per minute. Furthermore, of the 41 patients who met ATS criteria for NTM disease, weight was 51 ± 8.79 kg, the mean of body temperature was 36.93 ± 0.90 °C, the mean heart rate 108 ± 29.75 beats per minute and the mean respiratory rate was 29.75 ± 10.09 breaths per minute.
In addition, 17.07% (7/41) of those with NTM disease were HIV seropositive. Of the NTM specified, 50% (10/20) were identified as having M. avium, 15% Mycobacterium species (3/20) and 10% (2/20) Mycobacterium simiae (Fig. 2). Of the NTM/HIV co-infected participants, 71.43% (5/7) were living in the Commune VI of Bamako District and 71.43% (5/7) NTM/HIV were male with mean age of 43.48 ± 15.06 years.
NTM disease associated factors
In our univariate analysis, only fever (defined as body temperature of 38 °C or above) and cough were significantly associated with NTM disease (OR 0.41 (CI 0.21–0.81) and OR 0.8 (CI 0.04–0.17), respectively). Our analysis also found that HIV-positive patients had non-significant, but greater odds of having NTM disease compared with those without HIV (OR = 1.44; CI 0.59–3.50; P < 0.415).
Multivariate analyses found significant associations between not having cough and lower body weight with NTM disease (OR 0.05 (CI 0.02–0.13) and OR 0.32 (CI 0.11–0.93); Table 2). Our findings suggest that patients suspected of TB who are not coughing and who weigh <55 kg have a great probability of having NTM disease instead of TB.
Table 2. Risk factors associated with NTM infections, from 2006 to 2013 in Bamako, Mali
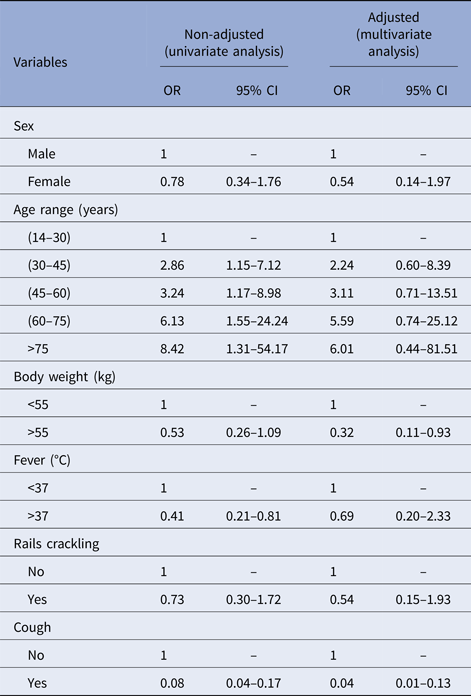
OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
In this study, we determined the prevalence of NTM among individuals suspected of having TB to be 9.3% (41/439) and 12.34% (41/332) among culture positives over the 7 years’ study period at the PGUTH. Conversely, the prevalence of M. tuberculosis have NTM and NTM target therapies. With testing of sputum for NTM, these patients would have received several months of unnecessary, expensive and toxic medications targeting TB. Our findings also highlight the need for better diagnostic tools for TB in low- and middle-income countries where NTM prevalence is potentially increasing [Reference Nishiuchi, Iwamoto and Maruyama8].
Similar findings have been reported by Zida et al. in Burkina Faso in 2014 [Reference Zida20] and Maiga et al. in Mali in 2012 [Reference Maiga4] with NTM prevalence of 11% and 12% in TB suspected cases, respectively.
The age range of those most affected by NTM was 30–40 years and males were more likely to have NTM disease. Similarly, a study by Gerogianni et al. [Reference Gerogianni21] in Central Greece found that 67% of NTM-infected individuals were men. We have also found that NTM infection was not driven by HIV infection in Mali with only 17.07% (7/41) of co-infection, although, our data suggest that HIV-infected patients are potentially more vulnerable than others. Another report in Kenya found that 46.7% of NTM disease cases were co-infected with HIV [Reference Nyamogoba22]. This difference is likely explained by the fact that HIV is at least six times more prevalent in the general population of Kenya than Mali (nearly 6% and 1%, respectively). Importantly, NTM disease appears to have a greater association with HIV infection in high-prevalence HIV regions, but in low-prevalence HIV areas, such as Mali, NTM remains highly present and possibly driven by other as yet unknown factors. Those factors could be genetics, environment and/or other immunodeficiency disorders. Notably, we found in our study that the absence of cough (with the presence of other symptoms suggestive of TB) and a body weight of <55 kg were associated with diagnosis of NTM disease in those suspected of having TB.
In our study, 50% of NTM cases were due to M. avium, which is similar to reports from France in 2004 with 47.7% M. avium among NTM cases [Reference Griffith5, Reference Nyamogoba22–Reference Prendki24]. M. avium and many NTM are present in water and often resistant to regular dosing of chloride in public drinking water [Reference Falkinham, Norton and LeChevallier25]. Other important NTM reservoirs/sources include soil, dust and bathrooms [Reference Nishiuchi, Iwamoto and Maruyama8].
This study had several limitations. First, asymptomatic burden of NTM disease could not be assessed due to the inclusion criteria. Second, our study was cross-sectional and without temporality we were unable to assess causality and can only determine associations. Finally, our study may lack generalisability given we utilised a recruitment centre in Bamako with clinically challenging suspected TB cases and those failing TB therapy. Despite these limitations, we consider the impacts of these limitations are minimal on the data interpretations.
Conclusion and recommendation
Our study was one of the first to assess the burden and risk factors of NTM disease in a TB endemic region of West Africa with low HIV seroprevalence. The prevalence of NTM disease was relatively high in Mali and requires better diagnostic tools to distinguish NTM from TB in this setting. Unlike other places, HIV was not a driving factor and further longitudinal studies are needed to determine the causal factors for the high prevalence of NTM disease we observed. Our data also reinforce that clinical symptoms and sputum smear are not sufficient to effectively diagnose and manage TB-suspected patients.
Authors contribution
BK, YSS, SS, MM BB, BD, NC, MP and SDi conceived and developed the protocols; BK, MM, BB, YSS, NC, AK, BD, MS, ACG, DG, MD, MB, SO, CJA, RLM and SS helped in the protocol development; BK, MM, BB, YSS, NC, AK, BD, MS, ACG, DG, MD, MB, SO and RLM led the study; BK, BB, YSS, NC, AK, BD, MS, ACG, DG, MB and SD were instrumental in data generation and laboratory assays; BK, BB, YSS, NC, AK, BD, MS, ACG, DG, MD, MB and SS collated, reviewed and interpreted data and contributed to the writing of the manuscript. BK, MM, BB, BD, YSS and SS wrote the first draft; and BK, MM, BB, BD, YSS and SS reviewed the drafts. SS, RLM, SDi and SDa provided mentoring for this work. All authors had access to the data and reviewed the manuscript.
Acknowledgements
The authors would like to acknowledge all SEREFO/UCRC staff, laboratory and clinical who contributed to patient recruitment, sample processing and data collection. The authors are immensely grateful to the volunteers who participated in the study. This work was supported by the National Institutes of Health (R01AI110386, D43TW010350 and D71 TW010428-01A1).
Declaration of Interest
None of the authors has a conflict of interest.






