Introduction
Malaria remains one of the most serious global infectious diseases. Only in 2017, it was responsible for 219 million cases and 435 000 deaths worldwide (World Health Organization, 2018). It is generally accepted that malaria elimination from the remaining endemic countries will not be possible with currently available strategies (Alonso et al., Reference Alonso, Brown, Arevalo-Herrera, Binka, Chitnis, Collins, Doumbo, Greenwood, Hall, Levine, Mendis, Newman, Plowe, Rodríguez, Sinden, Slutsker and Tanner2011). In this regard, a better understanding of the numerous gaps of knowledge in the complex biology of the parasite would facilitate the rationale design of new interventions.
The thrombospondin type 1 repeat (TSR) superfamily is a diverse group of proteins often involved in interactions with the extracellular matrix (Tucker, Reference Tucker2004; Morahan et al., Reference Morahan, Wang and Coppel2009). Particularly, the functional characterization of P. falciparum TSR domain-containing proteins revealed the essential roles that these effectors play in processes involved in cell invasion, egression and transmission of the parasite through the mosquito stages. Thus, circumsporozoite- and TRAP-related protein (CTRP) is essential for ookinete invasion of the mosquito midgut epithelium and oocyst development (Dessens et al., Reference Dessens, Beetsma, Dimopoulos, Wengelnik, Crisanti, Kafatos and Sinden1999), whereas thrombospondin-related protein 1 is required for the subsequent sporozoite egress from oocysts (Klug and Frischknecht, Reference Klug and Frischknecht2017). TRAP, circumsporozoite protein (CSP) and TRAP-related protein are all involved in the sporozoite invasion of the mosquito salivary glands; in the vertebrate host, CSP and TRAP also bind to the hepatocyte surface during sporozoite invasion (Ménard et al., Reference Ménard, Sultan, Cortes, Altszuler, van Dijk, Janse, Waters, Nussenzweig and Nussenzweig1997; Sultan et al., Reference Sultan, Thathy, Frevert, Robson, Crisanti, Nussenzweig, Nussenzweig and Menard1997; Combe et al., Reference Combe, Moreira, Ackerman, Thiberge, Templeton and Menard2009). Finally, TRAP-like protein and thrombospondin-related sporozoite protein have been described to play an important role in hepatocyte cell traversal (Labaied et al., Reference Labaied, Camargo and Kappe2007; Moreira et al., Reference Moreira, Templeton, Lavazec, Hayward, Hobbs, Kroeze, Janse, Waters, Sinnis and Coppi2008). Other TSR domain-containing proteins are expressed in the blood stages of the parasite. Previously described to be essential for the merozoite invasion of erythrocytes (Baum et al., Reference Baum, Richard, Healer, Rug, Krnajski, Gilberger, Green, Holder and Cowman2006), more recent studies revealed that the merozoite TRAP family protein (MTRAP) is completely dispensable for the viability of asexual parasites. Instead, MTRAP was demonstrated to be essential for gametocyte egress and, thus, for transmission to the mosquito (Bargieri et al., Reference Bargieri, Thiberge, Tay, Carey, Rantz, Hischen, Lorthiois, Straschil, Singh, Singh, Triglia, Tsuboi, Cowman, Chitnis, Alano, Baum, Pradel, Lavazec and Ménard2016). On the other hand, the thrombospondin-related apical merozoite protein (TRAMP) seems to be involved in the invasion of erythrocytes, since antibodies raised against this protein block merozoite invasion (Siddiqui et al., Reference Siddiqui, Dhawan, Singh, Singh, Gupta, Pandey, Mohmmed, Gaur and Chitnis2013). Interestingly, a secreted protein with altered thrombospondin repeat domain (SPATR) is expressed at multiple stages of the parasite such as sporozoites, asexual blood stages and gametocytes. Antibodies raised against SPATR block hepatocyte invasion by sporozoites; however, the biological relevance of SPATR during other stages of the parasite has not been addressed (Chattopadhyay et al., Reference Chattopadhyay, Rathore, Fujioka, Kumar, de la Vega, Haynes, Moch, Fryauff, Wang, Carucci and Hoffman2003).
The elucidation of the crystal structure of TSR domains revealed an unusual three-stranded fold known as the tryptophan ladder, also found in type 1 cytokine receptors and characterized by a motif of stacked aromatic and basic amino acids stabilized by disulfide bonds (Tan et al., Reference Tan, Duquette, Liu, Dong, Zhang, Joachimiak, Lawler and Wang2002; Olsen and Kragelund, Reference Olsen and Kragelund2014). In other organisms, TSR domains can be modified by two types of glycosylation, namely O-fucosylation and C-mannosylation. The protein O-fucosyltransferase 2 (PoFUT2) catalyses the O-fucose modification of TSR domains, which in turn may be elongated by a β3-glucosyltransferase (Kozma et al., Reference Kozma, Keusch, Hegemann, Luther, Klein, Hess, Haltiwanger and Hofsteenge2006; Luo et al., Reference Luo, Koles, Vorndam, Haltiwanger and Panin2006). On the other hand, DPY19 enzymes have been identified as the glycosyltransferases responsible for TSR C-mannosylation (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013; Shcherbakova et al., Reference Shcherbakova, Tiemann, Buettner and Bakker2017). Recent works revealed that TRAP and CSP are both modified in vivo by a deoxyhexose and a hexose (presumably forming a glucose-β1,3-fucose O-glycan) in P. falciparum and P. vivax salivary gland sporozoites. Additionally, TRAP was also shown to be modified by an additional hexose (presumably C-linked mannose) in P. falciparum (Swearingen et al., Reference Swearingen, Lindner, Shi, Shears, Harupa, Hopp, Vaughan, Springer, Moritz, Kappe and Sinnis2016) but, interestingly, not in P. vivax (Swearingen et al., Reference Swearingen, Lindner, Flannery, Vaughan, Morrison, Patrapuvich, Koepfli, Muller, Jex, Moritz, Kappe, Sattabongkot and Mikolajczak2017). These, and other works (Sanz et al., Reference Sanz, Bandini, Ospina, Bernabeu, Marino, Fernandez-Becerra and Izquierdo2013; Cova et al., Reference Cova, Rodrigues, Smith and Izquierdo2015; Bandini et al., Reference Bandini, Albuquerque-Wendt, Hegermann, Samuelson and Routier2019), are renewing the interest of the Plasmodium research community on the extent and relevance of the parasite glycosylation.
PfPoFUT2 was demonstrated to be dispensable for the asexual and sexual blood stage development of the parasite, in agreement with the absence of predicted acceptors with the TSR O-fucosylation consensus sequence (Lopaticki et al., Reference Lopaticki, Yang, John, Scott, Lingford, O'Neill, Erickson, McKenzie, Jennison, Whitehead, Douglas, Kneteman, Goddard-Borger and Boddey2017; Sanz et al., Reference Sanz, Aquilini, Tweedell, Verma, Hamerly, Hritzo, Tripathi, Machado, Churcher, Rodrigues, Izquierdo and Dinglasan2019). On the contrary, the relevance of PfPoFUT2 for the establishment of the infection in the mosquito host remains a controversial issue. While Lopaticki et al. reported that PfPoFUT2 gene disruption resulted in a deficient ookinete invasion and a reduced sporozoite gliding motility and cell traversal activity, Sanz et al. did not observe any evident phenotype secondary to PoFUT2 ablation, neither in P. falciparum nor in the murine model P. berghei. Interestingly, the phenotype described by Lopaticki et al. was attributed to a deficient secretion of certain PoFUT2 acceptors, in accordance with the proposed role of O-fucosylation in a protein folding quality control system (Vasudevan and Haltiwanger, Reference Vasudevan and Haltiwanger2014). However, to our knowledge, the relevance of the C-mannosylation of P. falciparum TSR-containing proteins has not been addressed before.
PfDPY19 was recently demonstrated to harbour a C-mannosyltransferase activity in vitro (Hoppe et al., Reference Hoppe, Albuquerque-Wendt, Bandini, Leon, Shcherbakova, Buettner, Izquierdo, Costello, Bakker and Routier2018). Interestingly, PfDPY19 exhibited a different acceptor specificity than the mammalian enzymes (see Table 1 for a list of putative PfDPY19 acceptors). In other organisms, C-mannosylation has been shown to be required for the efficient secretion of certain acceptors, in accordance with the role played by this modification in the stabilization of the tryptophan ladder (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013; Shcherbakova et al., Reference Shcherbakova, Tiemann, Buettner and Bakker2017). Taking into consideration the proposed roles for some of the putative acceptors of PfDPY19 in the asexual blood stages, we aimed to describe the relevance of the gene for the viability of P. falciparum by disrupting it and analysing the growth phenotype of ΔPfDPY19 mutant parasites.
Table 1. Conservation of the apicomplexan DPY19 C-mannosyltransferase consensus sequence among TSR domain-containing proteins expressed in P. falciparum

CSP, circumsporozoite protein; CTRP, circumsporozoite and TRAP-related protein; MTRAP, merozoite TRAP-like protein; PTRAMP, Plasmodium thrombospondin-related apical merozoite protein; SPATR, secreted protein with altered thrombospondin repeat; TLP, TRAP-like protein; TRAP, thrombospondin-related anonymous protein; TREP, TRAP-related protein; TRP1, thrombospondin-related protein 1; TRSP, thrombospondin-related sporozoite protein. Residues of the apicomplexan C-mannosylation consensus sequence are marked in bold, when conserved.
*TSR domains with or without a conserved C-mannosylation consensus sequence are marked as Y and N, respectively.
Material and methods
Construction of plasmids for CRISPR/Cas9-mediated PfDPY19 knockout
A single guide RNA (sgRNA) targeting the PfDPY19 genomic locus (PF3D7_0806200) was designed with the Eukaryotic Pathogen gRNA Design Tool (Peng and Tarleton, Reference Peng and Tarleton2015). To generate the plasmid expressing the Streptococcus pyogenes Cas9 and the sgRNA, the primers 5′-TAAGTATATAATATTTAAATTTAGGCCTTTCCATAGTTTTAGAGCTAGAA-3′ and 5′-TTCTAGCTCTAAAACTATGGAAAGGCCTAAATTTAAATATTATATACTTA-3′ were annealed and ligated into a BbsI-digested pDC2-Cas9-hDHFRyFCU plasmid (a generous gift from Ellen Knuepfer) (Knuepfer et al., Reference Knuepfer, Napiorkowska, van Ooij and Holder2017). To generate the rescue plasmid, two homology regions for PfDPY19 of ~530 bp were amplified from Pf3D7 1.2B genomic DNA with the primer pairs 5′-ATTCGAGCTCGGTACCCGGGAAATTATGTTGAGCAGAAACTTTCC-3′/5′CAGGTCGACTCTAGAGGATCCACGTCTCGAGCCCCAAGAATAATTTCTAAAAACAT-3′ and 5′-TTCTTGGGGCTCGAGACGTGCGGCCGCATTATATGTTTTCAGCTTGTCTTCC-3′/5′-CAGGTCGACTCTAGAGGATCCGCAATATGGAATATTACTTCCACAT-3′ and were cloned into a pUC19 backbone.
Parasite culture and transfection
Plasmodium falciparum 3D7 1.2B line (kindly provided by Cortés) (Cortés, Reference Cortés2005) was used for transfection and parasite maintenance. Pf3D7 1.2B was cultured with B+ human erythrocytes in Roswell Park Memorial Institute (RPMI) medium supplemented with Albumax-II at 37 °C under an atmosphere of 92% N2, 3% O2 and 5% CO2, following standard methods (Trager and Jensen, Reference Trager and Jensen1976). For PfDPY19 gene disruption, 60 µg of pDC2-Cas9-sgRNA-hDHFRyFCU plasmids and 15 µg of ScaI-linearized pUC19-PfDPY19 plasmids (Fig. 1A) were transfected into Percoll-purified segmented schizonts by electroporation with the Amaxa 4D system, as previously described (Moon et al., Reference Moon, Hall, Rangkuti, Ho, Almond, Mitchell, Pain, Holder and Blackman2013). Drug selection (i.e. 10 nm WR99210 from Jacobus Pharmaceuticals) was first applied ~20 h post-transfection and maintained for 4 days with daily media changes. The emergence of resistant parasites was monitored by visualizing Giemsa-stained blood smears by light microscopy. Viable parasites were screened by PCR for PfDPY19 gene disruption and treated for 1 week with 1 µ m 5-fluorocytosine for negative selection of parasites containing the pDC2-Cas9-sgRNA-hDHFRyFCU plasmid, prior to subcloning by limiting dilution.
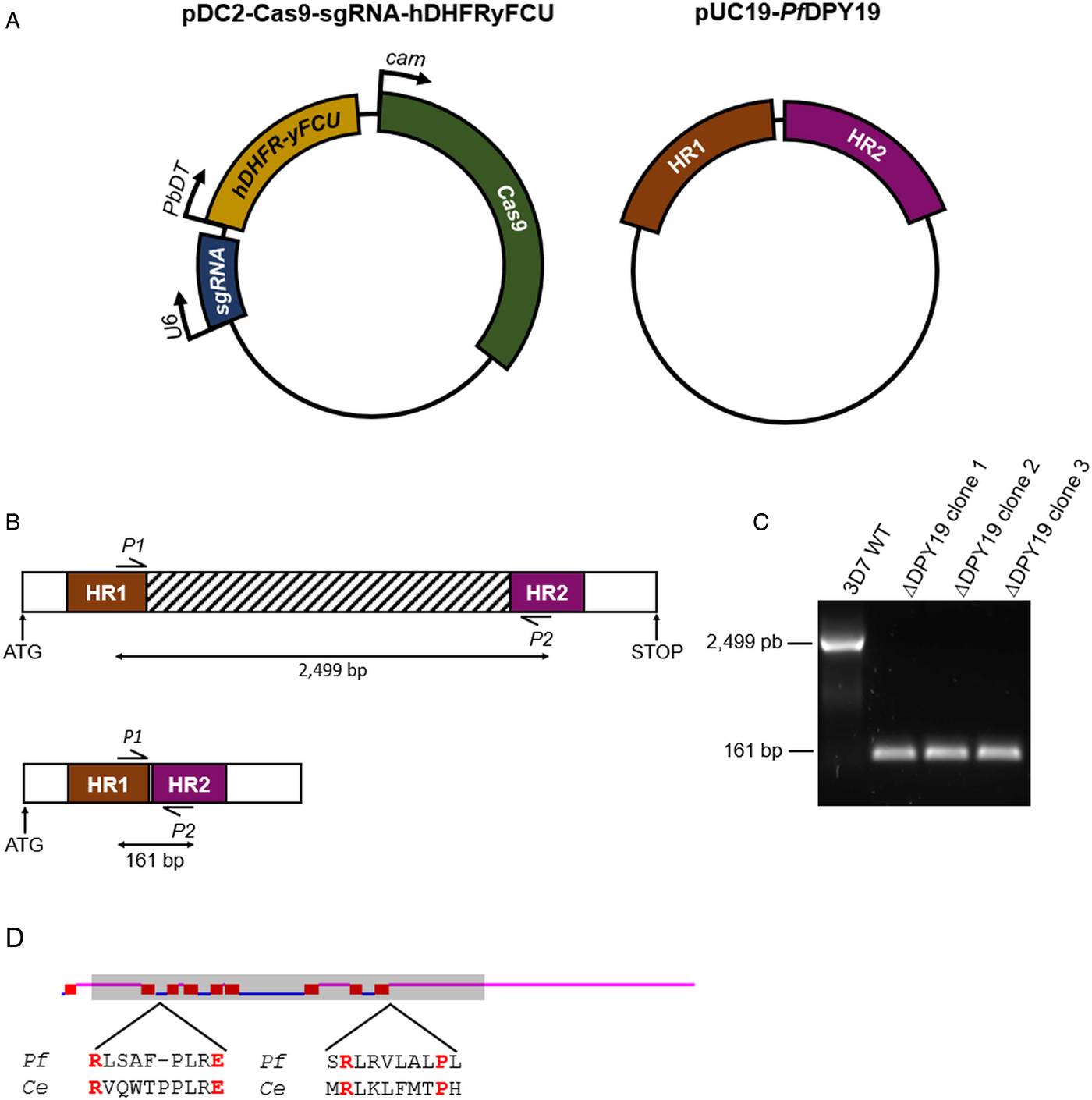
Fig. 1. PfDPY19 gene disruption. (A) The pDC2-Cas9-sgRNA-hDHFRyFCU expresses the S. pyogenes Cas9 endonuclease, the sgRNA targeting the PfDPY19 genomic locus and a fusion protein of the positive selectable marker human dihydrofolate reductase (hDHFR) and the negative selectable marker yeast cytosine deaminase/uridyl phosphoribosyl transferase (yFCU). The rescue plasmid (pUC19-PfDPY19) contains two homology regions of approximately 530 bp corresponding to the 5′ and 3′ ends of the PfDPY19 gene (PF3D7_0806200). (B) Schematic representation of PfDPY19 wild-type (up) and modified loci (down). In total, 2365 bp of the PfDPY19 coding region (which contain the locus targeted by the designed sgRNA) are excised after double homologous recombination with homology region 1 (HR1) and 2 (HR2) from the rescue plasmid. (C) Polymerase chain reaction (PCR) screening of PfDPY19 disruption of wild-type parasites (3D7 WT) and three different ΔPfDPY19 clones obtained by limited dilution. A negative control reaction (NC) was performed in the absence of template DNA to discard unspecific amplifications. (D) Predicted transmembrane helices (red boxes) of PfDPY19 protein with the TMHMM Server v. 2.0 software (Krogh et al., Reference Krogh, Larsson, von Heijne and Sonnhammer2001). Loops predicted to localize in the lumen of the ER and the cytoplasm are marked in pink and blue, respectively. The shaded area indicates the deleted gene fragment in ΔPfDPY19 mutants. Amino acids predicted to bind to dolichol-phosphate mannose in C. elegans (Ce) DPY19 are marked in red and are conserved in the P. falciparum (Pf) homologue (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013).
In vitro growth assay of parasite asexual development
To assess the biological relevance of PfDPY19 for the asexual blood stage development of the parasite, the growth of two independent clones of PfDPY19 null mutants was compared with that of the wild-type parental line in three biological replicates. Cultures were synchronized at ring stages by sorbitol synchronization and parasitaemias were determined by flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) using the Syto 11 dye to discriminate between infected and uninfected erythrocytes, as described elsewhere (Urbán et al., Reference Urbán, Estelrich, Cortés and Fernàndez-Busquets2011). Cultures were adjusted to ~1.5% parasitaemia and 1% haematocrit. After incubation for one complete intraerythrocytic cycle (~48 h), parasitaemias were determined again by flow cytometry. Multiplication rates for each strain were calculated as the ratio between the final and the initial parasitaemia.
Oxidative stress induction and parasite survival assay
To assess the ability of PfDPY19 null mutants to cope with the endoplasmic reticulum (ER)-stressing conditions, parasite cultures were subjected to different concentrations of dithiothreitol (DTT) as an oxidative stress proxy. Cultures were treated with DTT at a final concentration of 0.05 and 0.15 M or, alternatively, with phosphate buffered saline (PBS) as a negative control. After ~48 h of incubation, parasitaemia was determined by flow cytometry as described previously. The survival rate for each strain was expressed as the ratio between the parasitaemia of the cultures treated with DTT and the negative controls incubated with PBS.
Results
PfDPY19 is not essential for the asexual blood development of P. falciparum
To investigate the relevance of the PfDPY19 C-mannosyltransferase, we disrupted the gene coding for this glycosyltransferase (Fig. 1). As demonstrated by PCR, PfDPY19 gene was truncated in edited parasites. Remarkably, the deleted gene fragment (see striped box in Fig. 1B) codes for eight out of the nine predicted transmembrane domains of the native protein. Moreover, the delated fragment also codes for the conserved amino acids that have been predicted to bind to the dolichol-phosphate mannose precursor (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013). Given the magnitude of the gene deletion, it is highly unlikely that the generated PfDPY19 null mutants could yield a functional C-mannosyltransferase activity. Thus, the viability of these mutants strongly suggests that PfDPY19 is not essential for the asexual blood stage development of the parasite.
PfDPY19 gene disruption does not affect parasite growth
Plasmodium falciparum expresses three TSR domain-containing proteins during the blood stage development of the parasite, all of which contain at least one TSR domain that conserves the apicomplexan C-mannosylation consensus sequence W-X-X-W-X-X-C (see Table 1). Specifically, antibodies raised against TRAMP block the erythrocyte invasion of P. falciparum merozoites (Siddiqui et al., Reference Siddiqui, Dhawan, Singh, Singh, Gupta, Pandey, Mohmmed, Gaur and Chitnis2013). To assess if PfDPY19 gene disruption could have an impact on the secretion of TRAMP (or other acceptors) and, consequently, on parasite growth, we compared the multiplication rate of PfDPY19 null mutants with that of the parental wild-type line (Fig. 2A). However, we did not observe any difference across three biological replicates, suggesting that PfDPY19 disruption does not affect the parasite's asexual blood stage in vitro growth rate.
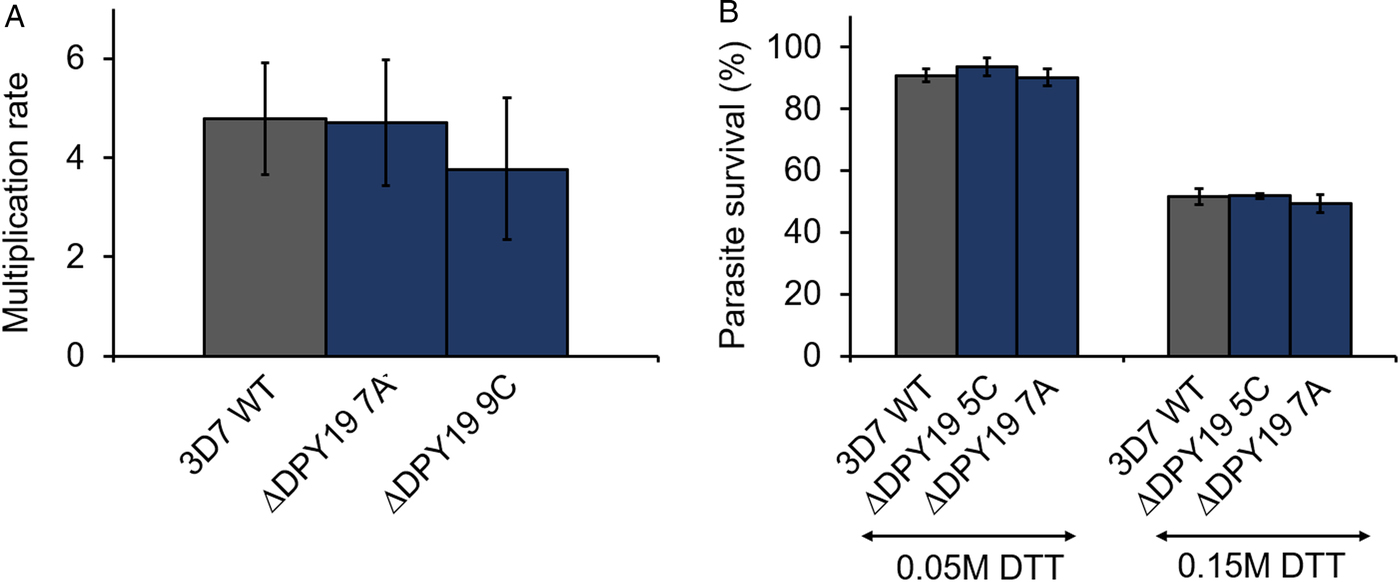
Fig. 2. PfDPY19 disruption does not alter parasite growth. (A) The growth rate of 3D7 1.2B wild-type (3D7 WT) parasites and PfDPY19 null mutants (ΔDPY19 7A and 9C clones) was monitored over a complete intraerythrocytic cycle. Values are the mean of three biological replicates and error bars represent the standard variation. (B) Growth inhibition of 3D7 1.2B wild-type (3D7 WT) parasites and PfDPY19 null mutants (ΔDPY19 5C and 7A clones) at two DTT concentrations (0.05 and 0.15 M). Values are the mean of three technical replicates and error bars represent the standard deviation.
During the intraerythrocytic development, the parasite is exposed to oxidative stress due to the host immune responses and the products generated during haemoglobin degradation. Thus, the parasite is especially vulnerable to redox imbalances that affect protein folding in the ER (Chaubey et al., Reference Chaubey, Grover and Tatu2014). Given the alleged role of C-mannosylation in the stabilization of the tryptophan ladder of TSR domains (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013; Shcherbakova et al., Reference Shcherbakova, Tiemann, Buettner and Bakker2017), we compared the survival rate of PfDPY19 null mutants with that of the parental wild-type line when subjected to different concentrations of DTT (Fig. 2B) to assess the impact of the absence of a PfDPY19 C-mannosyltransferase activity when coping with such conditions. However, no significant differences were observed at any of the DTT concentrations tested.
Discussion
The recent functional characterization of P. falciparum PoFUT2 suggests a critical role of O-fucosylation during the infection of the mosquito host (Lopaticki et al., Reference Lopaticki, Yang, John, Scott, Lingford, O'Neill, Erickson, McKenzie, Jennison, Whitehead, Douglas, Kneteman, Goddard-Borger and Boddey2017), although these results could not be replicated in an independent study (Sanz et al., Reference Sanz, Aquilini, Tweedell, Verma, Hamerly, Hritzo, Tripathi, Machado, Churcher, Rodrigues, Izquierdo and Dinglasan2019). According to the work conducted by Lopaticki et al., PoFUT2 disruption results in a deficient secretion of TRAP, which probably affects the parasite ability to invade hepatocytes. Moreover, it is likely that other acceptors are also affected by the absence of O-fucosyltransferase activity, as evidenced by the deficient invasion of the mosquito midgut epithelium. Although TRAP has also been demonstrated to be C-mannosylated in salivary gland sporozoites (Swearingen et al., Reference Swearingen, Lindner, Shi, Shears, Harupa, Hopp, Vaughan, Springer, Moritz, Kappe and Sinnis2016), the relevance of this modification remains unknown. The essential role of C-mannosylation for protein secretion observed in other organisms (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013; Shcherbakova et al., Reference Shcherbakova, Tiemann, Buettner and Bakker2017) prompted us to investigate the contribution of PfDPY19 to parasite survival and infectivity.
Contrary to O-fucosylation (Lopaticki et al., Reference Lopaticki, Yang, John, Scott, Lingford, O'Neill, Erickson, McKenzie, Jennison, Whitehead, Douglas, Kneteman, Goddard-Borger and Boddey2017; Sanz et al., Reference Sanz, Aquilini, Tweedell, Verma, Hamerly, Hritzo, Tripathi, Machado, Churcher, Rodrigues, Izquierdo and Dinglasan2019), C-mannosylation is also expected to occur during the blood stages of the parasite, as supported by the conservation of the apicomplexan consensus sequence (Hoppe et al., Reference Hoppe, Albuquerque-Wendt, Bandini, Leon, Shcherbakova, Buettner, Izquierdo, Costello, Bakker and Routier2018) in the TSR domain-containing proteins PTRAMP, MTRAP and SPATR (Table 1). However, the successful generation of a parasite line with a severely truncated PfDPY19 gene strongly suggests that this modification is not essential for the asexual development of the parasite. Furthermore, parasite growth was not affected in ΔPfDPY19 mutants, suggesting that the gene is not relevant during the asexual multiplication of the parasite, at least under the tested conditions. This finding is in partial agreement with a previous high-throughput screening study that described the gene coding for PfDPY19 as dispensable, but conferring significant fitness to P. falciparum in the asexual blood stages (Zhang et al., Reference Zhang, Wang, Otto, Oberstaller, Liao, Adapa, Udenze, Bronner, Casandra, Mayho, Brown, Li, Swanson, Rayner, Jiang and Adams2018). The discrepancy might arise from slight deviations associated with the comparison of sequencing reads as a proxy for competitive growth fitness in this particular gene or genomic region. The contribution of PfDPY19 to the secretion of the putative acceptors expressed in the blood stages has not been directly assessed. Nevertheless, if there is an effect on protein secretion secondary to PfDPY19 gene disruption, it does not seem to affect neither parasite growth nor the parasite's ability to cope with DTT-induced oxidative stress. Contrary to this, a recent work reports that Toxoplasma gondii DPY19 may be important for the growth fitness of T. gondii tachyzoites, a related apicomplexan (Gas-Pascual et al., Reference Gas-Pascual, Ichikawa, Sheikh, Serji, Deng, Mandalasi, Bandini, Samuelson, Wells and West2019).
The O-fucosylation dependence for protein secretion seems to be variable among the different acceptors that are expressed in the mosquito stages of P. falciparum (Lopaticki et al., Reference Lopaticki, Yang, John, Scott, Lingford, O'Neill, Erickson, McKenzie, Jennison, Whitehead, Douglas, Kneteman, Goddard-Borger and Boddey2017). These findings are in agreement with similar observations made with human TSR domain-containing proteins after the loss of the enzyme responsible for O-fucose elongation with glucose (Vasudevan et al., Reference Vasudevan, Takeuchi, Johar, Majerus and Haltiwanger2015). Similarly, C-mannosylation seems to be required for the secretion of only a subset of its acceptors in C. elegans (Buettner et al., Reference Buettner, Ashikov, Tiemann, Lehle and Bakker2013; Shcherbakova et al., Reference Shcherbakova, Tiemann, Buettner and Bakker2017). Thus, this modification may be more relevant in other stages of the parasite, where other putative effectors have also been described to play essential roles for parasite viability and infectivity. For instance, given MTRAP essentiality for gametocyte egress (Bargieri et al., Reference Bargieri, Thiberge, Tay, Carey, Rantz, Hischen, Lorthiois, Straschil, Singh, Singh, Triglia, Tsuboi, Cowman, Chitnis, Alano, Baum, Pradel, Lavazec and Ménard2016), a reduced secretion of this protein may result in a deficient transmission to the mosquito. Likewise, a reduced secretion of CTRP or TRAP as it has been reported after PfPoFUT2 gene disruption (Lopaticki et al., Reference Lopaticki, Yang, John, Scott, Lingford, O'Neill, Erickson, McKenzie, Jennison, Whitehead, Douglas, Kneteman, Goddard-Borger and Boddey2017) may cause a deficient mosquito midgut colonization and hepatocyte invasion, respectively. Furthermore, specific in vivo environmental conditions, such as temperature variations or other ER stress inducers, might affect the secretion of different acceptors along the parasite life cycle, including the asexual blood stages. Hence, further studies are required to define the function of PfDPY19 C-mannosyltransferase in Plasmodium parasites.
Acknowledgements
The authors thank Alfred Cortés and colleagues for the generous gift of the P. falciparum 3D7 1.2B line. We are also obliged to Ellen Knuepfer for providing us with the pDC2-based vector used for CRISPR/ Cas9-mediated generation of the PfDPY19 null mutant. We are grateful to R.R. Dinglasan, T. Hammerly, M. Ramírez and L. Lee for technical support, advice and useful suggestions.
Financial support
This work was funded by SAF2016-76080-R AEI/FEDER-UE to L.I.
Conflict of interest
None.
Ethical standards
Erythrocytes were obtained from the Banc de Sang i Teixits (Catalonia, Spain), after approval from the Comitè Ètic Investigació Clínica Hospital Clínic de Barcelona (Reg. HCB/2017/0413).






