Evidence now associates a number of adversities operating over intrauterine life and functional impairments in infancy and childhood with increased risk of schizophrenia. Reference Waddington, Lane, Larkin and O'Callaghan1–Reference Rapoport, Addington and Frangou3 However, the biology of developmental disturbance is poorly understood. Congenital anomalies constitute ‘hard’ biological evidence of dysmorphogenic events over embryonic and foetal life that are associated with a variety of early functional impairments. Reference Moore and Persaud4 These anomalies and related functional impairments can be examined prospectively from infancy for their ability to predict adverse adult outcomes, including schizophrenia. One study Reference Dalman, Allebeck, Cullberg, Grunewald and Köster5 has reported the presence of congenital anomalies to be associated with a doubling of risk of schizophrenia, and several other studies Reference Maki, Veijola, Jones, Murray, Koponen, Tienari, Miettunen, Tanskanen, Wahlberg, Koskinen, Lauronen and Ishohanni2 have reported a variety of early functional impairments to be associated with increased risk of this disorder. However, these relationships have yet to be considered together in detail.
Method
The Congenital Anomalies data-set in the Prenatal Determinants of Schizophrenia (PDS) study Reference Susser, Schaefer, Brown, Begg and Wyatt6 was used to conduct a systematic, prospective examination of the relationship between congenital anomalies, early functional impairments and risk of schizophrenia in adulthood. To our knowledge, this is the first such study to involve detailed examination of individual, physician-diagnosed congenital anomalies and related functional impairments.
Study cohort
The PDS study, including all methodologies relating to the present analysis, has been described previously in detail. Reference Susser, Schaefer, Brown, Begg and Wyatt6,Reference Bresnahan, Brown, Schaefer, Begg, Wyatt and Susser7 Briefly, the cohort members were enrolled into the Child Health and Development Study (CHDS), Reference Van den Berg8 which took place from 1959 to 1967. This study recruited nearly every pregnant woman under obstetric care from the Kaiser Permanente Medical Care Plan in Alameda County, California, USA, with the 19 044 live-born offspring of these women enrolled automatically into the Kaiser Permanente Medical Care Plan. The CHDS collected data from maternal medical records, maternal interviews and other sources described further below.
The PDS study cohort consists of the 12 094 live-born offspring who belonged to the Kaiser Permanente Medical Care Plan between 1 January 1981 (the year in which computerised registries became available) and 31 December 1997. The cohort was followed for 17 years. Thus, given that cohort enrolment involved births between 1959 and 1967, the ages of offspring ranged from 13 years to 38 years over the course of PDS study follow-up. Offspring who remained in the Kaiser Permanente Medical Care Plan and those lost to follow-up were similar to one another on most maternal and paternal characteristics, including occupation, education and ethnicity, as described previously in detail, Reference Susser, Schaefer, Brown, Begg and Wyatt6,Reference Bresnahan, Brown, Schaefer, Begg, Wyatt and Susser7 with the vast majority of individuals who left the Kaiser Permanente Medical Care Plan doing so before the age of 10 years.
Creation of the congenital anomalies data-set
The immediate source document for the congenital anomalies file, relating to live births in the CHDS, was the paediatric record card (‘pedicard’). This contained an abstract of all available medical information for each child. Information was abstracted on a routine basis over infancy, from the birth hospitalisation record through every visit made by each infant to any Kaiser clinic (95% of all anomalies), as well as for any hospitalisation or any special examination required, primarily over the first 5 years of life. On each occasion when new information was added to the pedicard, it was checked to see if any information pertaining to a congenital anomaly or early functional impairment had been added. If there was such an addition, the pedicard was reviewed by a physician to determine whether or not the diagnosis in question was one that belonged in that file. In this way, rare conditions not already listed were picked up and common conditions that were deemed ‘trivial’ and ‘defects of little or no consequence’ were screened out; thus, ‘minor physical anomalies’ were not captured. If the condition was determined to belong in the file, it was added.
This CHDS congenital anomalies data-set was initiated in the late 1950s to ascertain all frank cases of congenital anomalies and all occurrences of a group of early functional impairments that were considered to occur commonly as a symptom of a congenital anomaly. It encompassed structural abnormalities, related functional impairments, inborn errors of metabolism and chromosomal aberrations. Two physicians assigned code numbers to these congenital anomalies and early functional impairments; a third physician was co-opted in the event of disagreement. Coding was in accordance with the four-digit code numbers of ICD–7, 9 supplemented by a fifth digit to allow greater specification. The last modifications to the congenital anomalies file were made in 1972, prior to the beginning of the PDS study, hence it was finalised and codified substantially before determination of outcome in terms of schizophrenia spectrum-disorders.
Study classification of the congenital anomalies data-set
Using only ‘definite’ anomalies and/or functional impairments – 97% of all categories vis-à-vis 1% ‘probable’ and 2% ‘possible’ anomalies – the anonymised CHDS congenital anomalies file was categorised, blind to outcome measures, as follows. On the basis of previous work, Reference Maki, Veijola, Jones, Murray, Koponen, Tienari, Miettunen, Tanskanen, Wahlberg, Koskinen, Lauronen and Ishohanni2,Reference Moore and Persaud4,Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan10–Reference Vestergaard, Pedersen, Christensen, Madsen, Olsen and Mortensen15 an overall hypothesis-based category was applied to capture anomalies of craniofacial/midline structures and early functional–neural impairments that commonly occur as a symptom of a central nervous system (CNS) structural anomaly. A second overall category was applied to capture:
-
(a) possibly informative structural anomalies of other body regions;
-
(b) early functional–non-neural impairments that commonly occur as a symptom of a non-CNS structural anomaly;
-
(c) functional–genetic conditions that can be associated with congenital anomalies;
-
(d) astigmatism–myopia, as the most common single category in the data-set (present in 22% of the cohort);
-
(e) a category of ‘any other anomaly’ to capture all structural and early functional impairments in the data-set not included in any of the above categories.
Diagnosis of schizophrenia-spectrum disorders
The outcome was schizophrenia and other schizophrenia spectrum-disorders, defined on the basis of previous studies Reference Vestergaard, Pedersen, Christensen, Madsen, Olsen and Mortensen15 as any of the following: schizophrenia; schizoaffective disorder; delusional disorder; psychotic disorder not otherwise specified; and schizotypal personality disorder. Case ascertainment involved three steps: Reference Susser, Schaefer, Brown, Begg and Wyatt6 ascertainment of potential cases from computerised records; chart review of potential participants to confirm eligibility for assessment; diagnostic interview or chart review and consensus diagnosis. Case ascertainment was conducted by a computerised record linkage between the CHDS and Kaiser Permanente Medical Care Plan identifiers by using in-patient, out-patient and pharmacy registries: individuals from the hospital registry were screened for potential schizophrenia-spectrum disorder based on ICD–9 diagnoses 295–299 and psychiatrist review of all psychiatric and medical records; individuals from the out-patient registry were screened for potential schizophrenia-spectrum disorder based on ICD–9 diagnoses 295, 297, 298 or 299; individuals from the pharmacy registry were screened for potential schizophrenia-spectrum disorder based on a history of antipsychotic treatment.
There were 13 persons who had died among the 183 who screened positive for potential schizophrenia-spectrum disorder. From the 170 remaining individuals with a potential diagnosis of schizophrenia-spectrum disorder, 146 (86%) were contacted to schedule a diagnostic interview. Clinicians with at least a master's degree in a mental health field, and who were trained to reliability, administered the Diagnostic Interview for Genetic Studies (DIGS). Reference Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, Harkavy-Friedman, Severe, Malaspina and Reich17 This was completed by 107 (73%) of the 146 potential participants contacted; consensus of three experienced research psychiatrists was used to obtain DSM–IV diagnoses based on review of the DIGS narrative, medical records and discussions with the interviewer. For the 76 potential participants who were not interviewed (i.e. the 183 persons with a potential diagnosis of schizophrenia-spectrum disorder minus the 107 for whom an interview was completed), chart reviews by experienced clinicians were conducted; all diagnoses were confirmed by a research psychiatrist.
These procedures yielded a total of 71 persons having a schizophrenia-spectrum disorder, of whom 44 completed the DIGS and 27 were diagnosed by chart review. Among these 71 people, diagnoses were schizophrenia (n=43), schizoaffective disorder (n=17), delusional disorder (n=1), schizotypal personality disorder (n=5) and other schizophrenia-spectrum disorder (n=5), for whom a specific schizophrenia-spectrum psychosis diagnosis could not be made. Participant demographics were as follows: mean age at first hospitalisation 24.2 years (s.d.=4.8); 66% male, 34% female; maternal race, 42% White, 47% Black and minority ethnic, 11% other. Additional demographic factors, such as parental occupation, education and ethnicity, for both people with and without schizophrenia-spectrum disorder have been described previously in detail. Reference Susser, Schaefer, Brown, Begg and Wyatt6,Reference Bresnahan, Brown, Schaefer, Begg, Wyatt and Susser7
All those assessed in the PDS study provided written informed consent to their participation. The study protocol was approved by the institutional review boards of the New York State Psychiatric Institute and the Kaiser Foundation Research Institute.
Data analysis
Since the CHDS birth cohort contained siblings, only one sibling from each family was selected randomly to maintain independence of observations in the analyses. Owing to the limited number of offspring diagnosed with a schizophrenia-spectrum disorder during the course of the PDS study follow-up, if a sibship contained an affected sibling, that sibling was retained in the study and the unaffected siblings were excluded. However, if the sibship did not contain an affected sibling, then one unaffected sibling was randomly selected for inclusion into the sample. This selection process resulted in 7796 offspring. Five offspring diagnosed with a schizophrenia-spectrum disorder were subsequently excluded from the analyses reported here: four who were diagnosed prior to 1 January 1981 (the start date of the PDS study) and one who had an affected sibling who was a member of the PDS cohort. This gave a final total of 7791 PDS offspring.
Cox proportional hazards regression Reference Cox and Oaks18 was applied to analyse the data, since this statistical technique takes into account varying durations of follow-up, while similarly adjusting for multiple covariates. For offspring diagnosed with schizophrenia-spectrum disorders, the date of onset of the disorder was approximated by the date of first psychiatric admission or first psychiatric out-patient visit; thus, the length of follow-up for affected offspring was quantified as days elapsed from age 15 years until date of onset as defined above. Analogously, length of follow-up for unaffected offspring was quantified as days elapsed from age 15 years until the date of termination from the Kaiser Permanente Medical Care Plan or until the end of the PDS study, whichever was the sooner. To strengthen the evidence for causality, confounding was addressed through selection of covariates shown to be influential in previous studies. On this basis, maternal education, maternal race, maternal age, paternal age and infant gender were incorporated as covariates in Cox models for determination of risk ratios with associated 95% confidence intervals.
Results
The presence of any craniofacial/midline anomaly and/or early functional–neural impairment was associated with increased risk of schizophrenia-spectrum disorder (RR=2.18, 95% CI 1.11–4.28, P=0.023) (Table 1); individual anomalies and/or related functional–neural impairments present for each of the 13 participants with schizophrenia-spectrum disorder having one or more finding are presented in the Appendix. In planned analyses within this overall category, craniofacial/midline anomalies and related functional–neural impairments were each associated with a doubling risk of schizophrenia-spectrum disorder, although with a wider confidence interval, at trend level, for craniofacial/midline anomalies; this could reflect the smaller number of people with schizophrenia-spectrum disorder with such anomalies. Although the covariate of paternal age was also associated with risk of schizophrenia-spectrum disorder (e.g. RR=1.064 95% CI 1.011–1.119, P=0.016, when included in the analysis with any craniofacial/midline anomaly or related functional–neural impairment), these two risk associations were independent.
Table 1 Rate ratios for schizophrenia-spectrum disorders in adult offspring by congenital anomaly and functional impairment status
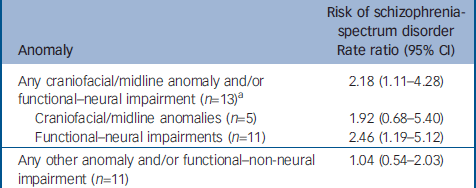
| Anomaly | Risk of schizophrenia-spectrum disorder Rate ratio (95% CI) |
|---|---|
| Any craniofacial/midline anomaly and/or functional–neural impairment (n=13) a | 2.18 (1.11–4.28) |
| Craniofacial/midline anomalies (n=5) | 1.92 (0.68–5.40) |
| Functional–neural impairments (n=11) | 2.46 (1.19–5.12) |
| Any other anomaly and/or functional–non-neural impairment (n=11) | 1.04 (0.54–2.03) |
a. Number of people with schizophrenia-spectrum disorder having one or more anomaly or functional impairment in that category
In contrast, having any other congenital anomaly and/or functional–non-neural impairment was not associated with risk of schizophrenia-spectrum disorder (Table 1). For example, the most common such category, astigmatism–myopia, was not associated with risk of schizophrenia-spectrum disorder: RR=1.32, 95% CI 0.59–2.97. A file documenting the rates for each individual congenital anomaly and functional impairment encountered in the study is available from the authors upon request.
Similar findings were apparent on confining analyses to people with schizophrenia, rather than schizophrenia-spectrum disorder.
Discussion
Using the PDS–CHDS population-based birth cohort study, we report the presence at birth or in infancy of craniofacial/midline anomalies and/or early functional impairments that commonly occur as a symptom of a CNS anomaly to be associated with a doubling of risk of schizophrenia-spectrum disorder in adulthood.
This category was selected from a diversity of entries within the PDS–CHDS congenital anomaly data-set. It derived from findings in schizophrenia of dysmorphology of craniofacial/midline regions Reference Waddington, Lane, Larkin and O'Callaghan1,Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan10–Reference Hennessy, Baldwin, Browne, Kinsella and Waddington13 and of functional impairments in infancy and childhood. Reference Maki, Veijola, Jones, Murray, Koponen, Tienari, Miettunen, Tanskanen, Wahlberg, Koskinen, Lauronen and Ishohanni2,Reference Qin, Xu, Laursen, Vestergaard and Mortensen14,Reference Vestergaard, Pedersen, Christensen, Madsen, Olsen and Mortensen15 In relation to the most frequent findings (see Appendix), febrile convulsions are a recognised antecedent for epilepsy in infancy in association with malformations of cortical development; Reference Baulac19–Reference Waruiru and Appleton21 our findings elaborate recent studies in which febrile seizures and epilepsy were associated with risk of adult schizophrenia. Reference Qin, Xu, Laursen, Vestergaard and Mortensen14,Reference Vestergaard, Pedersen, Christensen, Madsen, Olsen and Mortensen15 Deficits in language acquisition are believed to involve malformations of cortical development, although language delay can be influenced also by social factors; Reference Fisher and Marcus22 our findings elaborate well-recognised associations between schizophrenia and language disorder. Reference Maki, Veijola, Jones, Murray, Koponen, Tienari, Miettunen, Tanskanen, Wahlberg, Koskinen, Lauronen and Ishohanni2,Reference Condray23 Craniofacial/midline anomalies involve areas that share the embryological origins of the CNS, particularly frontal cortical regions. Reference Waddington, Lane, Larkin and O'Callaghan1,Reference Marcucio, Cordero, Hu and Helms24
Other congenital anomalies and related functional–non-neural impairments were not associated with risk of schizophrenia-spectrum disorder. These distinct relationships were evident on controlling for several potential confounders, including maternal education, maternal race, maternal age and infant gender. Although paternal age is associated with increased risk of schizophrenia in offspring in this Reference Brown, Schaefer, Wyatt, Begg, Goetz, Bresnahan, Harkavy-Friedman, Gorman, Malaspina and Susser25 and other Reference Malaspina, Harlap, Fennig, Heiman, Nahon, Feldman and Susser26 data-sets, as reprised here, the present findings were independent of paternal age.
To our knowledge, this is the first systematic study of schizophrenia in relation to anomalies and related functional impairments ascertained prospectively over infancy by physicians, with ascertainment and categorisation ‘blind’ to adult psychiatric outcome. These strengths have to be set against certain limitations. For example, the timing of assessments varied and individuals born in the later years of the study might have had less opportunity for anomalies and impairments to be detected. However, it is not clear how such variation in assessments over infancy could be related in any systematic way to the risk of schizophrenia in adulthood. To address the issue, we repeated Cox analyses using year of birth as an additional covariate and found this to have no effect on our results. Also, anomalies and related functional impairments constitute a diversity of abnormalities that may have varying manifestations and thresholds for detection, hence even experienced physicians might have differed in noting and specifying certain features. However, given the prominence and pervasiveness of the types of anomalies and functional impairments observed, and as all of these were diagnosed by paediatricians, it is unlikely that appreciable misclassification occurred. Moreover, as for variation in timing of assessments, it is not clear how variation between paediatricians in making those assessments could be related in any systematic way to risk of schizophrenia in adulthood.
Anomalies and/or functional impairments associated with a doubling of risk of schizophrenia-spectrum disorder appear to share some common relationship to brain dysmorphogenesis, which might result from the impact of genetic predisposition and environmental factors in the pathogenesis of schizophrenia.
Appendix
Individuals with schizophrenia-spectrum disorder having one or more craniofacial/midline anomalies and/or functional–neural impairments.
- Individual
-
Anomalies and/or functional impairments
- 1
-
(a) ‘Anomalies, teeth/developmental problems, teeth’
- 2
-
(a) ‘Ptosis, congenital’ (b) ‘Deficiency, mental’ (c) ‘Disturbance, speech’
- 3
-
(a) ‘Epilepsy – idiopathic seizure disorder’
- 4
-
(a) ‘Intelligence, borderline – IQ 68–85’ (b) ‘Minimal brain dysfunction’ (c) ‘Disturbance, speech’
- 5
-
(a) ‘Development, slow speech’
- 6
-
(a) ‘Development, slow speech’
- 7
-
(a) ‘Convulsions, febrile’
- 8
-
(a) ‘Convulsive disorder’
- 9
-
(a) ‘Cleft palate, with or without cleft uvula’
- 10
-
(a) ‘Anomalies, congenital – ribs’ (b) ‘Deficiency, mental’ (c) Convulsions, febrile'
- 11
-
(a) ‘Microcephaly’ (b) ‘Asymmetry, face, head, or skull’ (c) ‘Deficiency, mental’ (d) ‘Convulsions, febrile’ (e) ‘Convulsive disorder’
- 12
-
(a) ‘Convulsions, febrile’
- 13
-
(a) ‘Convulsions, febrile’
Acknowledgements
The authors thank Dr T. Clive Lee, Professor and Chairman of the Department of Anatomy, Royal College of Surgeons in Ireland, Dublin, for assistance in relation to the interpretation of rare congenital anomalies.
This study is supported by the Stanley Medical Research Institute, by National Institute of Mental Health grants 1-K02-MH65-422-01, 1-R01-MH60249 and 1-R01-MH63264, and by various NARSAD grants. The Child Health and Development Study has been supported by National Institute of Child Health and Human Development contracts N01-HD-1-3334 and N01-HD-6-3258 and is administered by the Public Health Institute, Berkeley, California, USA, principal investigator Barbara Cohn.




eLetters
No eLetters have been published for this article.