Folic acid (FA), also known as folate, is one of the B-complex vitamins and acts as an intermediate in lots of reactions which involves the transfer of one-C units; it is also required directly in the synthesis of purines, pyrimidines and methionine(Reference Bailey and Gregory1). Purines and pyrimidines are the building blocks of both types of nucleic acids, DNA and RNA, while amino acids (such as methionine) are the basic units of proteins. During their proliferation and differentiation, cells undergo extensive DNA as well as protein synthesis. Moreover, folate is an antioxidant that is capable of scavenging free radicals and their metabolic products to protect lipids from oxidative damage and maintain normal cellular physiology(Reference Gursu, Onderci and Gulcu2). Similarly, during the early stages of pregnancy, the addition of FA to the maternal diet leads to significantly higher serum antioxidant activity (glutathione and superoxide dismutase) in ewes compared with the non-treated control group(Reference El-Tarabany, Atta and Emara3). Therefore, a sufficiently high FA level is crucial to embryo development and rapid growth during gestation as well as the early stage. In addition, methionine can be transformed along with ATP to form S-adenosylmethionine, a major donor of methyl groups(Reference Li, Abouelezz and Cheng4). During embryonic development, when the visceral and somatic organs form the expression of genes with early organ-specific functions increase, while the expression of those related to cell division and general morphogenesis decrease(Reference Cardoso-Moreira, Halbert and Valloton5). Because the formation and development of embryos as well as the regeneration of adult tissues depend on cell proliferation, FA is essential for the development of organs and tissues. However, the optimal dose of FA supplementation is still unclear.
It is generally believed that the FA requirement in ruminants can be met through its production of the ruminal microflora(Reference Bechdel, Honeywell and Dutcher6); however, these requirements have never been thoroughly evaluated. Moreover, serum folate levels in dairy cows are known to decrease by 40 % during pregnancy(Reference Girard, Matte and Tremblay7). In addition, studies in ewes and lambs indicated that dietary FA supplementation can markedly improve their growth performance(Reference Suhdoon, Mohamed and Sultan8,Reference Li, Wang and Li9) . All this seem to suggest that rumenal FA synthesis cannot satisfy the demand in these animals. And when it was also reported that only a small amount of supplementary folate (about 3 %) could escape degradation in the rumen(Reference Zinn, Owens and Stuart10), the rumen-protected folic acid (RPFA) has come into the centre of attention.
Most of the energy requirement of animals is used by the visceral organs, especially the liver and gastrointestinal tract(Reference Ferrell and Jenkins11). Changes in the visceral organ mass may then alter the amount of energy and proteins available for growth(Reference Fluharty and Mcclure12). We reported previously that the average daily gain and feed conversion ratio improved after RPFA supplementation in lambs(Reference Li, Wang and Li9). Improving carcass characteristics such as live weight and dressing percentage of sheep have enormous economic benefits. For consumers, physical characteristics of meat is important that greatly impacts purchase decisions. Despite its importance, limited information is available on how RPFA supplementation affects slaughter performance and carcass composition of animals. Considering the vital functions of FA during pre- and postnatal development, we speculate that RPFA might modify visceral organ mass and animal carcass composition that in turn results in increased growth of the offspring. To test this hypothesis, we conducted a study to determine the effects of RPFA added to the maternal and/or lambs’ diet on the visceral organ mass and carcass characteristics of Hu lambs.
Materials and methods
Animals and experimental design
The animals were handled according to the protocol approved by the Animal Care and Use Committee of China Agricultural University. The present study was conducted at Jiangsu Qianbao Animal Husbandry Co. Ltd. For the experiment, 120 Hu ewes (24 ± 4·2 months of age and 44·6 ± 5·43 kg of body weight, mean ± SD) with two parity and showing signs of estrus were selected, mated and then randomly allocated into three groups (forty ewes in each group). The experimental animals received either 0 mg (M0F), 16 mg (M16F) or 32 mg (M32F) FA as RPFA per kg DM; they were raised separately in individual pens (1·5 × 3 m) and fed a total mixed ration (Table 1). The production method of the RPFA and its digestive performance have been described previously(Reference Li, Wang and Li9); here the disappearance rate in the rumen and the small intestine absorption rate of the RPFA were 92·6 and 85·6 %, respectively. On day 28 after mating, pregnancy diagnosis was done by means of type-B ultrasonography with a dual frequency 6·0/8·0 MHz linear probe (Tringa Linear Vet, Esaote-Pie Medical, Esaote China, Ltd.). The information about the number of ewes pregnant, lambed, those that gave birth to singletons, twins, triplets or quadruplets, their serum folate concentrations during pregnancy, their initial and postpartum weights, the lambing rates, mean birth weights and the litter weights at birth has been previously described(Reference Li, Wang and Li9).
Table 1. Ingredients and chemical composition of the diet for ewes and lambs (g/kg DM)

* Contained per kg milk replacer: 220 g crude protein, 180 g ether extract, 30 g Ca, 5 g P, 10 g lysine.
† Contained per kg premix: 145 g Ca, 20 g P, 1600 mg Fe, 200 mg Cu, 1300 mg Mn, 1300 mg Zn, 8·75 mg iodine, 3·75 mg Se, 3·75 mg Co, 100 000 IU vitamin A, 15 000 IU vitamin D, 1625 IU vitamin E, 250 mg niacin, 75 mg pantothenic acid and 5 mg biotin for pregnant ewes; 160 g Ca, 23 g P, 1800 mg Fe, 300 mg Cu, 1500 mg Mn, 1600 mg Zn, 10·0 mg iodine, 4·25 mg Se, 4·25 mg Co, 120 000 IU vitamin A, 17 500 IU vitamin D, 1550 IU vitamin E, 300 mg niacin, 90 mg pantothenic acid and 8 mg biotin for lactating ewes; 150 g Ca, 20 g P,1600 mg Fe, 200 mg Cu, 1750 mg Mn, 1150 mg Zn, 7·5 mg iodine, 3·75 mg Se, 3·75 mg Co, 75 000 IU vitamin A, 10 500 IU vitamin D, 1312 IU vitamin E, 150 mg niacin, 50 mg pantothenic acid and 3 mg biotin for lambs.
All newborn lambs were nursed by their dam and fed with a starter (Table 1) ad libitum until weaning at 50 d of age. At weaning, twenty-two offspring were selected from each maternal diet group and allocated into two groups based on litter size, sex and body weight to receive either 0 mg (OC) or and 4·0 mg (OF) FA addition as RPFA per kg DM in their diets. Altogether, 66 Hu lambs (50 ± 4·3 d of age and 16·4 ± 3·84 kg of body weight) were selected and divided into a total of six groups (M0F-OC, M0F-OF, M16F-OC, M16F-OF, M32F-OC and M32F-OF). The weaned lambs were fed twice per d (at 07.10 and 19.10 hours) with a total mixed ration (Table 1) and had free access to water in their individual pens. The feeding experiment of the weaned lambs lasted for 130 d, and included a 10-d adaptation period followed by a 120-d collection period.
Slaughter and sampling procedures
After the collection period (days 122–127), the lambs were slaughtered at an experimental abattoir. They were weighed individually to obtain pre-slaughter weight (PSW) after a 12-h fasting period. Slaughter was performed according to the following procedure: euthanasia; skinning; removal of head at the atlas-occipital joint; cutting of the limbs at the carpo-metacarpal and tarso-metatarsal joints; separation of the gastrointestinal tract (rumen, reticulum, omasum, abomasum, duodenum, jejunum and ileum) and its contents; removal of the heart, liver, spleen, lungs and kidney; excision of the omental, perirenal and tail fat deposits. The above elements were weighed and recorded. The foremost of these was that the weight of the stomach and small intestine that were determined after eliminating their contents. Empty body weight was calculated as 0·851 × PSW (kg) of lamb(Reference Cannas, Tedeschi and Fox13). The carcass of each lamb was weighed within 30 min after slaughter, and then the dressing percentage was calculated as (carcass weight/PSW) × 100. Each carcass was split in half, the right side was cut to obtain meat weight and bone weight, and then the meat percentage was calculated after determining the meat weight. Meanwhile, the left side of the carcass was chilled at 4°C for 24 h to measure the eye muscle area (EMA), girth rib (GR) value, pH, cooking loss, drip loss, shear force and meat colour. The length of duodenum, jejunum and ileum were determined using a tape measure.
Sample measurements and chemical analyses
Samples of total mixed ration were measured for DM (method 934.01), ash (method 942.05), N (method 976.05), ether extract (method 973.18) and acid-detergent fibre (method 973.18) according to AOAC(14). Organic matter content was estimated from the difference between DM and crude ash values. Neutral-detergent fibre content was measured based on methods described by Van Soest et al. (Reference Van Soest, Robertson and Lewis15), with heat stable α-amylase and sodium sulphite used in the neutral-detergent fibre procedure and expressed inclusive of residual ash. Folate contents were determined using HPLC as described by Alaburda et al. (Reference Alaburda, De Almeida and Shundo16).
The longissimus dorsi muscle excised from the left side carcass at the twelfth rib was used to determine the EMA by planimeter (QCJ-2000, Haerbin Optical Instrument factory, Haerbin, China) on traced outlines of a cross section of the eye of longissimus dorsi(Reference Gaunt, Ferrier and Tatham17). The GR value was measured at the site over the twelfth rib, 110 mm away from the midline(Reference Gaunt, Ferrier and Tatham17) using a vernier caliper. The pH was measured using a portable pH meter (205-PH1, Testo Instruments (Shenzhen) Co., Ltd.) with a penetrating glass electrode at 45 min and 24-h postmortem. Cooking loss was determined by calculating the difference in weight before and after a 15-min immersion in a 75oC water bath according to the method of Zhao et al. (Reference Zhao, Li and Su18). For drip loss analyses, 50 g of longissimus dorsi sample (10 cm × 5 cm × 1 cm) was placed in a polyethylene bag perpendicularly and away from the wall of the bag at 4oC for 24 h. The sample was then removed from the bag, dried on absorbent paper and reweighed. The amount of drip at 48 h postmortem was expressed as a percentage of weight change before and after drying(Reference Honikel19). Shear force was measured using a shear force instrument (TMS-PRO Food Texture Analyzer, Food Technology Corporation) (test speed: 60 mm/min; distance: 40 mm; and trigger force: 250 N) based on the method of Zhao et al. (Reference Zhao, Liu and Zhang20). Meat colour evolution, using the L*, a* b* system, was measured with a colorimeter (Chroma Meter CR 400/410, Konica Minolta Optics, Inc.) from a fat-free area and is shown as the average of three measurements.
Statistical analyses
All experimental data were analysed as a completely randomised block design with a 3 (0, 16 and 32 mg FA added to the maternal diets) × 2 (0 and 4·0 mg FA added to the lambs’ diets) factorial arrangement of treatments using the MIXED procedure of SAS (Proc Mixed)(21); the model was as follows:
where Y ijk is the dependent variable, μ is the overall mean, B i is the random effects of the ith block, M j is the fixed effects of RPFA added to the maternal diets (j = 0, 16 and 32 mg/kg), O k is the fixed effects of RPFA added in the lambs’ diets (k = 0 and 4 mg/kg), (MO) jk is the M × O interaction and ϵ ijk is the residual error. Mean separation was done using the PDIFF option of the LSMEANS statement in the case of interactions that were significantly different (P < 0·05). When the effects of RPFA added to the maternal diets reached 90 % level of significance, orthogonal contrasts (linear and quadratic) were compared using the CONTRAST statement of SAS with coefficients estimated based on the RPFA application rates. The main treatment effects were compared using the Duncan test. Differences were considered significant at P < 0·05.
Results
Slaughter performance
No significant M × O interaction was observed for PSW, weight of head, hoof, fur, carcass, meat and bone, dressing and meat percentage, and EMA (Table 2). The PSW increased quadratically (P = 0·043) with RPFA supplementation in the maternal diet and was higher for M16F lambs than lambs in the M32F and control groups; it also increased (P = 0·001) with RPFA supplementation in the lambs’ diets, whereas weights of head, hoof and fur were not affected by RPFA added to the maternal and/or lambs’ diets. Weights of carcass, meat and bone, as well as dressing and meat percentage also quadratically increased (P < 0·05) with RPFA supplementation in the maternal diet and were higher for M16F lambs than for those in the control and M32F groups; it also increased (P < 0·05) when RPFA was added to the lambs’ diets. The EMA only increased (P = 0·038) when RPFA was supplemented to the lambs’ diets.
Table 2. Effects of rumen-protected folic acid (RPFA) supplementation of maternal and offspring diets on slaughter performance in lambs
(Mean values with their standard errors)
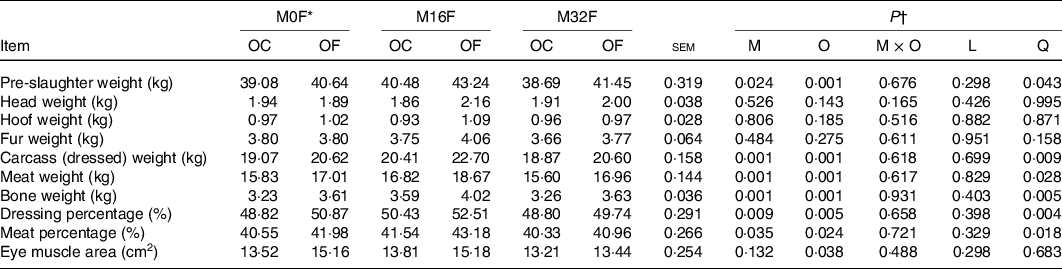
FA, folic acid.
* M0F, M16F and M32F with 0, 16 and 32 mg FA from RPFA per kg DM in maternal diets; OC and OF with 0 and 4·0 mg FA from RPFA per kg DM in offspring diets.
† M, main effect of RPFA supplementation in maternal diets; O, OC v. OF; M × O, interaction between RPFA supplementation in maternal diets and in offspring diets; L, linear; Q, quadratic.
Weight of visceral organs
No significant M × O interaction was observed for weights of liver, spleen and kidney, as well as organs coefficients (Table 3), but RPFA added to the lambs’ diets modified the response to maternal RPFA supplementation in the case of the weights of heart and lungs (M × O interaction, P < 0·05). Weights of the heart and lungs were greater for lambs born to M32F ewes when their diets were supplemented with RPFA, while weights of the heart and lungs of lambs from M0F or M16F ewes were not affected by RPFA added to the lambs’ diets. Liver weight quadratically increased (P = 0·042) with RPFA supplementation in the maternal diet and was higher (P = 0·021) for lambs born to M16F ewes than for those that derived from M0F and M32F ewes. Other selected visceral organ weights and their coefficients were not affected by RPFA added to the maternal and/or the lambs’ diets.
Table 3. Effects of rumen-protected folic acid (RPFA) supplementation of maternal and offspring diets on visceral organ mass in lambs
(Mean values with their standard errors)

EBW, empty body weight; FA, folic acid.
* M0F, M16F and M32F with 0, 16 and 32 mg FA from RPFA per kg DM in maternal diets; OC and OF with 0 and 4·0 mg FA from RPFA per kg DM in offspring diets.
† M, main effect of RPFA supplementation in maternal diets; O, OC v. OF; M × O, interaction between RPFA supplementation in maternal diets and in offspring diets; L, linear; Q, quadratic.
‡ EBW was calculated as 0·851 × pre-slaughter weight (kg) of lamb.
Stomach and intestine characteristics
Significant M × O interactions were observed between rumen and total stomach weights (P < 0·05), but no statistically significant M × O interactions were found for weights and coefficients of other gastrointestinal characteristics and small intestinal length (Table 4). Rumen and total stomach weights in lambs from M0F or M32F ewes increased (P < 0·05) when RPFA was added to the lambs’ diet, whereas in lambs born to M16F ewes these traits were not affected by RPFA added to the lambs’ diet. However, the rumen and total stomach coefficients were not affected by the presence of RPFA in the maternal and/or the lambs’ diet. Weights and coefficients of the reticulum and omasum quadratically increased (P < 0·05) with RPFA supplementation in the maternal diet and were higher (P < 0·05) for lambs from M16F ewes than for those born to M32F and M0F ewes, but they were not impacted by RPFA added to the lambs’ diet. Adding RPFA to the lambs’ diet increased the abomasum weight (P = 0·008). Weights of the duodenum and jejunum increased quadratically (P < 0·05) with RPFA supplementation in the maternal diet and were higher (P < 0·05) for lambs from M16F ewes than for lambs from M32F and M0F ewes. These weights also showed an increase (P < 0·05) when RPFA was added to the lambs’ diet, but their coefficients were not affected by RPFA present in the maternal and/or the lambs’ diets. The weight of the small intestine increased (P = 0·001) only by RPFA added to the lambs’ diet. No significant differences were found for the weight and coefficient of the ileum. The length of the duodenum increased (P = 0·001) by adding RPFA to the lambs’ diet but was unaffected by the presence of RPFA in the maternal diet. Finally, the lengths of the jejunum and ileum quadratically increased (P < 0·05) with RPFA added to the maternal diet and were higher (P < 0·05) for lambs born to M16F ewes than for those that derived from M32F and M0F ewes; they also increased (P < 0·05) when RPFA was added to the lambs’ diet.
Table 4. Effects of rumen-protected folic acid (RPFA) supplementation of maternal and offspring diets on gastrointestinal tissues in lambs
(Mean values with their standard errors)

EBW, empty body weight; FA, folic acid.
* M0F, M16F and M32F with 0, 16 and 32 mg FA from RPFA per kg DM in maternal diets; OC and OF with 0 and 4·0 mg FA from RPFA per kg DM in offspring diets.
† M, main effect of RPFA supplementation in maternal diets; O, OC v. OF; M × O, interaction between RPFA supplementation of maternal diets and offspring diets; L, linear; Q, quadratic.
‡ EBW was calculated as 0·851 × pre-slaughter weight (kg) of lamb.
Fat deposition
Significant M × O interaction was observed for weight and the coefficient of omental fat (P < 0·05), but there was no significant M × O interaction between weights and coefficients of tail fat and perirenal fat (Table 5). Tail fat weight and its coefficient quadratically increased (P < 0·05) with RPFA added to the maternal diet and were the highest in the case of lambs from M16F ewes, followed by lambs from M0F ewes, and they were the lowest for lambs born to ewes in the M32F group. However, only the tail fat coefficient decreased (P = 0·014) when RPFA was present in the offspring diet. Omental fat weight increased (P < 0·05) in lambs born to M0F or M16F ewes when RPFA was added to lambs’ diet, while it was not impacted by supplemental RPFA in the lambs’ diet in the case of lambs that were born to M32F ewes. The omental fat coefficient decreased (P < 0·05) in lambs from M32F ewes with RPFA added to the lambs’ diet, while the coefficient of omental fat of lambs born to M0F or M16F ewes was not impacted by RPFA added to lambs’ diet. Perirenal fat weight quadratically increased (P = 0·005) with RPFA added to the maternal diet and was the highest for lambs derived from M16F ewes, followed by lambs from M0F ewes, while it was the lowest for lambs from ewes in the M32F treatment group. Coefficient of perirenal fat quadratically increased (P = 0·031) with RPFA added to the maternal diet and was higher (P = 0·004) for lambs from M16F and M0F ewes than for lambs from M32F ewes. The weight of perirenal fat and its coefficient was not impacted by RPFA added to the lambs’ diet.
Table 5. Effects of rumen-protected folic acid (RPFA) supplementation of maternal and offspring diets on fat weight in tail, omentum majus and perirenal fat in lambs
(Mean values with their standard errors)

EBW, empty body weight; FA, folic acid.
* M0F, M16F and M32F with 0, 16 and 32 mg FA from RPFA per kg DM in maternal diets; OC and OF with 0 and 4·0 mg FA from RPFA per kg DM in offspring diets.
† M, main effect of RPFA supplements in maternal diets; O, OC v. OF; M × O, interaction between RPFA supplementation of maternal diets and offspring diets; L, linear; Q, quadratic.
‡ EBW was calculated as 0·851 × pre-slaughter weight (kg) of lamb.
Meat quality
A significant M × O interaction was observed for the GR value (P = 0·024; Table 6). The GR value decreased for lambs born to M16F ewes when RPFA was added to the lambs’ diet, whereas the GR value in the case of lambs from M0F or M32F ewes was not impacted by RPFA in the lambs’ diet. Addition of RPFA to the maternal diet did not affect meat pH, the GR value, cooking loss, drip loss, shear force and b* of meat colour, but linearly increased (P = 0·008) the L* value and quadratically increased (P = 0·037) the a* value of the meat colour. The L* value was higher (P = 0·009) for lambs from M32F ewes than for lambs from M16F and M0F ewes. The a* value was higher (P = 0·001) in the case of lambs born to M16F ewes than for lambs derived from M32F and M0F ewes. The addition of RPFA to the lambs’ diet did not affect meat pH, the GR value, cooking loss, shear force, L*, a* and b* of meat colour but tended to decrease (P = 0·077) drip loss.
Table 6. Effects of rumen-protected folic acid (RPFA) supplementation of maternal and offspring diets on meat quality in lambs
(Mean values with their standard errors)

FA, folic acid; GR, girth rib.
* M0F, M16F and M32F with 0, 16 and 32 mg FA from RPFA per kg DM in maternal diets; OC and OF with 0 and 4·0 mg FA from RPFA per kg DM in offspring diets.
† M, main effect of RPFA supplementation of maternal diets; O, OC v. OF; M × O, interaction between RPFA supplements in maternal diets and in offspring diets; L, linear; Q, quadratic.
‡ GR.
Discussion
Slaughter performance
The increase in PSW as a result of RPFA added to the maternal and/or lambs’ diets was in accordance with the higher average daily gain reported in our previous work(Reference Li, Wang and Li9). The result was consistent with the increased carcass weight, meat and bone weight. Carcass composition correlates with carcass weight, while carcass weight is dependent on the content of muscle, fat and bone(Reference Delfa and Teixeira22). The increase in meat weight may be due to the fact that RPFA supplementation can promote muscle development(Reference Wang, Li and Li23), which was also verified by the increase in EMA in the present study as EMA is known to be in a positive correlation with the total amount of muscle in sheep carcasses and is generally considered an indicator of muscle development(Reference Souza Júnior, Sousa and Pimenta Filho24). The higher dressing and meat percentage following RPFA addition to the maternal and/or lambs’ diets indicated that protein deposition was promoted by RPFA supplementation. The results might be due to the elevation in the apparent total tract digestibility of crude proteins(Reference Li, Wang and Li9) and hepatic gene expression responsible for protein synthesis, because according to an earlier study dietary RPFA addition could stimulate the transfer efficiency of methyl groups and the biosynthesis of amino acids, thereby facilitating protein synthesis(Reference Wang, Liu and Zhang25). In addition, La et al. (Reference La, Li and Wang26) demonstrated that hepatic gene expression related to protein synthesis was up-regulated by RPFA added to the diet of dairy calves. The increased EMA after RPFA addition to the lambs’ diet further confirmed that protein deposition was promoted by dietary RPFA supplementation.
Weights and coefficients of visceral organs
The weights of the internal organs and their weight coefficients reflect the health condition of the animals(Reference Smith, Gabler and Young27,Reference Zhang, Wang and Mo28) . Although liver weight was increased with RPFA added to the maternal diet, the weight coefficients of all selected visceral organs were not affected by the presence of RPFA in the maternal and/or lambs’ diets. The results indicated that dietary RPFA supplementation did not affect the health of selected visceral organs in lambs. The observed M × O interaction for the weights of heart and lungs suggested that the effect of RPFA added to the lambs’ diet was more pronounced in lambs from M32F ewes than in those from M0F or M16F ewes. The size of the visceral organs is related to feed intake(Reference Johnson, Johnson and Baldwin29) because the energy expenditure of these visceral organs elevates after feeding and is dependent on feed intake(Reference Seal and Reynolds30). However, there was no significant change in feed intake in the case of lambs during the study(Reference Li, Wang and Li9). A thorough explanation on the observed M × O interaction for weights of heart and lungs needs further studies.
Stomach and intestine characteristics
Stomach weight and the weight coefficient can reflect the development of the stomach(Reference Wang, Liu and Zhang31). In the present study, significant M × O interactions were observed between rumen and total stomach weights. Both weight and coefficient of the reticulum and omasum increased by added RPFA in the maternal diet. These results suggested that the development of the stomach in lambs was stimulated by RPFA added to the maternal and/or the lambs’ diets. Stomach development is due to an increase in ruminal fermentation products, such as acetate, propionate and butyrate, etc(Reference Wang, Liu and Zhang25). Li et al. (Reference Li, Wang and Li9) found that the ruminal total volatile fatty acids concentration and molar proportions of acetate and butyrate increased with RPFA addition to the maternal diet, and molar proportions of acetate elevated when RPFA was added to the lambs’ diet.
Small intestinal weight, weight coefficient and intestinal length can indicate the development of the small intestine(Reference Liu, Wang and Guo32). In the present study, the addition of RPFA to the maternal diet increased the length of the jejunum and ileum. The increased weight of the small intestine, duodenum, jejunum and the length of the duodenum, jejunum and ileum after RPFA supplementation of the lambs’ diet suggest that the development of the small intestine and the ability of the intestine to secrete enzymes might be promoted by RPFA addition to the maternal and/or lambs’ diets. Li et al. (Reference Li, Wang and Li9) found the activities of trypsin, chymotrypsin and lipase in the intestinal chyme became elevated by RPFA addition to the maternal diet, and chymotrypsin activity in the duodenum chyme as well as lipase in the jejunum chyme rose following RPFA addition to the lambs’ diet. Furthermore, Davidson and Townley(Reference Davidson and Townley33) reported that adequate FA was essential for maintaining the normal structure and function of the small intestine.
Fat deposition
The differences in the weights of perirenal fat, omental fat and tail fat among treatment groups were mainly related to metabolic processes, such as lipid metabolism and transport in response to RPFA added to diets. The increased weights and coefficients of tail and perirenal fat after the addition of RPFA to the maternal diet at a dose of 16 mg/kg indicate that RPFA might limit the deposition of fat in the liver and transfer it to the plasma and peripheral tissues, which in turn result in their deposition as tail and perirenal fat. This finding is consistent with previous results where supplemental RPFA in the maternal and/or lambs’ diets increased the level of very LDL in the serum(Reference Li, Wang and Li9), and it also caused more TAG being present in the liver that would be transported to the plasma(Reference Yao and Vance34) and then eventually deposited into tissues and organs. These results are in contrast with those reported by Graulet et al. who found that FA supplements given to dairy cows during early lactation stimulated fat deposition in the liver(Reference Graulet, Matte and Desrochers35). In lambs, RPFA supplementation led to the fattening of the animals, which indicates that a different lipid partitioning mechanism may exist between tissues; the reason needs to be clarified by additional research. In ruminants acetate, and to a lesser degree, propionate and butyrate are the principal precursors of de novo lipogenesis in the tissues(Reference Ladeira, Schoonmaker and Swanson36). Molar proportions of acetate and butyrate in the rumen increased with RPFA supplementation(Reference Li, Wang and Li9) that also contribute to more fat deposit. The significant M × O interaction for coefficient of omental fat indicates that the deposition of omental fat decreased in lambs from M32F ewes when they received RPFA supplementation in their diet, while the deposition of omental fat in lambs from M0F or M16F ewes was not influenced by RPFA added to the lambs’ diet. The decreased weights and coefficients of tail and perirenal fat with RPFA added to the maternal diet at a dose of 32 mg/kg suggest that excess FA alters lipid metabolism and transport in the liver. Studies have shown that excessive supplementation of FA in high-fat diets can lead to decreased expression of carnitine palmitoyl transferase-1 or to a deficiency in pseudo-methylenetetrahydrofolate reductase that damages the liver and leads to lipid accumulation(Reference Christensen, Mikael and Leung37,Reference McNeil, Hay and Rucklidge38) , resulting in limited fat deposition in tissues.
Meat quality
The pH value of lamb meat impacts meat quality by altering drip loss and tenderness(Reference Devine, Graafhuis and Muir39,Reference Watanabe, Daly and Devine40) . In the present study, the pH values were similar among treatments, suggesting that the meat of lambs in the different experimental groups had the same drip loss and tenderness. Water content is closely related to the juiciness of meat, while drip loss also affects meat quality(Reference Van Oeckel, Warnants and Boucqué41). The fact that drip loss and cooking loss did not change across treatment groups indicates that meat juiciness was not affected by the addition of RPFA to the maternal and/or lambs’ diet. Shear force is an indicator of meat tenderness and can be impacted by many factors, such as age, breed and muscle type(Reference Zhao, Li and Su18,Reference Abdullah and Qudsieh42) . In addition, higher fat diets are associated with lower shear force values.(Reference Sãnudo, Alfonso and Sánchez43). In the present study, the lack of difference in shear forces among treatment groups suggests that meat tenderness was not affected by RPFA supplementation. Usually, cooking loss of meat shows a positive correlation with fatness(Reference Kemp, Shelley and Ely44). However, fat can also act as a protective layer to avoid evaporation loss(Reference Tichenor45). As the GR value represents the fat content of the carcass(Reference Gaunt, Ferrier and Tatham17), the decreased GR value observed in lambs that derived from M16F ewes and received RPFA supplementation suggests that RPFA added to both the maternal and lambs’ diets can decrease the deposition of fat in carcasses. It is well known that colour and tenderness are some of the most important characteristics of meat that greatly affect consumer decisions; typically higher values of a* (>9·5) and L* (>34) of fresh lamb meat are preferred(Reference Khliji, De Ven and Lamb46). In the present study, the L* value for each group was greater than 34 and increased with RPFA added to the maternal diet, which implies that RPFA supplementation of the maternal diet increased meat lightness and its value at the consumer market. The a* value was greater than 9·5 for lambs from M16F ewes that also received RPFA supplementation. Literature data indicate that vitamin E improves meat colour by increasing antioxidant capacity, FA deficiency reduces serum α-tocopherol concentration(Reference Huang, Hsu and Lin47) which is the most common and biologically active form of vitamin E and elevated levels of vitamin E in the muscle systems increased redness of the meat(Reference Ponnampalam, Warner and Kitessa48). On the other hand, supplementary FA could improve mitochondrial activity, which stimulated the formation of deoxymyoglobin (instead of oxymyoglobin), prevented its conversion to metmyoglobin to reduce the L* and a* values of the meat(Reference Bjelanović, Grabež and Vučić49).
Conclusion
Supplementary RPFA in the maternal or lambs’ diets could elevate PSW, carcass and meat weight, dressing and meat percentage, as well as meat quality by promoting the morphological development of the gastrointestinal tract and also, the distribution of fat in the body. We found that the suitable dose of RPFA is 16 mg/kg DM in the maternal diet and 4 mg/kg DM in the lambs’ diets.
Acknowledgements
The authors thank the staff of the sheep unit of China Agriculture University for the care of the animals. All authors read and approved the manuscript.
This work was supported by a grant from the Ministry of Science and Technology of the People’s Republic of China (2018YFD0500402) and Agriculture Research System of China (CARS-38).
H.-Q. L. and H.-L. L. designed the experiment. H.-Q. L., B. W., Z. L., C. Z., L.-Y. J., Y.-F. G., W. L. and X.-G., Z. conducted the experiment and collected the data. H.-Q. L. analysed the data and wrote the manuscript. B. W. and H.-L. L. edited the manuscript.
The authors declare that no conflicts of interest exist.









