Methyl donor deficiency (MDD) leads to the accumulation of the toxic amino acid homocysteine (Hcy), which is associated with neural tube defects, intra-uterine growth retardation in the progeny( Reference Obeid and Herrmann 1 ) and neurodegenerative diseases in adulthood( Reference Tchantchou 2 ). The accumulation of Hcy can affect neurogenesis and synaptic plasticity during brain development( Reference Daval, Blaise and Guéant 3 ). Reduced neuroprogenitor proliferation and increased apoptosis occur in the fetal brain of folate-deficient rodents during late gestation( Reference Craciunescu, Brown and Mar 4 ). Indeed, Hcy is a metabolite of the essential amino acid methionine and can either be remethylated to methionine by enzymes requiring folate or vitamin B12 or be catabolised by cystathionine β-synthase to generate cysteine. In an animal model based on rats born to dams fed a MDD diet during gestation and lactation( Reference Blaise, Alberto and Nédélec 5 – Reference Blaise, Nédélec and Alberto 8 ), hallmarks of increased apoptosis were observed in selective brain structures of the 21-d-old progeny including the cerebellum and the pyramidal neurons of Cornu Ammonis (CA1) region as well as the main areas of persisting brain neurogenesis, i.e. the dentate gyrus in the hippocampus and the subventricular zone. The 21-d-old animals exhibited locomotor coordination impairment and cognitive defects, which mostly persisted in adulthood despite the restoration of normal diet feeding after weaning. Deficient pups also displayed growth retardation with dramatically reduced body weight( Reference Blaise, Alberto and Nédélec 5 , Reference Blaise, Nédélec and Schroeder 6 ) and visceral manifestations of fetal programming( Reference Garcia, Guéant-Rodriguez and Pooya 9 , Reference Pooya, Blaise and Moreno Garcia 10 ). We had also reported that some of these deleterious effects on growth could be linked to the MDD consecutive remodelling of gastric cellular organisation leading to ghrelin dysfunction( Reference Bossenmeyer-Pourié, Blaise and Pourié 11 ). This hormone plays a significant role in growth through its dual role as a growth hormone-releasing factor and as an orexigenic peptide( Reference Kojima and Kangawa 12 ).
Apoptosis in selective brain structures could also be involved in abnormal feeding behaviour and explain the dramatic growth retardation of MDD pups during the suckling period. Indeed, feeding disorders could be related to impaired olfactory discrimination, which plays a central role during the breast-feeding period. Early olfactory experience is critical for survival, and olfactory bulbs (OB) play a critical role during the postnatal period by allowing the pups to identify maternal odour( Reference Leon 13 , Reference Lledo and Saghatelyan 14 ). Newborn rats rapidly learn to identify maternal odours following the binding of noradrenaline to β-adrenoreceptors in the OB resulting from tactile stimulation( Reference Sullivan and Wilson 15 ). OB are defined as postnatal neurogenesis areas because subventricular zone neuronal progenitors migrate to these areas via the rostral migratory stream where they differentiate into bulbar interneurons. In rodents, the increased neurogenesis occurring in OB during pregnancy and the lactation period is essential for maternal care and offspring recognition( Reference Shingo, Gregg and Enwere 16 ).
Neurogenesis is closely related to neurosteroidogenesis. Besides their classical association with the neuroendocrine regulation of reproduction and sexual behaviour, most of the so-called neurosteroids originating from local brain synthesis are involved in developmental and adult cerebral plasticity( Reference Mellon 17 ). The synthesis of neurosteroids involves the recruitment of the rate-limiting step StAR protein (steroidogenic acute regulatory protein), which transfers cholesterol into the mitochondrial inner membrane to reach the cholesterol side-chain cleavage enzyme (CYP11A1) to transform it into pregnenolone( Reference Stocco 18 , Reference Midzak, Rone and Aghazadeh 19 ). StAR is widely distributed in the adult brain, especially in the neurogenic areas including OB( Reference Furukawa, Miyatake and Ohnishi 20 , Reference Lavaque, Sierra and Azcoitia 21 ). The androgen-to-oestrogen-converting enzyme cytochrome P450 aromatase (CYP19) represents the key enzyme of neurosteroidogenesis( Reference Garcia-Segura 22 ). The resulting oestradiol can modulate neurogenesis in the subventricular zone( Reference Brock, Keller and Veyrac 23 ) by allowing the recruitment of newborn cells in OB. This bears out the influence of oestradiol in olfactory discrimination.
In this context, in the present study, we investigated whether gestational MDD could affect olfactory performance through increased apoptosis and lower rates of neurogenesis in OB. For this purpose, 21-d-old rats born to dams fed a MDD diet and those born to dams fed a normal diet during the gestation and lactation periods were subjected to behavioural tests evaluating olfaction. Later, for the first time, in association with the classical determinants of Hcy metabolism, the expression levels of aromatase and the cholesterol-transfer protein StAR as well as oestrogen receptor (ER)α and the consecutive oestradiol content were monitored in the OB of the MDD pups and compared with those of the control pups.
Materials and methods
Animals
Experiments were carried out in Wistar rats (Janvier) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult female rats were maintained under standard laboratory conditions, under a 12 h light–12 h dark cycle, with access to food and water ad libitum. The rats were fed either a standard diet (Maintenance diet M20; Scientific Animal Food and Engineering) or a folate and choline-deficient diet 1 month before pregnancy, as described previously( Reference Blaise, Nédélec and Schroeder 6 , Reference Blaise, Alberto and Audonnet-Blaise 7 ) (Special Diet Service). The assigned diets were fed until weaning of the offspring at postnatal day 21.
Behavioural evaluation of olfaction
To monitor the acquisition of olfactory recognition in pups of dams fed the MDD diet (n 27) or the normal diet (n 24), their ability to return to their home cage successfully by means of environmentally sensitive information was recorded in a T-maze between postnatal days 5 and 19( Reference Heth, Nevo and Todrank 24 ). The animal model of gestational MDD is standardised and is widely used in different research projects. The differences between the number of weighed animals and that finally included in the behavioural tests are explained by the fact that a few of the animals were used for other protocols in the laboratory.
At each time point of postnatal development (see the Results section), each pup was subjected to a one-trial test consisting in behaving freely in a T-maze area (two arms of 30 cm length, walls of 10 cm height and a corridor of 5 cm width). The home cage (without the mother rat) of each pup was positioned at the end of one arm and a clean cage of the same size was positioned at the end of the other arm. These two cages were randomly placed between the right and left sides of the maze, and the corridors were carefully washed after each experiment. The test was considered successful when a pup returned directly to its home cage without visiting the arm containing the clean cage or without returning to the starting arm. The maximum time allowed for a pup to return to its home cage was 2 min. The time taken by the pups to reach the home cage was recorded. If a pup did not find its home cage within the allocated time, the maximum time (2 min) was affected.
Discrimination abilities at the time of weaning were tested through a place-preference test carried out in 21-d-old MDD (n 20) and control (n 24) pups. In accordance with previous studies( Reference Jacquot, Pourié and Buron 25 , Reference Buron, Hacquemand and Pourié 26 ), avoidance response to the irritant odour of toluene was evaluated in a corridor maze (80 cm in length, 8 cm in breadth and 15 cm in height). Both ends of the corridor contained a watch glass with a filter paper soaked in 15 μl of either pure toluene or distilled water (used as control). Toluene odour and water were randomly distributed in the right and left sides of the maze during each test. The odorant compartments were closed with a grid to avoid contact and covered to avoid the dispersion of odour. Each pup was placed in the middle of the corridor, facing a wall, and then allowed to move freely for 5 min. The movements of the pups were video-recorded and analysed with a video tracking system for automation of the behavioural experiments (View Point). Data on the total duration spent by each pup in each part of the corridor divided into three equal zones, defined as the repulsive toluene zone, the neutral central zone and the control water zone, were collected.
Tissue collection
Rat pups were weighed and killed at postnatal day 21 with an overdose of isoflurane. Whole brains were rapidly harvested. For immunohistochemical analyses, brains were immediately frozen in methylbutane previously chilled to − 30°C and stored at − 80°C. For biochemical analysis, brains were rapidly collected. The OB were dissected before freezing in liquid N2 and stored at − 80°C.
Isolation of mitochondrial and microsomal fractions
The mitochondrial and microsomal fractions of OB were isolated according to the following protocol. The samples were homogenised (1 g of tissue/3 ml buffer) in the isolation medium (0·32 m-sucrose, 10 mm-Tris–HCl and 1 mm-EDTA, pH 7·4). The homogenates were centrifuged at 2000 g for 3 min at 4°C (TLX ultracentrifuge OptimaTM, Beckman), and the pellet containing nuclei and cell debris was discarded. Centrifugation of the supernatant at 12 500 g for 10 min at 4°C resulted in a new supernatant, which was retained for the isolation of microsomes. The pellet was resuspended in the isolation medium and centrifuged at 11 000 g at 4°C for 10 min. The mitochondrial fraction was pelleted by additional centrifugation at 10 000 g for 10 min and suspended in radioimmunoprecipitation assay (RIPA) buffer (136 mm-NaCl, 1·7 mm-KH2PO4, 10 mm-Na2HPO4, 1 % Tergitol (v/v), 0·5 % deoxycholate (w/v), 0·1 % SDS (w/v), 100 mm-Na3VO4, 57 mm -phenylmethylsulfonyl fluoride and 0·14 % protease inhibitor) such that the mitochondrial fraction extracted from 0·1 g of brain tissue was suspended in 0·5 ml of RIPA buffer.
For the isolation of microsomal fractions, the supernatant collected previously was centrifuged at 15 000 g for 20 min at 4°C; the pellet was discarded and the supernatant was centrifuged again at 105 000 g for 90 min at 4°C. The microsomal pellet was resuspended in the storage buffer (80 mm-K2HPO4, 19 mm-KH2PO4, 0·2 mm-EDTA and 20 % glycerol (v/v), pH 7·4) such that 1 ml of suspension contained a microsomal fraction extracted from 3 g of tissue. The protein concentration of the subcellular fractions was assessed using a bicinchoninic acid protein determination kit (Interchim) with bovine serum albumin as a standard.
Measurement of vitamin B12, folate, S-adenosylmethionine and S-adenosylhomocysteine concentrations
The concentrations of vitamin B12 and folate were measured using a radioisotope dilution assay (simulTRAC-SNB; ICN Pharmaceuticals) as described previously( Reference Chéry, Barbé and Lequere 27 ). The concentrations of the classical determinants of Hcy metabolism in tissue homogenates were measured using HPLC adapted from Luippold et al. ( Reference Luippold, Delabar and Kloor 28 ). S-Adenosylmethionine (SAM) is demethylated to form S-adenosylhomocysteine (SAH) and Hcy is generated from the hydrolysis of SAH. Proteins were precipitated with 0·2 m-HClO4 before injection into the column (Lichrospher, 100 RP-C18, 5 μm, 250 × 4 mm inner diameter; Interchim). The mobile phase, consisting of 50 mm-sodium phosphate (pH 3·2), 10 mm-heptane sulfonate and acetonitrile (10–20 % from 0 to 20 min), was applied at a flow rate of 0·75 ml/min. The concentrations of SAM and SAH were quantified using a UV detector set at 254 nm.
DNA methylation
Global DNA methylation was quantified in the tissular extracts of OB obtained from male (n 5) and female (n 5) pups of the MDD diet-fed dams and pups of the normal diet-fed dams (n 5, respectively) using the Methylamp™ Global DNA Methylation Quantification Kit (Epigentek, Euromedex), as described previously( Reference Kerek, Geoffroy and Bison 29 ). Briefly, the methylated fraction of DNA is recognised by a 5-methylcytosine antibody and quantified through an ELISA-like reaction. The amount of methylated DNA is proportional to the intensity of absorbance.
Immunohistochemical analyses
Immunohistochemical analyses were carried out on cryostat-generated 20 μm sagittal brain sections mounted onto glass slides. For immunostaining, tissue sections previously fixed in 4 % paraformaldehyde (Sigma) were kept for 15 min at room temperature and were incubated for 10 min in PBS solution containing 0·1 % of Triton X-100. After washing the tissue sections three times with PBS for 5 min, non-specific binding was blocked with bovine serum albumin (10 %, w/v) dissolved in PBS and incubation was carried out for 1 h. The tissue sections were incubated for 72 h at 4°C with polyclonal rabbit antibodies against Hcy (1:200 dilution; Millipore), StAR (1:200 dilution; kindly provided by Professor D. Stocco, Texas Tech University), aromatase (1:200 dilution; BioVision Research Products) and oestradiol (1:200 dilution; US Biological) or a polyclonal mouse antibody against ERα (1:200 dilution; Santa Cruz Biotechnology). We used the monoclonal rabbit cleaved anti-caspase 3 antibody (1:200 dilution; Cell Signaling Technology) for the specific detection of apoptosis. The expression of the Ki-67 protein specifically associated with cell proliferation was analysed in OB sections using a polyclonal rabbit antibody against Ki67 (1:200 dilution; Abcam).
The tissue sections were then incubated for 1 h at room temperature in the presence of a secondary antibody (donkey anti-rabbit or donkey anti-mouse IgG conjugated to Alexa Fluor, 1:2000; Molecular Probes).
Cell nuclei were stained with the fluorescent dye 4,6-diamidino-2-phenylindole (5 μg/ml in distilled water). The tissue sections were then washed three times with PBS, coverslipped using Fluoromount mounting medium (Sigma) and kept in the dark until fluorescence analysis. The tissue sections were observed with a fluorescence microscope (Olympus) at × 20 magnification. The pictures were digitised with a monochromatic camera and analysed using the cellF software (Olympus).
Western blot analyses
The dissected cerebral regions were solubilised in RIPA lysis buffer containing 140 mm-NaCl, 0·5 % (w/v) sodium deoxycholate, 1 % (v/v) Nonidet P-40, 0·1 % (w/v) SDS and protease inhibitors (Roche Applied Science), lysed by three cycles of freezing/thawing and finally centrifuged at 4°C for 30 min at 15 000 g . The concentrations of proteins in the supernatant were determined using the BCA Protein Assay Kit (Interchim). Samples were mixed with an equal volume of 2 × Laemmli buffer and denatured by heating for 5 min at 100°C.
Mitochondrial fractions (30 μg of protein) for StAR detection, microsomal fractions (30 μg of protein) for aromatase detection and tissular extracts (50 μg of protein) for ERα detection were run on SDS–PAGE (10–12 %; Bio-Rad Mini Protean 3; Bio-Rad Laboratories, Inc.)( Reference Laemmli 30 ) and transferred onto polyvinylidene difluoride membranes (Immobilon-PS2, Millipore Corporation) in Tris–glycine transfer buffer with 20 % ethanol in a miniblotter (Bio-Rad Laboratories, Inc.). After being blocked in 5 % non-fat dry milk in Tris-buffered saline solution (200 mm-Tris and 1·5 m-NaCl, pH 7·4) and 0·1 % Tween 20 for 1 h under shaking, the membranes were incubated with various antibodies at 4°C overnight. The antibodies against StAR (1:1000 dilution), aromatase (1:1000 dilution) and ERα (1:200 dilution) were used for immunoblotting.
A horseradish peroxidase-labelled donkey anti-rabbit IgG against StAR and aromatase or anti-mouse IgG against ERα (1:2000 dilution; Santa Cruz Biotechnology) were used as secondary antibodies (incubation for 1 h at room temperature). Immunoreactivity was detected using a chemiluminescence kit (ECL Plus, Amersham Biosciences) and a chemiluminescence detector (Fusion FX7, Thermo Fisher Scientific). To normalise the total amount of protein per lane, the membranes were stripped and incubated with a monoclonal goat antibody against β-actin for StAR (1:1000 dilution; Santa Cruz Biotechnology) or a monoclonal chicken antibody against GAPDH for aromatase and ERα (1:1000 dilution; Millipore). Peroxidase-labelled donkey anti-goat IgG (1:2000 dilution; Santa Cruz Biotechnology) or peroxide-labelled donkey anti-chicken IgG (1:2000 dilution; Santa Cruz Biotechnology) were used as secondary antibodies. Densitometric analysis of the intensity of the Western blot bands was carried out using the Image J1.44p software (Freeware; nih.gov).
Quantitative real-time RT-PCR analysis
After grinding rat brain tissues in liquid N2, total RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen) following the manufacturer's instructions. RNA content was quantified by spectrophotometry at 260 nm (Microdrop, multiskan GO, X7, Thermo Fisher Scientific) and analysed by 2 % agarose gel electrophoresis. The first complementary DNA strand was reverse-transcribed from total RNA (1 μg) using the QuantiTect Reverse Transcription Kit (Qiagen) following the manufacturer's instructions.
Real-time PCR was carried out on the Lightcycler 2.0 Instrument (Roche Diagnostics) using the QuantiTect SYBR Green PCR Kit (Qiagen). Specific amplification of mRNA coding for StAR, P450 aromatase and ERα was carried out with specific pairs of primers. For StAR mRNA, 5′-AGGAAAGCCAGCAGGAGAAT-3′ and 5′-CTGTCCATGGGCTGGTCTA-3′ were used as the forward and reverse primers, respectively. For aromatase mRNA, 5′-TTGATTTTCGCTGAGAGACG-3′ and 5′-ACAGAGTGACGGACATGGTG-3′ were used as the forward and reverse primers, respectively. For ERα mRNA, 5′-ATGTGTCCAGCTACAAACCAA-3′ and 5′-CGTATCCCGCCTTTCATC-3′ were used as the forward and reverse primers, respectively. The internal control, a 90 bp product of polymerase II, was amplified using the forward primer 5′-GCATTAACATCAGGAACAATAAAGGC-3′ and the reverse primer 5′-GATCTCTCTAAAGTTGACCTCATTGG-3′. Temperature cycling consisted of one cycle to activate Taq DNA polymerase (95°C; 15 min), followed by forty-three cycles, each consisting of denaturation (95°C; 10 s), annealing (53°C for StAR and 56°C for both P450 aromatase and ERα; 15 s), and extension (72°C; 10 s) steps. Melting curve analyses were carried out by raising the temperature from 65 to 95°C. Cycle threshold (C t) was determined for each sample, and real-time PCR amplification efficiencies were obtained by calculating the ratio of crossing points of amplification curves, using the RelQuant software (Roche Diagnostics).
Statistical analysis
Data were prospectively collected and analysed using the SAS software (SAS Institute, Inc.). Raw data reported as means and standard deviations were compared using one-way ANOVA with Fisher's test. A P value < 0·05 was considered to indicate statistical significance.
Results
Effect of the methyl-donor-deficient diet on the body weight of newborn rats
During weaning, the body weight of female and male pups born to the MDD diet-fed dams was lower than that of pups born to the normal diet-fed dams (Table 1). The difference in weight between pups of the normal diet fed-dams and those of the MDD diet-fed dams was significantly higher (P< 0·01) in the females (56·4 %) than in the males (44·3 %). A smaller size of the MDD pups was also recorded, as reported previously( Reference Blaise, Alberto and Nédélec 5 ).
Table 1 Body weight (g) at weaning of 21-d-old rat pups born to dams fed a methyl donor-deficient (MDD) or a normal diet (Mean values and standard deviations, n 20 pups per group)
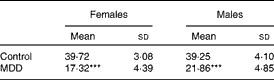
***Mean values were significantly different from the control rat pups for each sex (P< 0·0001, ANOVA).
Olfactory discrimination behavioural tests
The newborn rats were subjected to olfactory discrimination tests. Fig. 1(a) and (b) shows that the olfactory nest recognition ability of the deficient pups was affected irrespective of the sex. The acquisition of olfactory cues to return to their own nest was revealed by the shorter time taken to complete the test by both the control male and female pups between postnatal days 5 and 19 (from up to 100 s to less than 20 s). Despite a drop being achieved only by the end of the suckling period (postnatal day 19) in the deficient pups, the time necessary to complete the test remained higher than that in the control pups, starting at postnatal day 9 for the females and postnatal day 14 for the males.

Fig. 1 Behavioural evaluation of olfaction between postnatal days 5 and 19. Time required for (a) female pups and (b) male pups to reach the home cage at postnatal days 5 (D5), 7 (D7), 9 (D9), 14 (D14) and 19 (D19). CM (□), control males (n 12); DM (■), deficient males (n 10); CF (□), control females (n 12); DF (■), deficient females (n 17). Values are means, with standard deviations represented by vertical bars. Mean values were significantly different from the control pups: * P< 0·05, ** P< 0·01, *** P< 0·001 (ANOVA). Time spent in each zone of the corridor by (c) female pups and (d) male pups. ■, Toluene zone (tol); ![]() , water zone (water); □, central zone (central). ANOVA summary: for CF, n 12, F(2,24) = 21·2, and ***P< 0·0001 for tol v. central and tol v. water; for DF, n 8, F(2,21) = 0·199, and P>0·5438 for tol v. central and tol v. water; for CM, n 12, F(2,45) = 104·0, and ***P< 0·0001 for tol v. central and tol v. water; for DM, n 12, F(2,24) = 22·4, and ***P< 0·0001 for tol v. central and tol v. water.
, water zone (water); □, central zone (central). ANOVA summary: for CF, n 12, F(2,24) = 21·2, and ***P< 0·0001 for tol v. central and tol v. water; for DF, n 8, F(2,21) = 0·199, and P>0·5438 for tol v. central and tol v. water; for CM, n 12, F(2,45) = 104·0, and ***P< 0·0001 for tol v. central and tol v. water; for DM, n 12, F(2,24) = 22·4, and ***P< 0·0001 for tol v. central and tol v. water.
Fig. 1(c) and (d) also shows that the discrimination ability for a repulsive odorant, i.e. toluene, was significantly affected in the deficient female pups than in the control pups. Such a difference was not recorded in the male pups. The control female pups spent only 10 % of their time near the toluene source compared with the 90 % that they spent away from it (F(2,24) = 21·2; P< 0·0001). By contrast, female pups born to the MDD diet-fed dams spent up to 28 % of their time, evenly distributed among the three zones of the corridor, in the toluene zone (F(2,21) = 0·199; P>0·5438). By contrast, no sizeable differences were recorded in the male pups, since both the control and deficient pups succeeded in the discrimination test by spending only 5 and 2 % of their time in the toluene zone, respectively (F(2,45) = 104·0; P< 0·0001 and F(2,24) = 22·4; P< 0·0001, respectively).
S-Adenosylmethionine:S-adenosylhomocysteine ratio and vitamin concentrations in olfactory bulbs
The male and female pups born to the MDD diet-fed dams exhibited significantly lower concentrations of folate (Fig. 2(a)) and vitamin B12 (Fig. 2(b)) in the OB. The measurements of the determinants of Hcy metabolism indicated that, irrespective of the sex, there was no substantial variation in the concentrations of SAM (Fig. 2(c)). Yet, the concentrations of SAH were significantly higher in the OB of the MDD female pups than in those of the control female pups (Fig. 2(d)). The SAM:SAH ratio was consequently lower in the OB of the MDD female pups (Fig. 2(e)). Furthermore, global DNA methylation was reduced by 19·34 % in the OB of the deficient pups compared with the levels in the control female pups. No variation was observed between the control and MDD male pups.
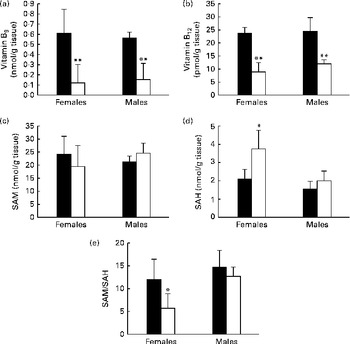
Fig. 2 Concentrations of (a) vitamin B9, (b) vitamin B12, (c) S-adenosylmethionine (SAM), and (d) S-adenosylhomocysteine (SAH) and (e) SAM:SAH ratio in olfactory bulbs of 21-d-old rat pups. Values are means, with standard deviations represented by vertical bars, n 5 per group. Mean values were significantly different from the control pups: * P< 0·05, ** P< 0·01 (ANOVA). ■, Control; □, methyl donor-deficient.
Homocysteine accumulation, apoptosis and cell proliferation in the olfactory bulbs of young rats
Hcy was detected in the glomerular cell layer of OB (Fig. 3(a)) and more specifically in the basal and apical dendrites. A strong accumulation of Hcy was observed in the OB of female pups born to the MDD diet-fed dams than in those of pups born to the normal diet-fed dams (Fig. 3(b)). This was not observed in the brain sections of the male pups (data not shown). The labelling of OB cells with a specific indicator of apoptosis, the antibody against cleaved caspase 3, revealed a low immunoreactivity in the glomerular cells of 21-d-old control female pups. It was dramatically higher in the OB sections of the deficient female pups (Fig. 3(c)). Cell proliferation was evaluated using the anti-Ki-67 antibody. As expected, the immunoreactivity of Ki-67 was nearly undetectable in the glomerular cell layer of OB of female pups born to the MDD diet-fed dams when compared with that in the pups born to the normal diet-fed dams (Fig. 3(d)).

Fig. 3 Immunostaining of homocysteine (Hcy), cleaved caspase 3 and Ki67 in the olfactory bulbs (OB) of 21-d-old female pups. (a) Image of the whole OB representing the glomerular cell layer. (b) Immunostaining of Hcy (green), (c) cleaved caspase 3 (green) and (d) Ki67 in the sagittal sections of OB. The cell nuclei of glomerular cells were stained with the fluorescent dye 4,6-diamidino-2-phenylindole (DAPI, blue). While the immunostaining of cleaved caspase 3 was more elevated, reflecting increased apoptosis in the OB of the methyl-donor-deficient female pups than in those of the control pups, labelling of Ki67 was almost undetectable, suggesting decreased cell proliferation. n 5 per group. Calibration bars = 200 μm. Original magnification × 20.
Expression of steroidogenic acute regulatory protein, aromatase, and oestrogen receptor α and immunostaining of oestradiol
Immunohistochemical staining with anti-StAR (Fig. 4(a)), anti-aromatase (Fig. 4(b)) and anti-ERα (Fig. 4(c)) antibodies revealed the most intense immunoreactivity in the basal and apical dendrites of the glomerular cell layer of OB in 21-d-old pups. The immunoreactivity of the three proteins was weaker in the OB of the deficient female pups. As a consequence, the immunoreactivity of oestradiol was lower in the glomerular cells of 21-d-old female pups of the MDD diet-fed dams (Fig. 4(d)).

Fig. 4 Immunostaining of steroidogenic acute regulatory protein (StAR), aromatase, oestrogen receptor α (ERα and oestradiol in the olfactory bulbs (OB) of 21-d-old female pups. (a) Immunostaining of StAR (red), (b) aromatase (green), (c) ERα (green) and (d) oestradiol (green) in the sagittal sections of OB. The cell nuclei of glomerular cells were stained with the fluorescent dye 4,6-diamidino-2-phenylindole (DAPI, blue). Immunoreactivity of the three proteins and oestradiol was lower in the OB of the methyl-donor-deficient female pups than in those of the control pups. n 5 per group. Calibration bars = 200 μm. Original magnification × 20.
Changes in the expression levels of StAR, aromatase and ERα were confirmed by Western blot analyses. A single band was observed for StAR in the mitochondrial fractions isolated from the OB, as illustrated in Fig. 5(a). Densitometric analysis indicated a substantial decrease in the levels of both mitochondrial StAR protein (Fig. 5(a)) and ERα (Fig. 5(c)) in female pups of the MDD diet-fed dams, which reached 60 % in the case of StAR, when compared the levels in pups of the normal diet-fed dams. A slighter but non-significant decline was observed in the control and deficient male pups. The expression of aromatase protein measured in the microsomal fractions of OB of the deficient female pups was also significantly lower. No difference was found between the MDD and control male pups (Fig. 5(b)).

Fig. 5 Effect of the methyl donor-deficient diet on the expression of steroidogenic acute regulatory protein (StAR), aromatase and oestrogen receptor α (ERα proteins in the olfactory bulbs (OB) of 21-d-old pups. (a) Representative set of Western blot showing immunodetectable StAR protein. From left to right, the lanes contain mitochondria from the OB (30 μg protein) of the 21-d-old control (C, ■) and deficient (D, □) female and male pups. Expression of StAR (30 kDa) was quantified and normalised against that of β-actin (43 kDa). (b) Representative set of Western blot showing immunodetectable P450 aromatase protein. The lanes contain microsomes isolated from the OB (30 μg protein) of the 21-d-old C and D pups. Expression of P450 aromatase (55 kDa) was quantified and normalised against that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (38 kDa). (c) Representative set of Western blot showing immunodetectable ERα protein. The lanes contain proteins from the OB (50 μg protein) of the 21-d-old C and D pups. Expression of ERα (66 kDa) was quantified and normalised against that of GAPDH. Densitometric data were obtained from five separate experiments (n 5 per group). Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different from the control pups (P< 0·05; ANOVA).
To examine the consequences of MDD on gene expression, the mRNA levels of StAR, aromatase and ERα were quantified in the OB of 21-d-old rat pups by quantitative RT-PCR analysis. There was no significant difference in the levels of the three proteins in the deficient male pups when compared with the levels in the control pups (Fig. 6). The mRNA level of StAR varied in the same manner as protein contents, and the mRNA expression of StAR measured in 21-d-old female pups born to the MDD diet-fed dams was significantly lower than that in female pups born to the normal diet-fed dams (Fig. 6(a)). Similarly, the mRNA expression of aromatase (Fig. 6(b)) and ERα (Fig. 6(c)) in the OB of the deficient female pups was lower.
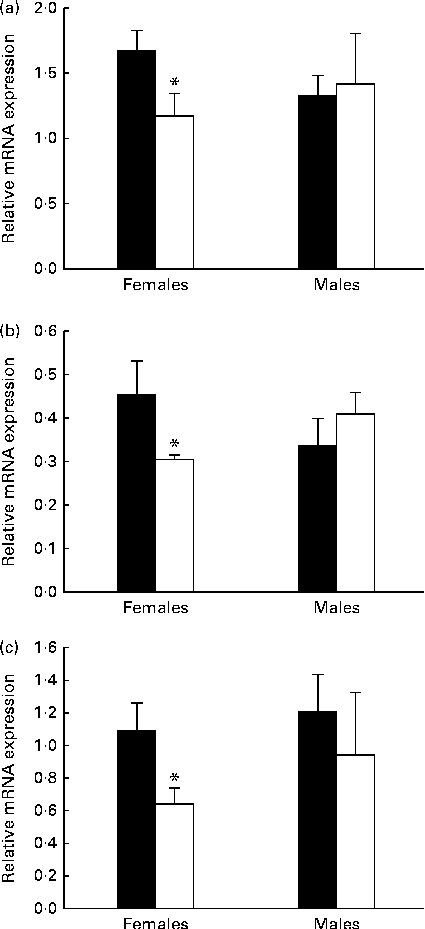
Fig. 6 Results of the quantitative RT-PCR analysis of the effect of the methyl donor-deficient diet on the expression of (a) steroidogenic acute regulatory protein (StAR), (b) aromatase and (c) oestrogen receptor α (ERα mRNA in the olfactory bulbs of 21-d-old female rats. Arbitrary unit refers to an internal standard in the olfactory bulbs of the control (■) and deficient (□) pups (n 5 per group, run in duplicate). Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different from the control pups (P< 0·05; ANOVA).
Discussion
Pups born to the MDD diet-fed dams exhibited lower body weight and impaired olfactory discrimination when compared with those born to the normal diet-fed dams. The exposure to the MDD diet during gestation and lactation was associated with significantly lower concentrations of vitamins B9 and B12 in the OB of 21-d-old pups. Vitamin B12 acts as a cofactor of the methionine synthase enzyme, which is involved in the synthesis of methionine, the precursor of SAM, which is the universal methyl donor. SAM is transmethylated into SAH and Hcy originates from SAH hydrolysis. Thus, it is easily understandable that a lack of nutritional intake of B vitamins will affect Hcy metabolism( Reference Blaise, Alberto and Nédélec 5 ). The concentrations of the classical determinants of Hcy metabolism have been reported previously for whole-brain homogenates, and enhanced concentration of total SAH associated with unchanged SAM concentrations have been measured( Reference Blaise, Nédélec and Schroeder 6 ). In the present study, we demonstrated for the first time that the concentrations of SAH were significantly higher in the OB of only female rats, with a consecutively reduced index of methylation capacity represented by the SAM:SAH ratio. This is in accordance with the lower amounts of methylated DNA quantified in the OB of the MDD female pups.
Hcy is a potent neurotoxic and pro-apoptotic compound, and its accumulation in neuronal cells has been reported to be associated with neurodegenerative diseases and with cognitive defects in the elderly( Reference Tchantchou 2 ). An association of cerebral accumulation of Hcy with functional defects as well as the hallmarks of apoptosis in hippocampal neurogenic areas has been reported previously( Reference Blaise, Nédélec and Schroeder 6 , Reference Blaise, Alberto and Audonnet-Blaise 7 ). Sustained Hcy immunoreactivity, concomitantly with both an increasing number of cells undergoing apoptosis and an impairment of cell proliferation in the glomerular cell layer of OB of the deficient pups, suggests that MDD also affects the OB of newborn rats.
We have recently hypothesised that MDD occurring in early life could be associated with altered synaptic plasticity and neurogenesis( Reference Daval, Blaise and Guéant 3 ). The postnatal period is crucial as neurogenesis is achieved around the third postnatal week in rats( Reference Kim, Park and Roh 31 ), which corresponds to the age of the pups assessed in the present study. During this period, high concentrations of ER are present in OB, whereas ERα transcripts are undetectable in adulthood( Reference Guo, Su and Sun 32 ). Moreover, the early postnatal period is characterised by a strong effect of oestrogens on neurogenesis and survival of OB neurons, whereas in adulthood oestrogens control both the survival of neurons and their functional integration( Reference Veyrac and Bakker 33 ). In the present study, we demonstrated that MDD impaired the critical steps of the neurosteroidogenic pathway, specifically only in female rats, by decreasing both the protein and transcription levels of StAR, aromatase and ERα in the OB. The reduced detection of oestradiol in the glomerular cell layer of OB appears to be a consequence of these changes and strengthens the hypothesis that the decrease in the expression levels of StAR, aromatase and ERα leads to poor neurosteroidogenesis in the OB of the deficient pups. These harmful effects of MDD on the synthesis of cerebral steroids are not surprising, since previous studies have reported an association between folate and steroid metabolism. In this respect, preconception folate deficiency affects fertility and follicular response to ovarian stimulation in association with depleted levels of oestradiol. According to the authors, this could result from the impaired methylation of aromatase DNA( Reference Twigt, Hammiche and Sinclair 34 ).
The behavioural evaluation of olfactory performance strongly suggests that the impaired neurosteroidogenesis in OB consecutively to gestational MDD could have functional consequences. If olfactory nest recognition ability is affected irrespective of the sex during the third week after birth, the 21-d-old deficient female pups would specifically exhibit a dramatically reduced odour discrimination performance. The T-maze test evaluates home-odour recognition and is based on both the olfactory and locomotor abilities of pups. Considering that both the male and female deficient pups displayed body-weight loss, it can be hypothesised that the mean time to complete the test would be lower in the MDD pups than in the control pups. Thus, we carried out the place-preference test, which is specifically based on olfactory functions. Indeed, a lack of clear discrimination is specific in pathophysiological damage affecting the neuro-olfactory tissues( Reference Buron, Hacquemand and Pourié 35 , Reference Zou, Pan and Wang 36 ). The learning of odour identification is essential not only during the postnatal period but also in adulthood, since clinical studies have reported less olfactory sensitivity( Reference Atanasova, Graux and El Hage 37 ) and fewer OB volumes in depressive patients( Reference Negoias, Croy and Gerber 38 ).
In addition to the greater weight difference between the MDD and control pups recorded in females than in the males, the patent functional and tissular consequences of MDD appeared to be sex dependent. The effects of oestradiol depend on various factors, especially strain and sex( Reference Bakker, Honda and Harada 39 , Reference Barker and Galea 40 ). A sexual dimorphic effect of oestrogens on the development of dendritic spines in cultured OB with significant effects of oestradiol treatment on primary cultures derived from brains of female rats has been reported( Reference Wu, Moriya-Ito and Iwakura 41 ). Interestingly, a previous study has revealed that neonatal repeated handling results in a loss of maternal odour preference, specifically in female rat pups than in control pups( Reference Raineki, De Souza and Szawka 42 ). These dimorphic variations seem to be associated with steroid-dependent molecular mechanisms that occur via the nuclear receptors and co-activators of steroids, and most of them are present at high levels in the OB of rats according to a sex-specific localisation, so as to regulate brain development and behaviour( Reference Bian, Zhang and Guo 43 ).
In conclusion, the present results provide the first evidence of the sex-dependent deleterious effects of perinatal MDD on olfactory discrimination performance associated with impaired neurosteroidogenesis, since the decreased expression levels of key proteins result in an apparent reduced content of oestradiol in the OB of 21-d-old female rat pups. This may contribute to their growth retardation and body-weight loss through impaired suckling. It has been shown that an early folate privation has not only developmental effects, but also life-long effects( Reference Hussain 44 ). Whether it is associated with neuropsychological disorders in adulthood requires further investigation.
Acknowledgements
The authors thank Professor D. Stocco (Texas Tech University, USA) for providing antibodies against StAR protein.
The present study received institutional grants from the French National Agency for Research (ANR Nutrivigene project) and the Region of Lorraine (France). ANR Nutrivigene and the Region of Lorraine had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: S. E. H. C, G. P., J.-L. D., J.-L. G. and B. L.-M. conceived and designed the experiments; S. E. H. C., G. P., N. M. and J.-M. A. carried out the experiments; S. E. H. C., G. P., J.-L. D., J.-L. G. and B. L.-M. analysed the data; S. E. H. C., G. P., J.-L. D., J.-L. G. and B. L.-M. wrote the article.
None of the authors has any conflicts of interest.









