Morbidity and mortality in developed countries have been shown to follow a socio-economic gradient, with higher rates of chronic disease observed among those of a lower socio-economic position (SEP)( Reference Stringhini, Sabia and Shipley 1 ). Diet, along with smoking, alcohol consumption and physical inactivity, is an important risk factor for many chronic diseases( 2 ) and a large number of dietary components have been shown to be socio-economically patterned( Reference Darmon and Drewnowski 3 – Reference Worsley, Blasche and Ball 5 ). Individuals of a higher SEP are more likely to consume foods associated with good health, such as nutrient-dense foods including whole grains, lean meats, fish, low-fat dairy products, nuts, fresh fruit and vegetables( Reference Darmon and Drewnowski 3 ). Conversely, individuals of a lower SEP are more likely to consume foods associated with higher disease risk such as energy-dense nutrient-poor foods including refined grains, fatty meats, cakes, added fats, full-fat dairy products and potatoes( Reference Darmon and Drewnowski 3 ). The majority of research describing the socio-economic patterning of diet has generally focused on investigating individual components of the diet, such as macronutrients, micronutrients and whole foods( Reference Stringhini, Sabia and Shipley 1 – Reference Darmon and Drewnowski 3 ). However, nutrients are not eaten in isolation, their intake may have synergistic effects and accurate measurement is difficult( Reference Hu 6 ). For this reason, measures of diet quality are being increasingly utilised to provide a broad insight into the effects of overall diet on health outcomes( Reference Russell, Flood and Rochtchina 7 ).
The term ‘diet quality’ is broadly used and poorly defined in the academic literature( Reference Alkerwi 8 ). We refer to diet quality herein as pertaining to the adherence to healthy eating guidelines. Indeed, the small number of studies that have analysed the relationship between markers of SEP and a diet quality index in adults commonly conceptualised diet quality as meeting national dietary guidelines due to the direct link with current dietary public health practice and policy( Reference Mullie, Clarys and Hulens 9 – Reference Collins, Young and Hodge 13 ). These studies come from Australia, Belgium, Denmark and the USA and have explored various markers of SEP including income, education and area-level socio-economic disadvantage. In general, these studies suggested that having a higher SEP is associated with higher diet quality( Reference Darmon and Drewnowski 3 ). However, the studies have reported variable findings for different age and sex groups and for different markers of SEP.
A better understanding of the relationship between SEP and diet quality may help explain some of the socio-economic inequalities in health. Therefore, the aim of the present study was to investigate and compare the association between three measures of SEP (income, education and area-level socio-economic disadvantage) and diet quality, using a diet quality index based on national dietary guidelines (Dietary Guideline Index (DGI)), in the Australian adult population. A secondary aim was to explore possible effect modification of this relationship by sex and by age.
Methods
Data source
Data from the baseline survey of the Australian Diabetes, Obesity and Lifestyle (AusDiab) study were used to analyse the relationship between each marker of SEP and diet quality. AusDiab is a national, population-based survey of 11 247 individuals aged ≥25 years at baseline (1999–2000). Participants were selected from forty-two randomly selected census collector districts from each of the six states and the Northern Territory. A household interview was conducted to collect information on sociodemographic details, health behaviours and dietary intake. Physical and biomedical examinations were conducted to collect anthropometric measures, blood pressure and blood samples. Household questionnaires were completed in 67 % of the households (n 11 479) that could be contacted and contained at least one eligible person. The response rate to the baseline biomedical testing among those who completed the household survey was 55 % (giving an overall response rate of 37 %). The study was approved by the ethics committee of the International Diabetes Institute. Detailed descriptions of the sampling and methodology used are published elsewhere( Reference Dunstan, Zimmet and Welborn 14 ). For the current analysis, we excluded participants with missing information on physical activity (n 86), smoking status (n 150), total energy intake (n 164) or with FFQ-reported energy intake outside plausible ranges according to established criteria (>16 800 kJ/d and <3360 kJ/d (>4015 kcal/d and <803 cal/d) for men and >14 700 kJ/d and <2100 kJ/d (>3513 kcal/d and <502 kcal/d) for women; n 1185)( Reference Black 15 ), DGI score (n 33), alcohol intake (n 1) or BMI (n 113). We additionally excluded participants with missing information on each SEP indicator of interest, income (n 139), education (n 2) and Socio-Economic Index for Areas (SEIFA; n 78). This resulted in a final sample size of 9296 participants for analyses.
Variables
Education
Education level was ascertained by asking the question ‘Which of these describes the highest qualification you have received?’ and categorised into the following four categories: primary school/never attended school, some secondary school completed, completed secondary school, and university/technical and further education (tertiary).
Income
Income was ascertained through the question ‘Which number best describes your total household income before tax?’ In order to adjust for the number of family members within a household, total household income was recorded and weekly individual income was then determined by using a modified version of the Organisation for Economic Co-operation and Development’s equivalence scale( Reference De Vos and Zaidi 16 ). For participants not living in a family unit, individual income was recorded. Income was categorised into quartiles derived from the data and expressed in Australian dollars: ≤$AU 230, $AU 230–465, $AU 465–700 and ≥$AU 701 per week.
Socio-economic index for areas
SEIFA is a score that ranks areas in Australia according to relative socio-economic advantage and disadvantage. It is derived by the Australian Bureau of Statistics using twenty variables collected in the census relating to education, income, employment, family composition, housing benefits, car ownership, ethnicity, English language proficiency and residential overcrowding( Reference Trewin 17 ). SEIFA was divided into quartiles ranging from the least disadvantaged (quartile 1) to the most disadvantaged (quartile 4).
Dietary intake
Dietary data were collected via a self-administered FFQ, which was developed and validated by the Cancer Council of Victoria( Reference Hodge, Patterson and Brown 18 ). The questionnaire included seventy-four food frequency questions covering intake of food groups during the previous 12 months. Each item had a choice of ten frequency categories: ‘never’, ‘less than once per month’, ‘1–3 times per month’, ‘once per week’, ‘twice per week’, ‘3–4 times per week’, ‘5–6 times per week’, ‘once per day’, ‘twice per day’ or ‘three or more times per day’. The frequency questions covered foods such as fruits, vegetables, cereals, dairy, meat, fish, snack foods and alcohol intake. Additionally, the questionnaire ascertained the usual type of milk, bread, spread and cheese consumed. The FFQ also contained questions and photographs regarding portion size, which were used in the calculation of intakes.
Diet quality score
Diet quality was measured using the DGI (as a continuous variable), which has been described in detail previously( Reference McNaughton, Ball and Crawford 12 ). Briefly, the DGI was developed to reflect adherence to the Dietary Guidelines for Australian Adults( 19 ). Food groups and cut-offs were guided by recommendations in the Australian Guide to Healthy Eating, which provides age- and sex-specific recommendations for the consumption of five core food groups (cereals, meats and alternative, fruits, vegetables and dairy) and ‘extra foods’. Because appropriate measures of salt use or fluid intake were not available in the AusDiab FFQ, the original DGI was adapted for use in the present study and reduced from the original fifteen components to thirteen components( Reference McNaughton, Dunstan and Ball 20 ).
The thirteen components included dietary indicators of vegetables and legumes, fruit, total cereals, meat and alternatives, total dairy, saturated fat, alcoholic beverages, added sugars and ‘extra foods’ and diet quality measures relating to wholegrain cereals, lean protein, reduced-/low-fat dairy and diet variety. The dietary indicators were based on the age- and sex-specific dietary guidelines, cut-off points and food groupings guided by the Australian Guide to Healthy Eating recommendations for the consumption of five core food groups (fruits, vegetables, cereals, dairy, meat and alternatives) as well as ‘extra foods’( Reference Smith, Kellett and Schmerlaib 21 ).
According to the Australian Guide to Healthy Eating, ‘extra foods’ are defined as foods that are not essential to provide nutrient requirements and contain too much fat, sugar and salt. This includes foods such as confectionery, chocolate, cakes, muffins, pies, pastries, puddings, ice cream, cream, biscuits, jams, mayonnaise and dressings, chips, meat pies, hamburgers, soft drinks, cordials, fruit juices and all alcoholic beverages. Each component of the DGI was scored between 0 and 10, where a score of 10 indicated that a participant met the recommendation. For example, if a participant reported eating 2 servings of fruit/d (recommended amount) he/she received 10 points for this component. A report of 1 serving/d would score 5 points and zero fruit consumption would score 0 points. The thirteen items were then summed for a total score, with a potential range of 0–130. Higher scores indicated a greater adherence to the dietary guidelines. A summary of the components of the DGI and criteria for minimum and maximum scores can be obtained elsewhere( Reference McNaughton, Ball and Crawford 12 , Reference McNaughton, Dunstan and Ball 20 ). Whole-grain cereal consumption was based only on the consumption of whole-grain and wholemeal bread, as other cereal items on the FFQ did not distinguish whole-grain varieties. Dietary variety was determined based on the proportion of foods for each core food group that were consumed at least once per week.
Demographic and other lifestyle information
Data on covariates such as age, sex, smoking status, country of birth and leisure-time physical activity were collected by self-report. Age was used on a continuous scale. Smoking status was categorised into current smoker, ex-smoker and never smoker. Country of birth was categorised into Australia/New Zealand, UK and Northern Ireland, and rest of world. Leisure-time physical activity was categorised as sedentary (0 min of physical activity time per week), insufficient (>0 and <150 min of physical activity time per week) and sufficient (≥150 min of physical activity time per week) based on self-reported frequency and duration of physical activity during the previous week, using the Active Australia Survey Questionnaire( 22 ). Total leisure-time physical activity time for the previous week was calculated as the sum of the time spent walking (if continuous and for 10 min or more) or engaging in moderate physical activity plus double the time spent during vigorous physical activity( Reference Armstrong, Bauman and Davies 23 ). At the time of data collection Australian public health guidelines recommended at least 150 min of physical activity per week for health benefits( 24 ).
Statistical analysis
Descriptive statistics were used to compare baseline characteristics across strata of SEP and are presented as means with 95 % confidence intervals, or proportions. Linear regression analyses were used to estimate the association between each indicator of SEP and DGI in the total sample and stratified by sex and by age (where age was dichotomised into ≤55 years and >55 years, close to the median age of 51 years). For each analysis two models were constructed, with model 1 adjusted for age and sex and model 2 adjusted for age, sex and country of birth. Beta coefficients from these models were used to calculate the relative difference in DGI score through comparison with the mean DGI score of each reference group (highest SEP group). These are reported for model 2 only. We additionally evaluated the relationship between SEP and DGI for each SEP indicator by treating the SEP variable as a continuous variable in regression models. A P value for a linear trend of <0·05 was considered significant. All statistical analyses were conducted using the statistical software package STATA version 10·1.
Results
The study sample was 45 % male, had a mean age 51 (sd 14·2) years and a mean DGI of 84 (sd 14·3). Table 1 describes the characteristics of the sample across markers of SEP. Individuals of a lower SEP were more likely to be women, older in age, current smokers and born in countries other than Australia, and less likely to engage in leisure-time physical activity. There was no clear pattern for mean DGI across levels of education and income. However, DGI appeared to improve with decreasing SEIFA quartiles.
Table 1 Baseline characteristics of the variables of interest across the categories for each measure of socio-economic position (un-weighted); adults (n 9296; age ≥25 years) from the Australian Diabetes, Obesity and Lifestyle Study
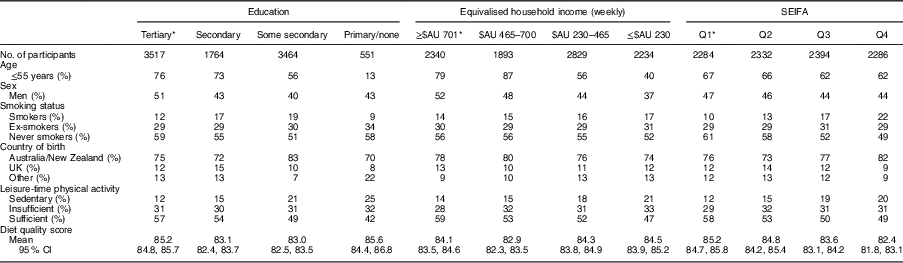
SEIFA, Socio-Economic Index for Areas; Q, quartile.
* Denotes group of least socio-economic disadvantage.
Results from all regression analyses were similar for model 1 (adjusted for age and sex) and model 2 (adjusted for age, sex and country of birth). For this reason we only present the results from model 2 (see Tables 2 and 3). For the total sample, higher levels of educational attainment, income and SEIFA were significantly associated with higher DGI. Those in the lowest education category had a mean (95 % CI) DGI score that was –4·7 (–6·0, –3·4) points lower than those in the highest SEP category (DGI score of 85·2 (84·8, 85·7)). The lowest income category had a DGI score that was –2·6 (–3·5, –1·8) points lower than those in the highest income category (DGI score of 84·1 (83·5, 84·6)) and those in the lowest SEIFA category had a DGI score that was –3·0 (–3·8, –2·2) points lower than those in the highest SEIFA category (DGI score of 85·2 (84·6, 85·8)). Relative inequality in DGI in the total sample ranged between 3 % and 6 % and was largest when education was used to indicate SEP. A significant P value for linear trend was observed for all SEP indicators, indicating a higher DGI across increasing levels of SEP. Interaction tests for age and for sex were not significant, although there was a suggestion of an interaction between sex and education (P=0·1; data not shown) and sex and income (P=0·1; data not shown). As we may have been underpowered to detect such interactions, we examined both sex-specific and age-specific analyses separately.
Table 2 The association between each measure of socio-economic position (SEP) and diet quality score in the total sample and stratified by sex; adults (n 9296; age ≥25 years) from the Australian Diabetes, Obesity and Lifestyle Study

SEIFA, Socio-Economic Index for Areas; Q, quartile; Ref., reference category; DGI, Dietary Guideline Index.
* Adjusted for age, sex, country of birth.
† Relative inequality: for the reference SEP category the mean DGI score is reported. For all other SEP categories we report the proportionate difference in diet quality score relative to the reference group.
‡ Adjusted for age and country of birth.
Table 3 The association between each measure of socio-economic position (SEP) and diet quality score, stratified by age; adults (n 9296; age ≥25 years) from the Australian Diabetes, Obesity and Lifestyle Study

SEIFA, Socio-Economic Index for Areas; Q, quartile; Ref., reference category; DGI, Dietary Guideline Index.
* Adjusted for age, sex, country of birth.
† Relative inequality: for the reference SEP category the mean DGI score is reported. For all other SEP categories we report the proportionate difference in diet quality score relative to the reference group.
In sex-specific analyses (Table 2), higher levels of education, income and SEIFA appeared to be associated with higher DGI for both men and women. Relative inequalities in DGI were slightly higher across SEIFA categories for men compared with women. Among men, those who had completed some secondary school had a lower DGI score than those who only completed primary school or never went to school. Nevertheless, a significant P value for linear trend, indicating a higher DGI across increasing level of SEP, was detected for each indicator of SEP for both men and women (P<0·01).
In age-specific analyses (Table 3), higher levels of education, income and SEIFA were again associated with a higher DGI score for both age groups. Relative inequalities were greater among those aged ≤55 years compared with those aged >55 years for each SEP indicator. Across all three SEP indicators the magnitude of difference in DGI between the highest and lowest SEP group was greater for those aged ≤55 years than for those aged >55 years. Among those aged ≤55 years, those who had completed some secondary school had a worse DGI score than those who only completed primary school or never went to school. This relationship was not seen among those aged >55 years. Conversely, among those aged >55 years, those of the second highest income quintile ($AU 465–700) had a lower DGI score than those of the second lowest quintile ($AU 230–465). Nevertheless, a significant P value for linear trend, indicating a higher DGI across increasing levels of SEP, was detected for each indicator of SEP for both age groups (P<0·05).
Discussion
The present study describes the association between multiple measures of SEP (income, education and SEIFA) and diet quality in a cohort of Australian adults using the DGI( Reference McNaughton, Ball and Crawford 12 ), a diet quality index that reflects the Australian dietary guidelines( 24 ). In the total sample, a clear and graded association between all indicators of SEP and DGI was demonstrated, in which a higher level of educational attainment and income and a lower level of area-level disadvantage were associated with higher diet quality.
The majority of the observed relationships were positively graded across each of the four categories of the SEP indicator. The few instances in which DGI did not increase with each increasing level of SEP may reflect the sensitivity of the SEP marker to discriminate differences in DGI scores. Socio-economic differentials in health are known to attenuate with age( Reference Martelin 25 ) so it is not surprising that we see variations in our subgroup aged over 55 years. This age group includes employed and retired individuals, and may render income a less accurate depiction of individual-level SEP in this subgroup. Furthermore, variation in educational attainment tends to be less in older age groups and the implications of different educational levels on health are likely to differ according to birth cohort( Reference Martelin 25 ). Area-level indicators of SEP also tend to be less sensitive than individual markers of SEP( Reference Galobardes, Shaw and Lawlor 26 ). Another reason for inconsistent results may arise as a result of random chance, due to the multiple testing.
The magnitude of association between indicators of SEP and DGI did not vary considerably by sex, but relative inequalities in DGI were slightly stronger among men using SEIFA and among women using education to measure SEP. In contrast, the association between indicators of SEP and DGI were consistently stronger among those aged 55 years or less compared with their older counterparts, possibly indicating that SEP has a greater influence over diet quality for younger men and women. This was particularly the case for education, and may reflect changes in educational attainment levels over time along with a weakening of the importance of education as a marker of disadvantage as people age. To our knowledge, while the relationship between age and diet quality has been previously examined( Reference Malon, Deschamps and Salanave 27 ), the modifying role of age on the relationship between SEP and diet quality is a novel contribution to the literature.
The graded relationships that we observe between SEP and our diet quality score in the total sample are congruent with previous studies, that have been conducted in various populations, with a range of different diet quality indices( Reference Mullie, Clarys and Hulens 9 – Reference McNaughton, Ball and Crawford 12 ).
The sex differences observed in the literature appear to be mixed. Consistent with our observations, Le et al. ( Reference Le, Dallongeville and Wagner 28 ) reported that higher educated adults complied more closely with French national dietary guidelines than lower educated adults and that this relationship appeared to be similar for men and women. Conversely, in an earlier study Malon et al. ( Reference Malon, Deschamps and Salanave 27 ) found that adherence to French national guidelines was not significantly associated with education, but was significantly associated with economic level. Dynesen et al.( Reference Dynesen, Haraldsdottir and Holm 11 ) observed a significant association between level of education and diet quality for men, but not women (using a modified version of the Healthy Eating Index (HEI)). In that study, although the diet quality index represented Danish dietary guidelines, it was based only on intakes of fruit, vegetables, fish and type of spread used on bread and did not take into account intakes of other types of meat, dairy products, breads, cereals, pasta, rice and potatoes. In the present study, our diet quality index encompassed a large variety of food items and may provide a more comprehensive measure of diet quality, which may strengthen the observed association with SEP. In a smaller sample of 491 American women from the 1991–1994 survey of the Market Research Corporation of America Information Services, a significant association between level of education and diet quality was demonstrated using a modified version of the HEI( Reference Loughrey, Basiotis and Zizza 10 ). The relationship between income and diet quality was also investigated; however, unlike the positive association observed in our study, no significant association was observed. The discrepancies may arise from lack of regression analyses used in the American study and therefore the inability to adjust for potential confounding factors.
In the Australian context, only one other study has quantified the association between SEP (income and SEIFA) and diet quality. In a study of 8220 Australian men and women using data from the 1995 Australian National Nutrition Survey, McNaughton et al. ( Reference McNaughton, Ball and Crawford 12 ) found a significant positive association between income and diet quality for men and women using the DGI. An association between lower SEIFA (lower level of socio-economic disadvantage) and a higher diet quality score was also detected for women, but not men. In contrast, we observed a strong association between lower SEIFA and higher DGI among both men and women.
The accumulated evidence suggests a higher SEP, as indicated by both individual- and area-level markers, is associated with a higher diet quality, for both men and women. Our results, combined with others, suggest that age is an important modifier of this relationship, particularly with regard to the use of education as an indicator of SEP.
Studies have shown that factors such as lack of nutrition knowledge, inequitable access to healthy foods and different social norms are likely to explain some of the observed associations between SEP and diet quality( Reference Wardle, Parmenter and Waller 29 – Reference Ball, Crawford and Mishra 31 ). Furthermore, some of the observed associations in terms of level of income and diet quality may be explained by food costs, where people with lower levels of income may be more likely to buy less expensive foods, which tend to be less healthy( Reference Drewnowski and Darmon 32 , Reference Turrell and Kavanagh 33 ). Health beliefs, weight control and nutrition knowledge may also explain the observed sex differences in diet quality( Reference Oakes and Slotterback 34 – Reference Baker and Wardle 36 ). To our knowledge, no study to date has examined the moderating role of age on the association between SEP and diet quality. In view of the different relationships observed in the present study between SEP and diet quality at younger and older ages, age stratification should be implemented in future research. Such stratification is likely to account for changes in both diet quality and the sensitivity of SEP indicators to discriminate differences in DGI scores over the life course.
Strengths of our study include the use of a large national population-based study with a diet quality index intended for use in the Australian population. Rather than focusing on single nutrients, the DGI takes into account whole foods, types of foods and dietary variety, which has the advantage of representing cumulative effects of a large number and range of nutrients( Reference Hu 6 ). Use of diet quality indices more generally involves comparing dietary intakes with existing guidelines, principles or criteria to generate scores( Reference Hu 6 ). As diet quality indices can be based on local guidelines they are useful to assess compliance with, and effectiveness of, dietary recommendations, and may be easier to compare scores across studies( Reference Michels and Schulze 37 , Reference McNaughton 38 ). The majority of previous studies that have investigated the association between SEP and dietary intake have used methods other than diet quality indices as their measures of food intake and have analysed children rather than adults( Reference Giskes, Lenthe and Brug 39 – Reference Lazarou and Newby 44 ).
The present study also has several limitations. The dietary information used to calculate the DGI was obtained via a self-administered FFQ. While FFQ are a valid and widely used method to obtain dietary information( Reference Hodge, Patterson and Brown 18 ), participants may have under- or over-reported their intake of certain foods( Reference Cade, Thompson and Burley 45 ). Further, the FFQ we used did not include questions on sugar-sweetened beverages, which may have led to an underestimation of the differences in DGI across SEP groups in our study, due to the previously observed negative association between SEP and sugar-sweetened beverage intake( Reference Darmon and Drewnowski 3 , Reference Clifton, Chan and Moss 46 ). Additionally, the DGI in itself has limitations, as it does not define upper limits for serving frequencies for some of the dietary components, which is important when considering foods such as meat and dairy that have a U-shaped association with health( Reference Waijers, Feskens and Ocke 47 ). However, this approach to scoring is consistent across diet quality scores in the literature( Reference Waijers, Feskens and Ocke 47 ) and the DGI is considered to be an improvement on previous food-based scores because it does include indicators of excess consumption. Consequently, while use of other diet quality scores may have led to small differences in results, it is unlikely that different conclusions would have been reached. Supporting this, Waijers et al. found that the predictive capacity of several diet quality scores was comparable( Reference Waijers, Feskens and Ocke 47 ). Further, the DGI is subject to the same limitations as other indicators of diet quality. The development of diet quality scores is commonly linked to national dietary guidelines, which rely on varying grades of evidence for what actually constitutes a healthy diet. Moreover, many arbitrary choices are included in the development of diet quality scores and they may fail to recognise the different interrelationships between dietary components( Reference Arvaniti and Panagiotakos 48 ). However, while individuals with similar diet quality scores may have quite different contributing components, this is what makes diet quality scores particularly useful: they are able to identify a poor diet due to a variety of reasons, rather than on the basis of single dietary components. Additionally, recent evidence suggests that an emphasis on diet quality, rather than individual nutrients and energy, may be more effective for the long-term prevention of obesity and non-communicable diseases( Reference Ludwig and Friedman 49 , Reference Mozaffarian 50 ). Finally, the AusDiab study had a modest response rate, which may give rise to participation bias as those from lower SEP groups are commonly under-represented in epidemiological surveys( Reference Ekholm, Gundgaard and Rasmussen 51 ). This under-representation of lower SEP groups may result in a more homogenous low SEP population in our sample and thus lead to an underestimation in the magnitude of difference in DGI scores across SEP groups.
The present study has implications for nutrition promotion interventions. A consistent and significant socio-economic gradient in DGI scores was observed across all markers of SEP in the total sample, for men and women and particularly for people aged 55 years or less. The magnitude of difference ranged between 2 and 5 DGI units and is likely to be associated with observable differences in health risk between SEP groups. McNaughton et al.( Reference McNaughton, Dunstan and Ball 20 ) previously demonstrated significant relationships between a 10-unit increase in the DGI score and a range of cardiometabolic risk factors, for both men and women. While McNaughton et al. did not examine this relationship using smaller units for the DGI score, their results are highly significant (P<0·0001). Future research should explicitly examine the mediating role of diet quality in the relationship between SEP and a range of morbidity outcomes. Such analysis would determine the relevance of diet quality in the policy context of reducing socio-economic inequalities in health.
In the present study we observed a socio-economic gradient in DGI scores, rather than simply a gap between the most and least disadvantaged. For this reason dietary interventions should aim to improve overall diet quality across the whole of society, with a scale and intensity that are proportionate to the level of socio-economic disadvantage (a concept known as proportionate universalism( Reference Marmot, Goldblatt and Boyce 52 )). This will require whole-of-population approaches to improve diet quality in addition to targeting the most disadvantaged. It will be essential that interventions, particularly where the reach and effectiveness are at least equally effective across all socio-economic strata, be prioritised and implemented. Where a nutrition intervention is effective, but to a greater degree for those with a higher SEP, it will be important that complementary strategies are employed to ensure that lower socio-economic groups also benefit in our attempts to improve population diet quality. Given the known tracking of health behaviours from childhood through to adulthood, it will also be important to support interventions that have the potential to improve diet quality across the life course, such as mandated nutrition policies in childhood and workplace settings. We have recently demonstrated that obesity prevention interventions reliant primarily on information delivery are more likely to be more effective in those with higher SEP than those interventions that change aspects of the structural environment( Reference Beauchamp, Backholer and Magliano 53 ). It follows that prioritising nutrition interventions that target the nutrition environment, such as banning the marketing of energy-dense nutrient-poor foods to children, and improving the availability and affordability of healthy foods, have the potential to improve diet quality in an equitable manner. It is essential that interventions and policies are continually evaluated for their health equity impact, so that those most likely to reduce the socio-economic gradient can be prioritised. Improving diet quality and reducing its associated socio-economic gradient are likely to lead to reduced inequalities in other health outcomes.
In conclusion, the present study determined that a higher level of SEP, as measured by educational attainment, level of income or area-level disadvantage, is associated with higher levels of diet quality in Australian adults. Healthy eating initiatives need to address overall diet quality and to act both across the population as a whole and with a proportionate focus on those with the greatest level of socio-economic disadvantage.
Acknowledgements
Acknowledgements: The authors are most grateful to the following for their support of the study: the Commonwealth Department of Health and Aged Care; Abbott Australasia Pty Ltd; Alphapharm Pty Ltd; AstraZeneca; Aventis Pharmaceutical; Bristol-Myers Squibb Pharmaceuticals; Eli Lilly (Aust) Pty Ltd; GlaxoSmithKline; Janssen-Cilag (Aust) Pty Ltd; Merck Lipha s.a.; Merck Sharp & Dohme (Aust); Novartis Pharmaceutical (Aust) Pty Ltd; Novo Nordisk Pharmaceutical Pty Ltd; Pharmacia and Upjohn Pty Ltd; Pfizer Pty Ltd; Roche Diagnostics; Sanofi Synthelabo (Aust) Pty Ltd; Servier Laboratories (Aust) Pty Ltd; BioRad Laboratories Pty Ltd; HITECH Pathology Pty Ltd; the Australian Kidney Foundation; Diabetes Australia; Diabetes Australia (Northern Territory); Queensland Health; South Australian Department of Human Services; Tasmanian Department of Health and Human Services; Territory Health Services; Victorian Department of Human Services; Victorian OIS Programme; and Health Department of Western Australia. Also, for their invaluable contribution to the set-up and field activities of AusDiab, the authors are enormously grateful to A. Allman, B. Atkins, S. Bennett, S. Chadban, S. Colagiuri, M. de Courten, M. Dalton, M. D’Embden, T. Dwyer, D. Jolley, I. Kemp, P. Magnus, J. Mathews, D. McCarty, A. Meehan, K. O’Dea, P. Phillips, P. Popplewell, C. Reid, A. Stewart, R. Tapp, H. Taylor, T. Welborn, F. Wilson and P. Zimmet. Financial support: This work was funded by an Australian Research Council (ARC) Linkage grant (number LP120100418) and in part by the Victorian Government’s Operational Infrastructure Support (OIS) Programme. K.B. is supported by the ARC Linkage grant (number LP120100418) and an Australian National Preventive Health Agency grant (number 188PEE2011). A.P. is supported by a National Health and Medical Research Council Career Development Fellowship (grant number 1045456). S.A.M. is supported by an Australian Research Council Future Fellowship (grant number FT100100581). J.E.S. is supported by a National Health and Medical Research Council Senior Research Fellowship (grant number 586623). No funding bodies had any role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Conflict of interest: The authors declared that no competing interests exist. Authorship: K.B. and E.S. contributed equally to the study. A.P. contributed to formulation of the research question, study design, analysis, manuscript preparation and finalisation, and takes responsibility for the final analyses. K.B. contributed to formulation of the research question, study design, analysis, manuscript preparation and finalisation. E.S. contributed to study design, analysis, manuscript preparation and finalisation. E.G., D.J.M., S.A.M. and J.E.S. contributed to data acquisition, data analysis, manuscript preparation and finalisation. Ethics of human subject participation: Ethics approval for the current analysis was obtained from Alfred Hospital Ethics Committee (Alfred ethics project number 55/12).






