The intestinal epithelium is a single layer of columnar epithelial cells that has two critical functions: acting as a barrier to harmful substances and as a selective filter to essential nutrients(Reference Blikslager, Moeser and Gookin1, Reference Kunzelmann and Mall2). It can synthesise and secrete many mucosal barrier factors, such as diamine oxidase (DAO), trefoil factor family (TFF) and transforming growth factor-α (TGF-α), which are considered as central factors to maintain and restore intestinal mucosal integrity(Reference Playford3–Reference Agostino, Argenio and Ciacci5). Intestinal tight junctions are the apical-most adhesive junctional complexes defining paracellular permeability of the intact intestinal epithelium, which consist of integral transmembrane proteins (occludin and claudin)(Reference Turner6). Changes in the intestinal barrier can affect the invasion of harmful substances and the absorption of nutrients. Intestinal barrier dysfunction is thought to result in many intestinal diseases, including diarrhoea, inflammatory bowel disease, ischaemic disease, food allergy and Crohn's disease(Reference Kucharzik, Walsh and Chen7, Reference Meddings, Jarand and Urbanski8).
Specific dietary nutrients have been shown to affect intestinal functioning, one of which is dietary fibre. Recent studies have shown that dietary fibre is a beneficial nutrient for preventing intestinal disease (diarrhoea, constipation and the irritable bowel syndrome) and improving intestinal health in human subjects and animals(Reference Ingvar9). However, due to resistance to digestion and absorption in the foregut, the effect of dietary fibre on intestinal health has been primarily attributed to a mutual effect on intestinal bacteria(Reference Iain10), especially in the hindgut. Previous studies have indicated that dietary fibres could selectively regulate intestinal bacteria, including the stimulation of beneficial bacterial species and the suppression of pathogenic bacterial species(Reference Gibson, Beatty and Wang11, Reference Zhong, Cai and Cai12).
Commensal bacteria have been shown to change intestinal barrier integrity. In vitro, occludin and cingulin gene mRNA levels of Caco-2 cells were up-regulated by Lactobacillus (Reference Anderson, Cookson and McNabb13). Escherichia coli could result in tight junction disruption through the destabilisation and dissociation of tight junction proteins from the epithelial cells(Reference Spitz, Yuhan and Koutsouris14, Reference Muza-Moons, Schneeberger and Hecht15). These results suggest that bacteria could affect intestinal barrier integrity by regulating the gene expression level of tight junction proteins. Meanwhile, dietary fibres (wheat bran, pea fibre, cellulose and mixed fibre) have been shown to increase the excretion of intestinal mucin by stimulating the capacity of mucosal protein synthesis in many species including human subjects(Reference Fuller, Cadenhead, Verstegen, Huisman and den Hartog16, Reference Lien, Sauer and He17). However, few studies have shown the effect of dietary fibre on intestinal mucosal barrier factors (DAO, TFF and TGF-α). Therefore, it is probable that dietary fibre improves gut mucosal barrier function, such as intestinal mucosal tight junctions and mucosal barrier factors, through supporting a better intestinal microbiota.
Because piglets often suffer major stress at weaning, accompanied by intestinal mucosal damage and diarrhoea in conventional pig production, we chose weaned piglets as the experimental animal. Generally, maize fibre (MF), wheat bran fibre (WBF), soyabean fibre (SF) and pea fibre (PF) are increasingly incorporated in human food and animal diets as dietary fibre sources. Previous studies have shown that these fibre sources possess different physico-chemical properties (solubility, fermentability) and components, which results in discordant intestinal health(Reference Hamberg, Rumessen and Gudmandhoyer18, Reference Titgemeyer, Bourquin and Fahey19). In order to further determine the mechanism by which dietary fibre affects intestinal health, we hypothesise that dietary fibre could change intestinal health by the regulation of intestinal mucosal tight junctions and barrier factors. Therefore, in the present study, complex fibre sources purified from cereals (maize and wheat) and legumes (soyabean and pea) were selected and added to weaning piglet diets. The study aimed to assess whether supplementation with dietary fibres affects intestinal mucosal tight junctions and barrier factors, and regulates intestinal bacterial populations.
Materials and methods
The experimental protocols used in the present study were approved by the Sichuan Agricultural University Institutional Animal Care and Use Committee.
Preparation of dietary fibres
MF, SF, WBF and PF were purified from maize, soyabean, wheat and pea, respectively, by Chinese Food Companies. MF was provided by Ci Yuan Biotech Company Limited, SF was from Winway Biotech Company Limited, WBF was from Shangyidao Science and Technology Company Limited and PF was from Jianyuan Food Company Limited. Crude protein, crude fibre, neutral-detergent fibre (NDF) and acid-detergent fibre (ADF), cellulose and hemicellulose contents of the fibre sources are summarised in Table 1. The contents of crude fibre, NDF and ADF were assayed in our laboratory by using the method of Van Soest(Reference Van Soest, Robertson and Lewis20). To avoid protein contamination, sodium sulphite was added to a NDF solution. Hemicellulose and cellulose were calculated as follows:
Table 1 Crude protein (CP), crude fibre (CF), neutral-detergent fibre (NDF), acid-detergent fibre (ADF), cellulose and hemicellulose contents of the fibre sources

Experimental design and animal management
The experiment followed a randomised block design. A total of 125 cross-bred (Duroc × Landrace × Yorkshire) piglets (weaned at 28 (sem 2) d) were blocked and assigned to one of five experimental diets based on their body weight and litters. Each treatment was replicated with five pens of five pigs per replicate pen. The five treatments included a control group and four dietary fibre groups. The temperature was maintained at 26°C for the first 3 d after weaning and then reduced by 2°C/week for the remainder of the experiment. Piglets consumed the diets and water ad libitum for 30 d. All pigs were checked daily for general health and diarrhoea during the experimental period. Pigs showing loose faeces/diarrhoea were recorded. The incidence of diarrhoea was calculated as follows:
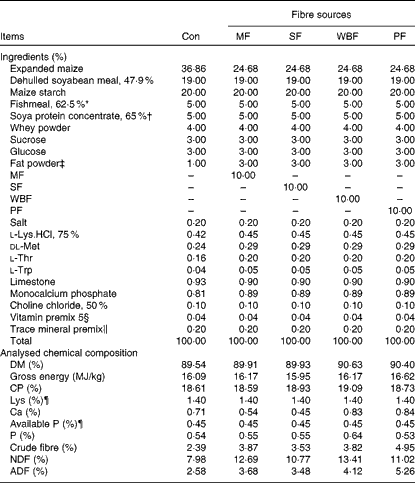
Piglets were fed maize–soyabean meal diets (Table 2) formulated to meet the nutrient recommendations of the National Research Council (1998). The five experimental diets included a control diet without a supplemental fibre source and four diets in which expanded maize was replaced by 10 % MF, 10 % SF, 10 % WBF or 10 % PF. To ensure similar energy levels in all the diets, expanded maize was reduced and 2 % fat powder was added in the fibre source diets.
Table 2 Composition of the experimental diets (as-fed basis)

Con, control; MF, maize fibre; SF, soyabean fibre; WBF, wheat bran fibre; PF, pea fibre; CP, crude protein; NDF, neutral-detergent fibre; ADF, acid-detergent fibre.
* Imported Peruvian red fishmeal.
† Produced by WILMAR & ADM J.V.
‡ Made of palm oils, produced by Shandong Tianjiao Biotech Company Limited.
§ Provided the following per kg diet: 4·05 mg vitamin A; 52·25 μg vitamin D3; 24 mg vitamin E; 3 mg vitamin K3; 3 mg vitamin B1; 6 mg vitamin B2; 3 mg vitamin B6; 24 μg vitamin B12; 15 mg pantothenic acid; 1·2 mg folic acid; 150 μg biotin; 1 g choline.
∥ Provided the following per kg diet: 110 mg Fe (as FeSO4.7H2O); 10 mg Cu (as CuSO4.5H2O); 110 mg Zn (as ZnSO4.7H2O); 6 mg Mn (as MnSO4.H2O); 0·3 mg I (as KI); 0·3 mg Se (as Na2SeO3).
¶ Calculated value.
Sample collection
After 30 d, five pigs per diet were anaesthetised by a lethal injection of sodium pentobarbital (200 mg/kg body weight), and the abdomen was immediately opened to remove the ileum and the colon, emptied and sampled. The middle section (2 cm) of the ileum and colon was collected and fixed in 10 % formaldehyde-phosphate buffer for intestinal histology analysis. Intestinal segments of the ileum and colon were collected and immediately frozen at − 80°C for quantitative real-time PCR. Mucosal scrapings from the ileum and colon were prepared and stored at − 80°C until ELISA analysis. Approximately 3 g of the digesta from the middle section of the ileum and colon were kept in sterile tubes and immediately frozen at − 80°C until analysis for microbial DNA and volatile fatty acid (VFA) concentrations.
Histological measurements
Measurements of villous height and crypt depth were conducted as described by Shen et al. (Reference Shen, Piao and Kim21). Fixed intestinal segments were packed with paraffin wax. Consecutive sections at 5 μm thickness were stained with haematoxylin–eosin for histomorphological examination. Intestinal mucosal morphology including villous height and crypt depth was measured at 40 × magnification with an Olympus CK 40 microscope (Olympus Optical Company). Positively stained goblet cells were counted in ten randomly selected mucous layers using Image-Pro Plus software, version 6.0 (Media Cybernetics).
Volatile fatty acid analysis
VFA concentrations in the intestinal digesta were determined using a gas chromatographic method described by Franklin et al. (Reference Franklin, Mathew and Vickers22). Digesta samples (1 g) were thawed and suspended in 2 ml of distilled water in a screw-capped tube. After being vortexed, the sample was centrifuged (12 000 g) at 4°C for 10 min. The supernatant (1 ml) was transferred into centrifuge tubes (2 ml) and mixed with 0·2 ml metaphosphoric acid. After 30 min at 4°C, the tubes were centrifuged (12 000 g) again at 4°C for 10 min. Aliquots of the supernatant (1 μl) were analysed using a Varian CP-3800 gas chromatograph (Agilent Technologies). A flame ionisation detector was used with an oven temperature of 100–150°C. The polyethylene glycol column was operated with highly purified N2, as the carrier gas, at 1·8 ml/min. The lower detectable limit for all VFA was 0·1 mmol/l.
ELISA analysis of diamine oxidase activities, transforming growth factor-α, trefoil factor family, MHC-II and secretory IgA concentration
Approximately 1 g of mucosal scrapings was homogenised by hand after being suspended in 9 ml PBS. After centrifugation at 700 g for 10 min, the supernatant was removed and centrifuged at 12 000 g for 15 min. Then, the supernatant was taken for the measurement of intestinal factors by an anti-swine ELSIA kit (BlueGene Biotech). The total protein content of the intestinal samples was measured by the Bradford brilliant blue method simultaneously. The concentration of intestinal factors was expressed as μg/mg or g protein(Reference Zhong, Cai and Cai12).
DNA isolation, design and validation of primers for Lactobacillus, Escherichia coli and Bifidobacterium
Bacterial DNA was extracted from the intestinal digesta using the Stool DNA Kit (Omega Bio-tek) according to the manufacturer's instructions.
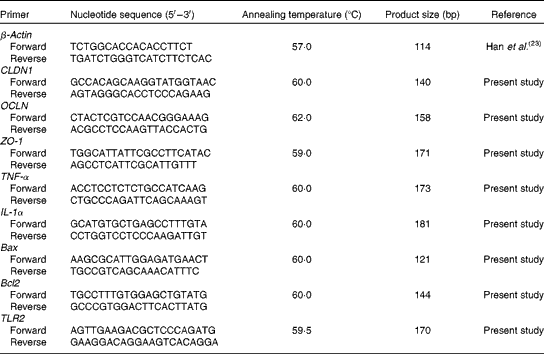
For quantitative detection of Lactobacillus, E. coli and Bifidobacterium, primers and fluorescent oligonucleotide probes (Table 3) were designed following 16S rRNA sequences of maximum species of each genus encountered in the pig intestinal tract downloaded from the GenBank database, EMBL and DDBJ. To avoid any non-specific amplification, the sequences of all the genera fetched from the database were submitted to DNAStar (MegAlign) program (DNASTAR, Inc.), as described by Han et al. (Reference Han, Xiang and Yu23). It was revealed that the Lactobacillus, E. coli and Bifidobacterium blocks of hypervariable regions comprised all other genera. These sequences were submitted to a second round of alignment where the maximum number of species belonging to one genus was aligned and the conservative regions were selected as genus-specific primers and probes of Lactobacillus, E. coli and Bifidobacterium. Furthermore, to ensure that the oligonucleotide sequences were complementary pairing with the target genus only, they were checked with the GenBank program BLAST (NCBI BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the Ribosomal Database Project (RDP) program Check-Probe (details on RDP data and analytical functions can be found at http://rdp.cme.msu.edu/). Primers (Table 3) for total bacteria were obtained from Lee et al. (Reference Lee, Lee and Shin24). All the primers and probes(Reference Qi, Xiang and Han25, Reference Xiang, Qi and Han26) were commercially synthesised by Life Technologies Limited.
Table 3 Sequences of primers and probes for intestinal bacteria

Microbial quantitative PCR conditions
Quantitative real-time PCR was performed by conventional PCR on the Opticon DNA Engine (Bio-Rad). Each reaction was run in a volume of 25 μl with 1 μl of forward and 1 μl of reverse primers (100 nm), 12·5 μl SYBR Premix Ex Taq (2 × concentrated), 1 μl template DNA and 9·5 μl of double-distilled water for detecting total bacteria. The PCR conditions were as follows: one cycle of pre-denaturation at 95°C for 30 s; forty cycles of denaturation at 95°C for 5 s; annealing at 60°C for 30 s and extension at 72°C for 60 s. The PrimerScriptTM PCR kit (Perfect Real Time; Takara) was used for Lactobacillus, E. coli and Bifidobacterium. The reaction protocol was composed of one cycle of pre-denaturation at 95°C for 2 min; fifty cycles of denaturation at 95°C for 15 s; annealing at 60°C for 30 s and extension at 72°C for 50 s. Each reaction was run in a volume of 20 μl with 8 μl RealMasterMix (2·5 × ), 1 μl of forward and 1 μl of reverse primers (100 nm), 1 μl probe enhancer solution (20 × ), 0·3 μl probe (100 nm), 1 μl DNA and 7·7 μl of double-distilled water in each reaction for detecting Lactobacillus, E. coli and Bifidobacterium.
Standard curve
For the quantification of bacteria in the test samples, specific standard curves were generated by constructing standard plasmids, as presented by Han et al. (Reference Han, Xiang and Yu23). The standard strains of Lactobacillus, E. coli and Bifidobacterium were cultured anaerobically or aerobically in the respective culture, including 1 % glucose at 37°C from 12 to 48 h. The specific PCR product of all bacteria was purified using the QIAQuick Gel Extraction Kit (Qiagen), and cloned into a pGEM-T Easy Vector (Promega). After verification of the sequence, the recombinant plasmid was isolated using the Endo-Free Plasmid Kit I (OMGA), and standard plasmids for all bacteria were constructed successfully. DNA concentration of the standard plasmids was measured using a spectrophotometer (Coulter DU 800; Beckman). The copies were calculated by the following formula: (6·0233 × 1023 copies/mol × DNA concentration (μg/μl))/(660 × 106× DNA size (bp)). A 10-fold serial dilution series of plasmid DNA was used to construct the standard curves for total bacteria, Lactobacillus, E. coli and Bifidobacterium. Each standard curve was constructed by a linear regression of the plotted points, and cycle threshold (CT) values were plotted against the logarithm of template copy numbers.
Real-time PCR for quantification of claudin 1, occludin, zonula occludens 1, TNF-α, IL-1α, B-cell lymphoma/leukaemia-2-associated X protein, B-cell lymphoma/leukaemia-2 and Toll-like receptor 2
Frozen intestinal tissue (0·1 g) was homogenised in 1 ml TRIzol reagent (Invitrogen) and total RNA was extracted following the manufacturer's instructions. The concentration and purity of RNA were analysed spectrophotometrically (Beckman Coulter DU800; Beckman Coulter Inc.), and the OD260:OD280 ratio (where OD is the optical density) ranged from 1·8 to 2·0 for all samples. The integrity of RNA was measured by formaldehyde gel electrophoresis and the 28S:18S ribosomal RNA band ratio was determined as ≥ 1·8. The RNA samples were reverse transcribed into complementary DNA using the PrimeScript™ RT reagent kit (Takara) according to the manufacturer's instructions. The primers were synthesised commercially by Life Technologies Limited, which are listed in Table 4. Real-time PCR for quantification of claudin 1, occludin (OCLN), zonula occludens 1 (ZO-1), TNF-α, IL-1α, B-cell lymphoma/leukaemia-2 (Bcl-2)-associated X protein, Bcl-2 and Toll-like receptor 2 (TLR2) were carried out on the Opticon DNA Engine (Bio-Rad) using SYBR Green PCR reagents (Takara). β-Actin was chosen as the reference gene transcript, and the relative expression ratio of the target gene in comparison with the reference gene was calculated as described previously(Reference Michael27). Each standard and sample was run simultaneously in duplicate on the same PCR plate, and the average of each duplicate value expressed as the number of copies was used for statistical analysis.
Table 4 Sequences of primers for the intestine-related genes

CLDN1, claudin 1; OCLN, occludin; ZO-1, zonula occludens 1; Bax, B-cell lymphoma/leukaemia-2-associated X protein; Bcl2, B-cell lymphoma/leukaemia-2; TLR2, Toll-like receptor 2.
Statistical analysis
The pen was considered as the experimental unit for all analyses. Bacterial copies were transformed (log10) before statistical analysis. Litters and body weight of piglets were used as blocking factors, and five blocks were included. All data were subjected to one-way ANOVA for a randomised block design using the GLM procedure of SAS 9.0 (SAS Institute Inc.). Statistical differences among the treatments were separated by Tukey's multiple-range tests. For significance determination, the α-level was set as 0·05. All data are presented as means with their pooled standard errors.
Results
Effects of fibre sources on diarrhoea incidence
There was no difference in diarrhoea incidence between the pigs fed the control diet (10·00 %) and the fibre source diets. However, lower diarrhoea incidence was observed in pigs fed the WBF (8·56 %) and PF diets (8·72 %) compared with those fed the MF (11·47 %) and SF diets (12·40 %).
Effects of the fibre sources on intestinal mucosal morphology
The results of intestinal mucosal morphology analysis are presented in Table 5. In the ileum, pigs on the WBF diet had a higher villous height than those fed the SF diet (P= 0·031). The ileal villous height:crypt depth ratio was higher in WBF diet-fed pigs than in those supplemented with the MF (P= 0·018) and SF diets (P= 0·023). In pigs fed the WBF and PF diets, the number of mucosal goblet cells increased in the colon compared with pigs fed the control (P< 0·001 and < 0·001, respectively), MF (P= 0·001 and 0·008, respectively) and SF diets (P< 0·001 and P= 0·005, respectively). However, a difference in ileal goblet cells was only observed in pigs fed the WBF diet when compared with those on the control (P= 0·023), MF (P= 0·025) and SF diets (P= 0·032).
Table 5 Effects of the dietary fibre sources on the intestinal morphology of weaning piglets (Mean values with their pooled standard errors)

Con, control; MF, maize fibre; SF, soyabean fibre; WBF, wheat bran fibre; PF, pea fibre.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Effects of the fibre sources on volatile fatty acid concentrations
VFA concentrations in the intestinal digesta are presented in Table 6. In the colon, there was no difference in VFA concentrations between the pigs fed the control diet and the fibre source diets; however, pigs on the MF diet had the lowest VFA concentration when compared with those fed the other diets. Higher acetate, propionate, butyrate and total VFA concentrations were observed in pigs on the SF diet when compared with those fed the MF diet (P= 0·026, 0·005, 0·013 and 0·010, respectively). The concentration of butyrate was higher in pigs fed the WBF diet than in those on the MF diet (P= 0·036). Meanwhile, an increase in acetate (P= 0·035) and total VFA (P= 0·035) concentrations was observed in PF diet-fed pigs compared with those on the MF diet.
Table 6 Effects of the dietary fibres on the volatile fatty acid (VFA) concentrations of weaning piglets (Mean values with their pooled standard errors)

Con, control; MF, maize fibre; SF, soyabean fibre; WBF, wheat bran fibre; PF, pea fibre.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Effects of the fibre sources on intestinal mucosal diamine oxidase activities, transforming growth factor-α, trefoil factor family and MHC-II concentration
Concentrations of the intestinal factors are presented in Table 7. In the ileum, DAO activity was higher (P= 0·022) in pigs fed the WBF diet than in those on the control diet. In the colon, pigs on the PF diet had higher TFF (P= 0·003), TGF-α (P= 0·041) and MHC-II (P= 0·020) concentrations than those on the control diet. Meanwhile, pigs supplemented with the WBF and PF diets had higher TFF concentrations than those on the MF (P< 0·001 and P= 0·006, respectively) and SF diets (P< 0·001 and P= 0·004, respectively). MHC-II concentrations were higher in pigs fed the PF diet than in those on the SF diet (P= 0·013). However, there was no effect on SIgA concentrations between the diets in the ileum and colon.
Table 7 Effects of the dietary fibres on the intestinal factors of weaning piglets (Mean values with their pooled standard errors)

Con, control; MF, maize fibre; SF, soyabean fibre; WBF, wheat bran fibre; PF, pea fibre; DAO, diamine oxidase; TGF-α, transforming growth factor-α; TFF, trefoil factor family; SIgA, secretory IgA.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Effects of the fibre sources on intestinal bacteria
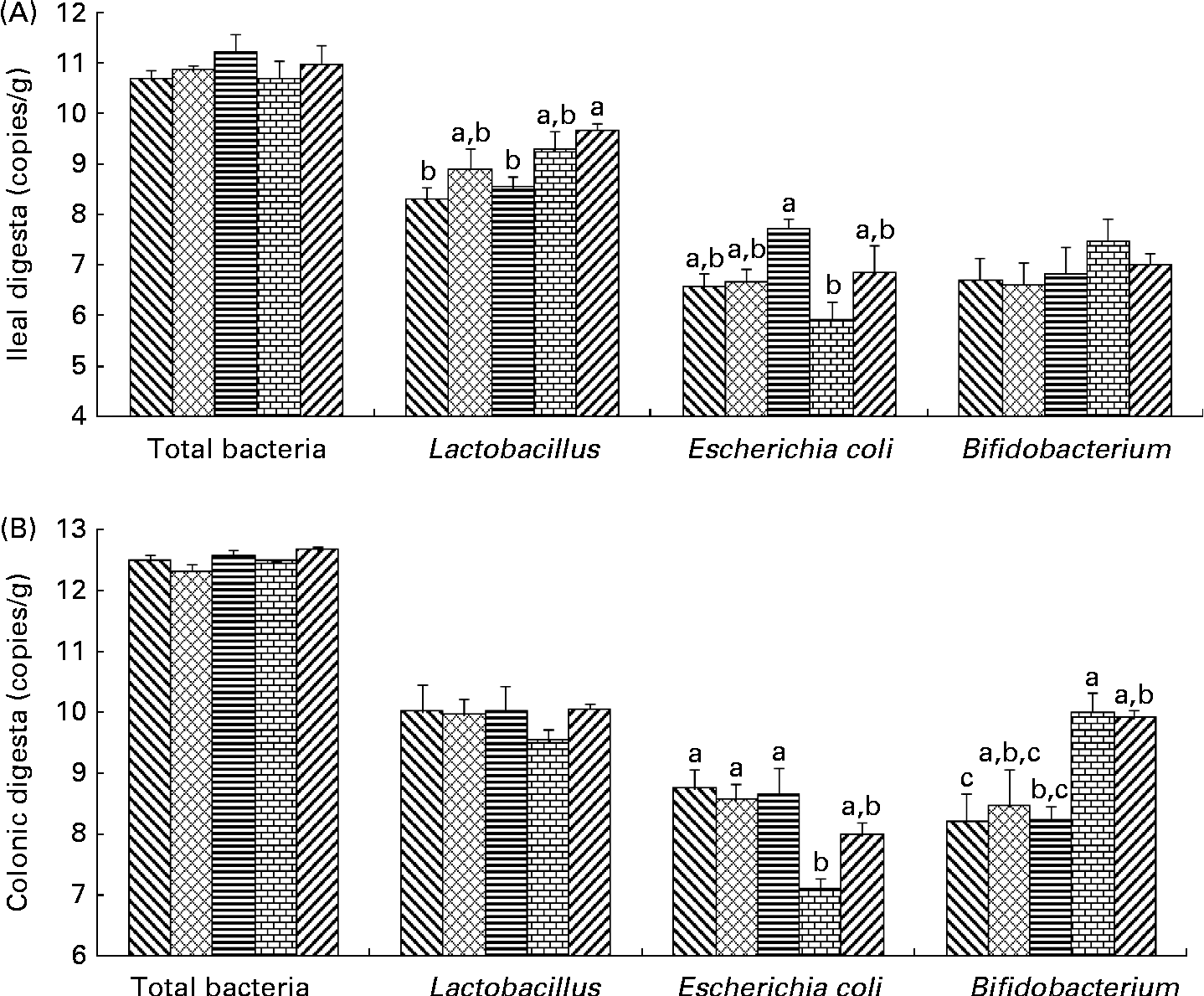
Bacterial populations in the intestinal digesta were affected by the dietary fibres (Fig. 1). There was no effect of the diets on total bacterial populations in pigs. In the ileum, Lactobacillus populations were higher in pigs fed the PF diet than in those on the control (P= 0·012) and SF diets (P= 0·037). In the colon, an increase in Bifidobacterium populations was found in pigs fed the WBF diet compared with those on the control (P= 0·035) and SF diets (P= 0·040). Higher colonic Bifidobacterium populations were observed in pigs fed the PF diet compared with those on the control diet (P= 0·047). A difference in E. coli populations was observed in the ileum and colon. Pigs supplemented with the WBF diet had lower (P= 0·021) ileal E. coli populations than those on the SF diet. In addition, colonic E. coli populations decreased in pigs fed the WBF diet compared with those on the control (P= 0·010), MF (P= 0·024) and SF diets (P= 0·016).

Fig. 1 Effects of the dietary fibres on intestinal bacteria in the (A) ileal and (B) colonic digesta. CT (![]() ), control; MF (
), control; MF (![]() ), maize fibre; SF (
), maize fibre; SF (![]() ), soyabean fibre; WBF (
), soyabean fibre; WBF (![]() ), wheat bran fibre; PF (
), wheat bran fibre; PF (![]() ), pea fibre. Values are means (n 5), with standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within a cluster of bars, not across the clusters of bars (P< 0·05).
), pea fibre. Values are means (n 5), with standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within a cluster of bars, not across the clusters of bars (P< 0·05).
Effects of the fibre sources on intestinal gene expression
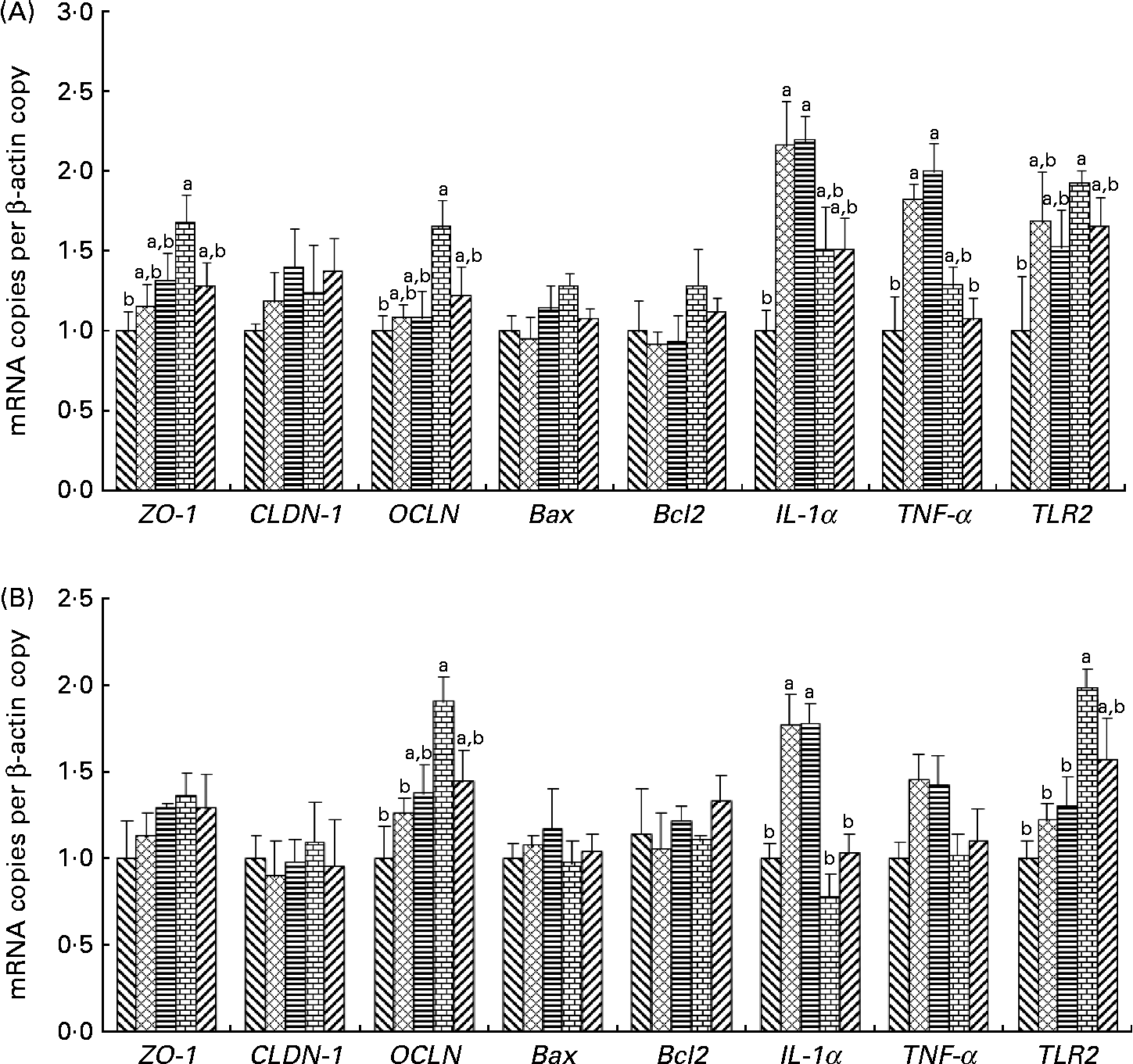
The effects of the dietary fibres on intestinal gene expression are presented in Fig. 2. In the ileum and colon, an increase in OCLN and TLR2 mRNA levels was observed in pigs fed the WBF diet compared with those on the control diet (P= 0·049 and 0·037, respectively). Pigs supplemented with the WBF diet had higher ileal ZO-1 mRNA levels than those on the control diet (P= 0·045). Meanwhile, the WBF diet increased colonic OCLN mRNA levels compared with the MF diet (P= 0·004). Pigs fed the WBF diet showed higher colonic TLR2 mRNA levels when compared with those on the MF (P= 0·020) and SF diets (P= 0·039). However, pigs supplemented with the MF and SF diets had higher IL-1α mRNA levels in the colon compared with those on the control (P= 0·005 and 0·004, respectively), WBF (P< 0·001 and < 0·001, respectively) and PF diets (P= 0·007 and 0·006, respectively), and had higher IL-1α mRNA levels in the ileum compared with those on the control diet (P= 0·014 and 0·011, respectively). Meanwhile, higher ileal TNF-α mRNA levels were also observed in pigs fed the MF and SF diets compared with those on the control (P= 0·014 and 0·019, respectively) and PF diets (P= 0·027 and 0·036, respectively). However, no differences in Bcl-2-associated X protein and Bcl2 mRNA levels were observed in pigs fed all the diets.

Fig. 2 Effects of the dietary fibres on intestinal gene expression in (A) the ileum and (B) the colon. CT (![]() ), control; MF (
), control; MF (![]() ), maize fibre; SF (
), maize fibre; SF (![]() ), soyabean fibre; WBF (
), soyabean fibre; WBF (![]() ), wheat bran fibre; PF (
), wheat bran fibre; PF (![]() ), pea fibre; ZO-1, zonula occludens 1; CLDN-1, claudin 1; OCLN, occludin; Bax, Bcl-2-associated X protein; Bcl2, B-cell lymphoma/leukaemia-2; TLR2, Toll-like receptor 2. Values are means (n 5), with standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different within a cluster of bars, not across the clusters of bars (P< 0·05).
), pea fibre; ZO-1, zonula occludens 1; CLDN-1, claudin 1; OCLN, occludin; Bax, Bcl-2-associated X protein; Bcl2, B-cell lymphoma/leukaemia-2; TLR2, Toll-like receptor 2. Values are means (n 5), with standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different within a cluster of bars, not across the clusters of bars (P< 0·05).
Discussion
Dietary fibre has been reported to decrease diarrhoea incidence in pigs and improve gut health(Reference Wellock, Fortomaris and Houdijk28, Reference Molist, Gómez and Gasa29). According to Molist et al. (Reference Molist, Gómez and Gasa29), wheat bran has been shown to decrease the number of pathogenic E. coli in the faeces and reduce the incidence of post-weaning diarrhoea. Pea fibre (pea hulls and pea inner fibre) has also been shown to improve intestinal health in animals by reducing the adhesion and increasing the excretion of enterotoxigenic E. coli (Reference Becker, Wikselaar and Jansman30). However, in the present study, no effect on diarrhoea incidence was observed between the pigs fed the control diet and the fibre source diets. A difference in diarrhoea incidence was observed among the fibre diets. The result indicates that the effects of dietary fibres on intestinal health are related to fibre sources, maybe resulting from inconsistent intestinal function in regulating intestinal bacteria (such as E. coli).
Previous studies have shown that a lower villous height:crypt depth ratio is associated with microbial challenges and antigenic components of the feed(Reference Huang, Lee and Shih31). Therefore, measurement of intestinal mucosal morphology was used to evaluate the surface area of the intestine undertaken for mucosal integrity. The present results show that supplementation with the WBF diet elevated ileal mucosal integrity by improving ileal villous height and the villous height:crypt depth ratio, in agreement with a previous study showing that feeding pigs with high-insoluble fibre diets might be better protected against pathogenic bacteria by increasing the villous length(Reference Hedemann, Eskildsen and Lærke32).
Previous studies have found that pigs fed soluble and insoluble dietary fibres had more goblet cells in the ileum than those in a fibre-free group(Reference Shingo, Naoki and Kei33), in agreement with the WBF and PF diets in the present study. Goblet cells have been found to play an important protective role in the intestine by synthesising and secreting several mediators, including mucin and peptide trefoil factors (TFF)(Reference Kirk, Julian and Mohammad34). In the present study, a higher colonic TFF concentration was observed in pigs fed on the WBF and PF diets with an increase in goblet cells. TFF, which is found mainly in the small and large intestine, is relatively resistant to proteolytic digestion and can stimulate the repair process(Reference Raymond, Subrata and Asif35). DAO activities in the intestinal mucosa have been determined as a marker for small-intestinal mucosal maturity and integrity in rats(Reference D'Agostino, D'Argenio and Ciacci36). According to Kiyoshi & Yoshihisa(Reference Kiyoshi and Yoshihisa37), DAO activities in the small intestine have been found to be higher in rats fed a fibre diet, such as a mixture of crystal cellulose and carboxymethyl cellulose sodium salt, than in rats fed a fibre-free diet, in agreement with pigs fed the WBF diet in the present study. Previous studies have reported that the function of mucosal TGF-α is to maintain normal epithelial integrity as the ligand of the common epidermal growth factor/TGF-α receptor on adjacent cells(Reference Romano, Polk and Awad38). In the colon, TGF-α concentration was higher in pigs fed the PF diet than in those on the fibre-free diet, suggesting that pigs fed the PF diet had better epithelial integrity. Due to the ability to present peptide antigens to CD4+ T-lymphocytes, the MHC class II molecule is necessary to initiate the immune response(Reference Tjadine, Erik and Peter39). In the present study, pea fibre could support a stronger intestinal immunity defence response against extracellular pathogens by increasing colonic MHC-II concentration. Therefore, the present study indicates that pea fibre and wheat bran fibre could improve intestinal barrier function by increasing the concentration of intestinal barrier factors.
Dietary fibres have been shown to selectively regulate intestinal bacteria, including stimulating the growth of health-promoting bacterial species (bifidobacteria and lactobacilli) and suppressing the growth of potential pathogenic bacterial species (E. coli and clostridia)(Reference Gibson, Beatty and Wang11, Reference Zhong, Cai and Cai12), in agreement with wheat bran and pea fibres in the present study. Previous studies have shown that lactobacilli are a predominant group in the small intestine(Reference Bin, Joshua and Qi40); however, bifidobacteria are a predominant group in the colonic microbiota(Reference Jing, Baoguo and Yanping41), which could be the reason for the difference in Lactobacillus populations observed only in the ileum, and the difference in bifidobacteria populations found only in the colon in the present study. With an increase in Lactobacillus populations in the ileum and bifidobacteria in the colon of piglets fed the WBF diet, the establishment of E. coli was inhibited possibly by a phenomenon known as competitive exclusion, first referred to as colonisation resistance(Reference van der Waaij, Berghuis-de Vries and Lekkerkerk-v42). However, we found that not all of the dietary fibres selected could establish a better intestinal microflora, and there was a difference in E. coli populations between the pigs fed the WBF and SF diets. According to Castillo et al. (Reference Castillo, Skene and Roca43), the host's intestinal bacteria changes in response to dietary fibre composition due to specific substrate preferences of bacteria. Supplementation with specific dietary fibre allows the regulation of the composition of intestinal bacteria(Reference Barbara, Seema and Robert44). These results indicate that more fibre components easily used by bifidobacteria and lactobacilli exist in wheat bran and pea fibres than in maize and soyabean fibres. On the contrary, more fibre components easily used by E. coli exist in soyabean fibre, compared with wheat bran fibre in the present study.
Tight junction proteins (ZO-1, CLDN1 and OCLN) play a crucial role in intestinal barrier integrity, which seal the paracellular space between epithelial cells, thus preventing the paracellular diffusion of intestinal bacteria and other antigens across the epithelium(Reference Dulantha, Rachel and Warren45). In vitro, the probiotic Lactobacillus also have shown to up-regulate occludin and cingulin gene mRNA levels of Caco-2 cells, suggesting that bacteria could affect intestinal barrier integrity by regulating the gene expression level of tight junction proteins(Reference Anderson, Cookson and McNabb13). In contrast, E. coli has been reported to trigger tight junction disruption through destabilisation and dissociation of the ZO-1, occludin and claudin 1 tight junction complex from the epithelial cells(Reference Spitz, Yuhan and Koutsouris14, Reference Muza-Moons, Schneeberger and Hecht15). Previous studies have shown that occludin mRNA levels were higher in rats fed a galacto-oligosaccharide diet than in those fed a fibre-free diet, and the beneficial effect of galacto-oligosaccharides on occludin mRNA levels has been considered to be related to the greater number of bifidobacteria and lactobacilli(Reference Zhong, Cai and Cai12), in agreement with wheat bran fibre in the present study. However, molecular mechanisms by which intestinal bacteria mediate tight junction alterations are still unclear.
Toll-like receptor 2 (TLR2) has been shown to play a key role in microbial recognition and the control of adaptive immune responses(Reference Cario46). TLR2 activated by specific microbial ligands is necessary to preserve intestinal epithelial tight junction-associated integrity against toxic or inflammatory stress-induced damage in rats(Reference Cario47, Reference Mankertz, Tavalali and Schmitz48). According to Gibson et al. (Reference Gibson, Ma and Rosenberger49), TLR2− / − mice suffered impaired epithelial barrier function mediated via ZO-1 and claudin 3, suggesting that TLR2 plays a critical role in maintaining intestinal mucosal integrity during infection by a bacterial pathogen. These results suggest that TLR2 could mediate barrier function via tight junction protein gene expression in pigs fed a wheat bran fibre diet, as specific microbial ligands in the present study. Meanwhile, we found that pigs fed the MF and SF diets up-regulated intestinal pro-inflammatory cytokine (IL-1 and TNF-α) mRNA levels as interference factors of the intestinal barrier(Reference Boivin, Ye and Kennedy50). Pro-inflammatory cytokines have been shown to increase intestinal permeability through the dysregulation of tight junction proteins(Reference Zund, Madara and Dzus51, Reference Berin, Yang and Ciok52), which is the partial reason why lower ZO-1 and OCLN mRNA levels were found in pigs fed the MF and SF diets than in those on the WBF diet.
In the present study, a difference in VFA concentrations in the fibre diets was observed only in the colon, but not in the ileum, which is possibly because the digesta is retained longer in the large intestine than in the small intestine and because microbial populations are greater in the colon than in the ileum(Reference Simpson, McCracken and White53). According to Taciak et al. (Reference Taciak, Pastuszewska and Tuśnio54), a change in total VFA concentrations was observed in the middle colon when pigs were fed different fibre sources (potato fibre or cellulose). A previous study has indicated that butyrate concentration was higher in pigs fed a wheat bran fibre diet than in those on a maize fibre diet(Reference Carneiro, Lordelo and Cunha55), in agreement with the present study. Lower fermentable components observed in the MF diet were due to VFA concentrations being lower in pigs fed the MF diet than in those on the other fibre diets, resulting in lower energy supply for the growth of the intestinal epithelium. However, fermentability of the fibre sources could not explain why wheat bran and pea fibres, and not soyabean fibre, improved intestinal barrier function.
From the composition analysis of fibre sources, it has been found that cereal (maize and wheat bran) fibres have a higher NDF content than legume (soyabean and pea) fibres, which suggests a higher insoluble fibre content in cereal than in legume fibres(Reference Van Soest, Robertson and Lewis20). However, positive effects of wheat bran and pea fibres on ileal and colonic barrier integrity appear to be related to high ADF and cellulose contents of fibre sources rather than to their botanical origin and solubility.
Meanwhile, there is an interesting difference in Ca: a higher Ca content in wheat bran and pea fibre diets, in agreement with the present results showing changes in epithelium integrity. Previous studies have shown that an increase in dietary Ca content has a beneficial impact on intestinal alkaline phosphatase(Reference Brun, Brance and Rigalli56), which is a key enzyme involved in the dephosphorylation (and detoxification) of pro-inflammatory bacterial components resulting in the down-regulation of intestinal inflammation(Reference Lalles57). Also, beneficial effects of dietary Ca have been documented in animal models of colonic inflammation(Reference Schepens, Schonewille and Vink58–Reference van Ampting, Schonewille and Vink60). Incidentally, the protective effects of Ca seem to depend on dietary P(Reference Schepens, ten Bruggencate and Schonewille59), and the WBF diet also displayed a higher P content in the present study.
In conclusion, complex fibre sources could affect intestinal mucosal barrier function and regulate intestinal bacteria in weaning piglets. Also, the present results show that piglets fed the different fibre sources did not have the same qualitative or quantitative effects on ileal and colonic barrier functions. Wheat bran and pea fibres improved intestinal barrier function, probably mediated by changes in microbiota composition and concomitant changes in TLR2 gene expression. Additionally, fibre composition is considered as a more important factor to affect intestinal barrier function in piglets than their botanical origin, solubility and fermentability.
Acknowledgements
The present study was supported by the earmarked fund for the China Agriculture Research System. Each author fully contributed to the design, experiment and interpretation of the results of the study. There is no conflict of interest.