Introduction
Despite major medical research advancements over the past 50 years, CVD remains the leading cause of death worldwide and is responsible for 39 % of non-communicable disease deaths in populations aged under 70 years( 1 ). The leading non-communicable disease risk factor is hypertension, which is responsible for 13 % of global deaths each year and is a major risk factor for coronary artery disease (CAD), IHD and stroke( 1 ).
The pathogenesis of CVD is influenced by a variety of risk factors that can be broadly categorised as either modifiable or non-modifiable( Reference Buttar, Li and Ravi 2 ). Non-modifiable risk factors cannot be controlled through intervention and include advancing age, sex (men at greater risk than premenopausal women; postmenopausal women at greater risk than men), ethnicity and family history of CVD( Reference Buttar, Li and Ravi 2 ). Modifiable risk factors, on the other hand, have the ability to be manipulated through intervention in order to control, treat or modify the risk factor( Reference Buttar, Li and Ravi 2 ). Established modifiable risk factors for CVD include hypertension, tobacco use, raised blood glucose, physical inactivity, unhealthy diet, raised blood cholesterol/lipids and overweight and obesity( Reference Buttar, Li and Ravi 2 ).
Implementation of various lifestyle strategies which target specific modifiable risk factors can reduce the risk of CVD by up to 80 %( 1 , Reference Buttar, Li and Ravi 2 ), thus indicating that CVD is a chronic and mostly lifestyle-induced disease, to which the majority of current mortality is the consequence of previous exposures to behavioural risk factors such as inappropriate nutrition, insufficient physical activity and tobacco exposure( Reference Buttar, Li and Ravi 2 – Reference Clair, Rigotti and Porneala 5 ). In addition, excess weight and central obesity, increased blood pressure, dyslipidaemia, diabetes and low cardiorespiratory fitness are among the factors contributing principally to CVD risk( Reference Buttar, Li and Ravi 2 , Reference Isomaa, Almgren and Tuomi 6 ).
Given the scope and prevalence of CVD within our current food and lifestyle environment, it is clear that preventative measures are the most appropriate to deal with this global health issue in order to reduce the costs to both the community (through improved quality of life) and governments through a reduction in hospitalisations, medication use and rehabilitation( Reference Buttar, Li and Ravi 2 ). Although behavioural factors such as smoking cessation and increased physical activity appear relatively straightforward targets for public health preventative interventions, the definition of a perceived ‘healthy’ diet has changed over time, leading to a general sense of public confusion and uncertainty surrounding the topic( Reference Carpentier and Komsa-Penkova 7 , Reference Harnack, Block and Lane 8 ).
Currently, the most compelling dietary evidence for CVD prevention is linked to whole-diet approaches such as the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets( Reference Carpentier and Komsa-Penkova 7 , Reference Sofi, Cesari and Abbate 9 ). Although the cardioprotective effects of these diets may be credited to a whole-diet/whole-food effect, some individual nutritive components of these foods have also been extensively investigated.
The investigation of single nutritive components demonstrates that the evidence is less clear; this is especially noticeable for fruit and vegetable constituents. While whole fruit and vegetable consumption has been consistently shown to reduce CVD risk, as evidenced by various prospective studies showing a direct inverse association between fruit and vegetable intakes and the development of CVD events such as myocardial infarction (MI) and stroke( Reference Dauchet, Amouyel and Hercberg 10 – Reference Bazzano, He and Ogden 13 ), the various constituents of fruits and vegetables such as vitamin C, polyphenols, fibre and antioxidants are yet to clearly demonstrate a beneficial link or a physiological pathway for their individual effect( Reference Wang, Ouyang and Liu 14 – Reference Vivekananthan, Penn and Sapp 18 ).
A recent and biologically plausible hypothesis for the cardioprotective and blood pressure-lowering effect of vegetables has been linked to their inorganic nitrate (NO3 –)/nitrite (NO2 –) content( Reference Hord 19 ). Support for this hypothesis has been implied in studies indicating that nitrate-rich green leafy vegetables and vitamin C-rich fruits and vegetables contribute most to the apparent cardiovascular protective effect of total fruit and vegetable intake( Reference Bhupathiraju, Wedick and Pan 20 , Reference Joshipura, Ascherio and Manson 21 ). Additionally, cardioprotective diets including the DASH, Mediterranean and traditional Japanese diets have been shown to naturally contain high quantities of inorganic nitrate (147–1222 mg/d) relative to a typical Western-style diet (about 75 mg/d)( Reference Hord, Tang and Bryan 22 – Reference L’Hirondel and L’Hirondel 24 ).
Within the human body, inorganic nitrate/nitrite (NOx) can be metabolised to produce NO (Fig. 1)( Reference Lundberg and Weitzberg 25 , Reference McKnight, Smith and Drummond 26 ). NO is a highly valuable signalling molecule and has been demonstrated to mediate favourable effects on blood pressure control, platelet function, vascular health and exercise performance( Reference Kapil, Weitzberg and Lundberg 27 – Reference Bailey, Winyard and Vanhatalo 30 ). In addition, the utility of inorganic NOx as an NO donor may be of particular relevance given that one serving of nitrate-rich vegetables (such as beetroot) has been estimated to produce more NO under specific conditions than can be endogenously formed by the classical l-arginine–nitric oxide synthase (NOS) pathway each day( Reference Hord 19 , Reference Kelm 31 , Reference Hotchkiss 32 ) (Fig. 1 ( Reference Webb, Patel and Loukogeorgakis 33 )).
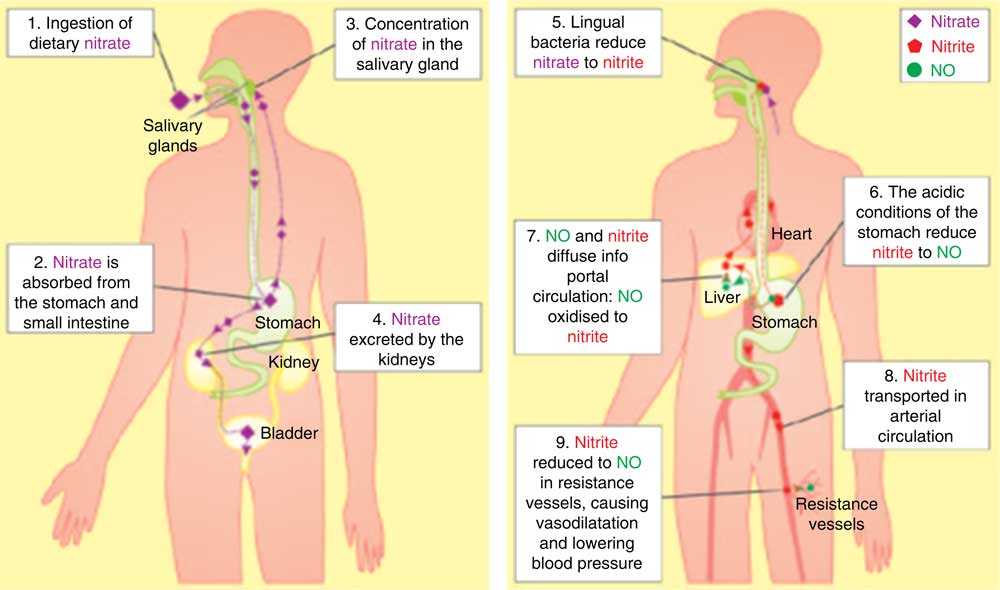
Fig. 1 The fate of dietary nitrate. Nitrate is systematically absorbed becoming concentrated in the salivary glands and part of the salivary circulation. Salivary nitrate is reduced to nitrite by oral bacteria. In the stomach nitrite may produce NO. Nitrite transported in arterial circulation can be reduced to NO in low oxygen concentrations which can lead to vasodilation and reductions in blood pressure (Webb A, Patel N, Loukogeorhakis S, et al. Acute blood pressure lowering,vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension, vol. 51, pp. 784–790, from http://hyper.ahajournals.org/content/51/3/784.short ( Reference Webb, Patel and Loukogeorgakis 33 )).
Currently, the true effect that dietary/inorganic NOx may have on CVD risk factors and outcomes is poorly understood, but it is a highly worthwhile line of investigation given that an increased daily consumption of nitrate intake represents a potential low-cost and simple treatment option for reducing CVD burden.
Production of nitric oxide in the body
Endogenous production via the l-arginine–nitric oxide synthase pathway
The notion that NOx could be produced endogenously in the body was first considered in the early 1980s, upon finding that NOx excretion was exceeding quantities of ingestion in animal and human models( Reference Li and Förstermann 34 , Reference Green, De Luzuriaga and Wagner 35 ). Later it was demonstrated that l-arginine was the substrate for synthesising nitrogen oxides endogenously via the action of NOS enzymes( Reference Palmer, Ashton and Moncada 36 ).
In healthy individuals the l-arginine–NOS pathway can produce sufficient quantities of NO to maintain health (approximately 1·7 mmol/d)( Reference Kelm 31 , Reference Hotchkiss 32 ). However, conditions such as diabetes mellitus, ageing, hypercholesterolaemia and tobacco exposure have been found to make an impact on the bioactivity of endogenously produced NO via one or more of the following functions( Reference Guzik, Mussa and Gastaldi 37 – Reference Ichiki, Ikeda and Haramaki 42 ):
-
(1) Increased degradation of NO( Reference Taddei, Virdis and Ghiadoni 38 , Reference Ichiki, Ikeda and Haramaki 42 , Reference Bryan and Loscalzo 43 );
-
(2) Altered phosphorylation and activation of NOS( Reference Taddei, Virdis and Ghiadoni 38 , Reference Bryan and Loscalzo 43 );
-
(3) Increased production of NOS inhibitors (for example, asymmetric dimethylarginine; ADMA), leading to disruption of NOS activation( Reference Taddei, Virdis and Ghiadoni 38 , Reference Feron, Dessy and Moniotte 39 , Reference Böger, Bode-Böger and Szuba 41 – Reference Bryan and Loscalzo 43 );
-
(4) Deficiency of the NOS substrate, l-arginine( Reference Li and Förstermann 34 , Reference Taddei, Virdis and Ghiadoni 38 , Reference Böger, Bode-Böger and Szuba 41 );
-
(5) Reduced availability of one or more cofactors essential for NOS function( Reference Li and Förstermann 34 , Reference Taddei, Virdis and Ghiadoni 38 ).
While appropriate medical management, consumption of a healthy diet and moderate exercise can somewhat reverse these effects, it has been postulated that supplementing parts of the NOS pathway may enhance NOS activity and NO production( Reference Taddei, Virdis and Ghiadoni 38 , Reference Böger, Bode-Böger and Szuba 41 , Reference Bryan and Loscalzo 43 ). This has been of particular importance given that increased ADMA levels inhibit NOS function and have been cited as the strongest risk predictor of cardiovascular events, and all-cause and cardiovascular mortality in individuals with CAD( Reference Sibal, Agarwal and Home 44 ). Although it remains unclear whether a change in ADMA can alter CVD risk, interventions such as l-arginine supplementation have been shown to improve endothelial-mediated vasodilation in individuals with elevated ADMA levels( Reference Böger, Bode-Böger and Szuba 41 , Reference Sibal, Agarwal and Home 44 ).
As a result, the effect of l-arginine supplementation has been investigated and short-term supplementation has been shown to improve endothelial function and relieve symptoms in patients with CHD( Reference Creager, Gallagher and Girerd 45 ). Long-term (6 months) supplementation, however, demonstrated no beneficial effect( Reference Bednarz, Jaxa-Chamiec and Maciejewski 46 ). In fact the long-term l-arginine supplementation led to increased rates of death and less cardiovascular improvement compared with the placebo due to the development of arginine toxicity and hyperkalaemia (abnormally high serum K)( Reference Schulman, Becker and Kass 47 , 48 ). In addition, the utility of supplementing arginine is questionable given that arginine is classified as a ‘semi-essential’ or ‘conditionally essential’ amino acid, depending on the developmental stage or health status of the individual( Reference Nakaki and Hishikawa 49 ). However, it is generally accepted that healthy adults should not need to supplement with arginine as their bodies produce physiologically sufficient amounts( 48 ). Arginine is also highly abundant in the diet, as rich dietary sources include meat, dairy products, vegetables, legumes and whole grains( 48 , Reference Nakaki and Hishikawa 49 ).
The ‘arginine paradox’ appears to address this notion, as it refers to the phenomenon that exogenous arginine causes NO-mediated biological effects, despite the fact that NOS are theoretically saturated in the substrate l-arginine( Reference Nakaki and Hishikawa 49 ). A recently published cross-sectional study including 2771 men and women investigated whether regular dietary intakes of l-arginine were associated with serum NOx, as an indicator of systemic NO production( Reference Mirmiran, Bahadoran and Ghasemi 50 ). This study found that increased dietary l-arginine intakes were strongly associated with serum NOx, which was independent of the overall dietary patterns of the study participants and other dietary factors, including intakes of high-nitrate-containing foods (probably due to collection of fasting blood samples)( Reference Mirmiran, Bahadoran and Ghasemi 50 ). Therefore, although there may be some utility in consuming adequate amounts of arginine, which is readily achieved by consumption of a healthy balanced diet, there appears to be no great benefit for the general population to be using arginine supplements. However, dietary intervention to also consume nitrate-rich foods holds much promise for supplementing the NOS pathway via the alternative nitrate–nitrite–NO pathway.
The nitrate–nitrite–nitric oxide pathway
Up until the early 1990s, plasma NOx were considered to be biologically inactive endproducts of NO production in the human body. However, it is now clear that under specific conditions nitrate and nitrite anions can be recycled in vivo back to NO( Reference McKnight, Smith and Drummond 26 , Reference Kapil, Weitzberg and Lundberg 27 , Reference Weitzberg and Lundberg 51 , Reference Zweier, Samouilov and Kuppusamy 52 ).
With a bioavailability of 100 %, ingested inorganic nitrate is swiftly absorbed in the proximal small intestine leading to significantly raised plasma nitrate concentrations for a period of up to 5–6 h post-nitrate ingestion( Reference Kapil, Weitzberg and Lundberg 27 , Reference Webb, Patel and Loukogeorgakis 33 , Reference Hobbs, George and Lovegrove 53 – Reference Lundberg and Govoni 55 ). About 75 % of this nitrate is excreted at the kidneys; however, the other 25 % of plasma nitrate is actively extracted by the salivary glands, leading to salivary nitrate concentrations which are ten to twenty times higher than plasma nitrate concentrations( Reference Kapil, Weitzberg and Lundberg 27 , Reference Bryan and Loscalzo 43 , Reference Lundberg and Govoni 55 – Reference Bondonno, Liu and Croft 57 ). Salivary nitrate accumulation must occur in order for nitrate to be reduced to nitrite, as anaerobic bacteria in the oral cavity use nitrate as an alternative electron acceptor to oxygen during respiration( Reference Kapil, Weitzberg and Lundberg 27 , Reference Lundberg and Govoni 55 , Reference Pannala, Mani and Spencer 56 , Reference Tannenbaum, Weisman and Fett 58 ). When this nitrite-rich saliva is swallowed it is reduced in the acidic stomach to produce nitrogen oxides including NO( Reference McKnight, Smith and Drummond 26 , Reference Kapil, Weitzberg and Lundberg 27 , Reference Zweier, Samouilov and Kuppusamy 52 , Reference Benjamin, O’Driscoll and Dougall 59 ). Today, this process is widely known as the nitrate–nitrite–NO pathway, and is thought to be one of the body’s major sources of NO generation, especially in situations when NO bioavailability via the conventional l-arginine–NOS pathway is compromised. In addition it has been suggested that the nitrate–nitrite–NO pathway may play a significant role in maintaining levels of bioactive NO and may be critical for maintaining cardiovascular homeostasis in the body( Reference Kapil, Weitzberg and Lundberg 27 , Reference Hobbs, George and Lovegrove 53 , Reference Coggan, Leibowitz and Spearie 60 ).
Noteworthy factors other than inorganic nitrate and nitrite consumption which have been shown to facilitate the nitrate–nitrite–NO pathway include:
-
(1) Entero-salivary nitrate cycling. Approximately 25 % of plasma nitrate is actively taken up by the salivary glands leading to significant nitrate accumulation in the saliva. Within the oral cavity, anaerobic bacteria reduce nitrate to nitrite via the action of nitrate-reductive enzymes. Nitrite-rich saliva must be swallowed to produce NO in the acidic stomach. The importance of this salivary nitrate cycling has been demonstrated in studies where subjects spat after a dietary load of inorganic nitrate, preventing the opportunity for nitrate to accumulate in the saliva and be reduced to nitrite, therefore preventing NO production and any beneficial effects( Reference Lundberg and Weitzberg 25 , Reference Webb, Patel and Loukogeorgakis 33 , Reference Lundberg, Weitzberg and Gladwin 61 ).
-
(2) Presence of anaerobic bacteria. Mammalian bacteria can utilise nitrate as an alternative electron acceptor to oxygen during respiration, and is a vital component of the nitrate–nitrite–NO pathway as human cells lack the required nitrate reductase enzymes( Reference Lundberg, Weitzberg and Gladwin 61 ). The importance of these bacteria has been further established in studies of germ-free rats, in which gastric NO formation was negligible post-dietary nitrate load( Reference Sobko, Reinders and Norin 62 ). Additionally, human studies have demonstrated that the use of commercial antibacterial mouthwash in human subjects abolished any blood pressure-lowering effects of a dietary nitrate load, indicating that the mouthwash killed off the commensal facultative bacteria in the mouth, thus preventing the production of nitrite and NO leading to a loss of beneficial health effects( Reference Bondonno, Liu and Croft 63 – Reference Duncan, Dougall and Johnston 65 ).
-
(3) Hypoxic conditions. The rate in which nitrate is reduced to nitrite is thirty times greater during conditions of low oxygen tension, as the oral bacteria use salivary nitrate as an alternative electron acceptor to oxygen during respiration( Reference Duncan, Dougall and Johnston 65 ). Xanthine oxidoreductase has also been shown to catalyse the reduction of nitrite to NO in hypoxic conditions( Reference Zhang, Naughton and Winyard 66 – Reference Webb, Bond and McLean 68 ). This could also account for the increased production and utility of NO seen in exercising skeletal muscle or during myocardial ischaemia( Reference Zweier, Samouilov and Kuppusamy 52 , Reference Lundberg, Weitzberg and Gladwin 61 , Reference Duranski, Greer and Dejam 69 ). It is also important to note that plasma nitrite can be reduced to NO along the physiological oxygen gradient of the circulatory system( Reference Gladwin, Raat and Shiva 70 ). Specifically, deoxygenated Hb in the peripheral circulation can act as a nitrite reductase for NO production, as it has been revealed that as Hb deoxygenation increases, more NO is produced( Reference Cosby, Partovi and Crawford 71 – Reference Doyle, Pickering and DeWeert 73 ). This provides an explanation for how various human studies have observed vasodilation after a NOx load, in healthy subjects at rest( Reference Webb, Patel and Loukogeorgakis 33 , Reference Ashworth, Mitchell and Blackwell 74 ).
-
(4) Acidic conditions. Nitrite in the acidic stomach has been shown to spontaneously decompose to NO, a reaction that appears to increase in conditions of reduced pH (increased acidity)( Reference McKnight, Smith and Drummond 26 ). The importance of an acidic stomach for this reaction has been demonstrated in a study showing that NO production via nitrite protonation was inhibited in individuals using proton pump inhibitors (medications which reduce the acidity of gastric juices)( Reference Lundberg, Weitzberg and Lundberg 75 ).
-
(5) Presence of reducing agents including vitamin C and polyphenols. Both vitamin C and polyphenols are abundant in a vegetable-rich diet, and their presence in the diet has been shown to favour the formation of NO via the nitrate–nitrite–NO pathway and prolong the half-life of NO in the stomach( Reference Mowat, Carswell and Wirz 76 , Reference Gago, Lundberg and Barbosa 77 ).
Sources of dietary inorganic nitrate and nitrite
N is vital to life on Earth and can undergo many chemical and biological changes in order to be amalgamated into living and non-living material. An essential form of environmental N includes inorganic nitrate, as an adequate nitrate supply in the soil is essential for plant growth( Reference Bryan and Loscalzo 43 , Reference Crawford 78 ).
The two major determining factors of the nitrate content of vegetables and fruit include their species and the amount of available nitrate in the soil( Reference Bryan and Loscalzo 43 ). Some species of vegetables such as green leafy vegetables (mean nitrate about 975–3624 mg/kg) and beetroot (mean nitrate about 1992 mg/kg) are naturally high in nitrate; however, environmental factors can lead to great variation among samples( Reference Hord, Tang and Bryan 22 ). These factors include seasonal differences and disruption to normal plant growth, leading to nitrate accumulation in the plant leaves, stems and stalks, due to changes in the photosynthetic conversion of plant nitrate to amino acids( Reference Crawford 78 – Reference Kaiser and Brendle-Behnisch 80 ). Therefore, established factors shown to effect the normal growth of plants include drought conditions, high temperatures, shady and cloudy conditions, deficiency of soil nutrients, and excessive soil N( Reference Bryan and Loscalzo 43 ). Additionally, farming practices leading to damaged produce, early harvest, storage and transport conditions, processing and cooking practices will also result in significant variation in vegetable and fruit nitrate content( Reference Bryan and Loscalzo 43 ).
European-based studies have demonstrated that organically grown vegetables have a lower nitrate content than conventionally grown crops, despite the fact that organic fertilisers may cause high nitrate levels in vegetables, depending on the types and amount of organic fertilisers applied( Reference Muramoto 81 ). A California-based study by Muramoto( Reference Muramoto 81 ) reiterated this notion, as it found that spinach grown and harvested during the same season and under the same farming practices had a wide range of nitrate contents. This range appeared greatest in organic spinach, in which the maximum nitrate content measured was 3000 mg/kg, which was five times higher than the minimum (600 mg/kg)( Reference Muramoto 81 ). However, this study also demonstrated that conventionally grown spinach contained on average 30 % more nitrate than spinach grown organically, a result most probably explained due to the wide use of N-containing fertilisers in conventional farming( Reference Muramoto 81 ).
Muramoto( Reference Muramoto 81 ) also found a statistically significant seasonal difference in the nitrate content of iceberg lettuce, as winter samples were found to have on average 52 % more nitrate than summer samples( Reference Muramoto 81 ). This finding is consistent with Ekart et al. ( Reference Ekart, Gorenjal and Madorran 82 ), which found lettuce harvested during summer had a statistically significant lower nitrate content than lettuce harvested during winter (summer harvest: 1209 mg/kg; winter harvest: 2164 mg/kg). In addition, Ekart et al. ( Reference Ekart, Gorenjal and Madorran 82 ) found that washing leafy greens reduced the nitrate content of foods on average by 19 %. Other processing, such as boiling, blanching and sautéing, were found to significantly reduce the nitrate content of spinach by 53, 36 and 30 %, respectively( Reference Ekart, Gorenjal and Madorran 82 ), a finding which could be partly explained due to the water-soluble nature of inorganic nitrate( Reference Omar, Artime and Webb 83 ).
Due to the high variability of nitrate within plant species, accurate and reliable nitrate intake measured from fruit and vegetable consumption is difficult to predict. Despite this, combined vegetable and fruit intake is the major source of exogenous inorganic nitrate exposure and is predicted to constitute 30–90 % of total nitrate intake( Reference Du, Zhang and Lin 84 ). Other sources of nitrate intake include drinking water and meat products; however, their nitrate content is highly regulated to comply with strict government limits( 85 – Reference Shuval and Gruener 89 ).
Nitrate occurs naturally in the water supply; however, in most developed countries water nitrate is generally present in concentrations much lower than allowed in the water guidelines (≤50 mg/l)( 85 , 86 , Reference Ward, DeKok and Levallois 88 ). Therefore, nitrate from the water supply is unlikely to contribute significantly to total nitrate intake in comparison with food sources.
Nitrate and nitrite salts (for example, potassium nitrite/sodium nitrate) have been used as food additives in cured meats for many years due to their effectiveness in ensuring microbial safety and their ability to enhance the flavour and appearance of the product( Reference Bryan and Loscalzo 43 ). The maximum levels of nitrate and nitrite allowed as food additives have been defined (Table 1)( 85 , Reference Jukes 90 – 92 ).
Table 1 Permissions for nitrate and nitrite in food productsFootnote *

* Nitrate salt: potassium nitrate and sodium nitrate. Nitrite salt: potassium nitrite and sodium nitrite.
It has been estimated that approximately 60–80 % of dietary nitrates are derived from vegetables (mainly green leafy and root vegetables), indicating that vegetable intake tends to contribute the greatest quantities of dietary nitrate (Table 2)( Reference Hord, Tang and Bryan 22 , Reference Weitzberg and Lundberg 93 ). This has been further implied by dietary patterns such as the DASH diet, Mediterranean, vegetarian and traditional Japanese diets which tend to include high quantities of vegetables (five or more serves per d) and provide approximately 147–1222 mg nitrate per d( Reference Hord, Tang and Bryan 22 – Reference L’Hirondel and L’Hirondel 24 ). This is a relatively high nitrate intake compared with the typical Western-style diet which tends to be low in vegetables (one to three serves per d) and provides about 60–75 mg nitrate per d( Reference L’Hirondel and L’Hirondel 24 ). In addition, processed and cured meats are frequently cited as the major dietary source of nitrite (Table 3)( Reference Hord, Tang and Bryan 22 , Reference Lundberg and Weitzberg 25 , Reference Du, Zhang and Lin 84 , Reference Machha and Schechter 94 ), followed by various fruits and vegetables (Tables 2, 4 and 5) that have been physically damaged or poorly stored, as enzymes present in the plant tissues and/or contaminating bacteria facilitate the reduction of nitrate to nitrite( Reference Bryan and Loscalzo 43 , 85 ).
Table 2 Vegetable sources of nitrate and nitrite with estimated nitrate and/or nitrite contentsFootnote * (Mean values and ranges)

NA, data not available; ND, not detected.
* Data are combined nitrate and nitrite estimates from various published papers, government documents and reviews.
Table 3 Meat-based sources of nitrate and nitrite with estimated nitrate and/or nitrite contentsFootnote * (Mean values and ranges)

ND, not detected; NA, data not available.
* Data are combined nitrate and nitrite estimates from various published papers, government documents and reviews.
Table 4 Fruit sources of nitrate and nitrite with estimated nitrate and/or nitrite contentsFootnote * (Mean values and ranges)

NA, data not available; ND, not detected.
* Data are combined nitrate and nitrite estimates from various published papers, government documents and reviews.
Table 5 Nitrate- and nitrite-containing herbs with estimated nitrate and/or nitrite contentsFootnote * (Mean values and ranges)

ND, not detected.
* Data are combined nitrate and nitrite estimates from various published papers, government documents and reviews.
Nitric oxide in the cardiovascular system
Within the cardiovascular system, basal endothelial NO has a critical role in maintaining cardiovascular health as it controls vascular tone, smooth muscle cell proliferation and growth, platelet activity and aggregation, leucocyte trafficking, expression of adhesion molecules and inflammation( Reference Li and Förstermann 34 , Reference Machha and Schechter 94 – Reference De Caterina, Libby and Peng 99 ). However, when the bioavailability of NO is compromised, the beneficial effects of NO are lost and endothelial dysfunction predominates due to the imbalance created between the release of vasoconstrictors and vasodilators (such as NO)( Reference Hobbs, George and Lovegrove 53 , Reference Versari, Daghini and Virdis 100 , Reference Vanhoutte 101 ). This idea has been supported in a study conducted by Kleinbongard et al. ( Reference Kleinbongard, Dejam and Lauer 102 ) which found that plasma nitrite levels are a reliable indicator of endothelial dysfunction and correlate with cardiovascular risk factors in humans. Additionally, endothelial dysfunction has been strongly linked with atherosclerosis development and a number of cardiovascular disorders such as hypertension, CAD, congestive heart failure and peripheral artery disease in multiple longitudinal studies( Reference Hobbs, George and Lovegrove 53 , Reference Vanhoutte 101 , Reference Landmesser and Drexler 103 – Reference Gokce, Keaney and Hunter 107 ).
While in the past most of the evidence suggesting a relationship between endothelial dysfunction and clinical events from atherosclerosis development was considered ‘circumstantial’, more recently conducted cross-sectional studies have indicated that severe endothelial dysfunction of the arteries can trigger events of unstable angina and MI( Reference Vita and Keaney 108 , Reference Schächinger, Britten and Zeiher 109 ). Al Suwaidi et al. ( Reference Al Suwaidi, Hamasaki and Higano 104 ) studied 157 patients with mild CAD for 2·3 years, and found an increased incidence of cardiovascular events in patients with impaired endothelium-dependent vasodilation (NO production of endothelium) of the coronary arteries. In another study by Katz et al. ( Reference Katz, Hryniewicz and Hriljac 110 ), 259 subjects with chronic heart failure were assessed prospectively, to which endothelial dysfunction in chronic heart failure was found to significantly increase risk of mortality, thus supporting the notion that coronary endothelial dysfunction plays a role in the pathogenesis of coronary atherosclerosis, risk of cardiac events and death( Reference Al Suwaidi, Hamasaki and Higano 104 , Reference Katz, Hryniewicz and Hriljac 110 ).
Many factors are known to predispose to endothelial dysfunction, due to reductions in NO concentrations and bioavailability in humans( Reference Li and Förstermann 34 , Reference Lidder and Webb 111 , Reference Lundberg, Gladwin and Weitzberg 112 ). These factors are consistent with the modifiable and non-modifiable risk factors for CVD, including hypertension, hypercholesterolaemia, diabetes, tobacco use, physical inactivity, consumption of unhealthy diets and increased age and sex (NO bioavailability is reduced in postmenopausal women, a period in which CVD risk is drastically increased in women)( Reference Li and Förstermann 34 , Reference Lundberg, Gladwin and Weitzberg 112 – Reference Celermajer, Sorensen and Spiegelhalter 120 ). Interestingly, improved endothelial function is a common feature of experimental intervention studies, which have shown reductions in cardiovascular risk and improvements in endothelial-dependent vasodilation in the coronary and peripheral circulation( Reference Vita and Keaney 108 ). Such interventions commonly include the use of lipid- and blood pressure-lowering medications, smoking cessation and increased physical activity( Reference Vita and Keaney 108 , Reference Tsuchiya, Asada and Kasahara 117 , Reference Celermajer, Sorensen and Georgakopoulos 121 – Reference Hornig, Maier and Drexler 124 ). However, the notion that inorganic nitrate and nitrite either consumed from dietary sources such as green leafy vegetables or supplements is relatively new, and their therapeutic potential as an NO donor via the nitrate-nitrite–NO pathway remains unclear( Reference Lundberg, Gladwin and Weitzberg 112 , Reference DeVan, Brooks and Evans 125 ).
Cardiovascular protective actions of nitric oxide
NO is non-polar and can diffuse freely across cell plasma membranes and is a key signalling molecule capable of many important functions, acting primarily by stimulating intra-cellular receptors within the target cell( Reference Wilson and Hunt 126 ).
Within the vasculature of the cardiovascular system, the primary role for NO’s action is for the regulation of vascular function and blood pressure, a notion which has been clearly demonstrated in animal models in which synthesis of NO was blocked leading to persistently elevated blood pressure( Reference Lundberg, Gladwin and Weitzberg 112 , Reference Channon, Qian and George 127 ). In addition, this interaction has been demonstrated in some recently conducted short-term dietary nitrate trials in human subjects, which showed that peak blood pressure-lowering effects were achieved in synchronisation with peak plasma concentrations of NO (NOx) after a dietary nitrate load( Reference Hobbs, Kaffa and George 28 , Reference Webb, Patel and Loukogeorgakis 33 , Reference Hobbs, Goulding and Nguyen 128 ).
The cellular pathway in which NO exerts this vasodilatory action is well established. NO rapidly diffuses across vascular smooth muscle cell membranes. Within the smooth muscle cells, NO binds to and activates guanylyl cyclase to produce cyclic GMP( Reference Wilson and Hunt 126 ). Once produced, cyclic GMP can have a number of effects in the cells, but many of these effects are mediated through the activation of protein kinase G. Activation of protein kinase G via cyclic GMP leads to the activation of myosin phosphatase which in turn leads to smooth muscle cell relaxation and vasodilation( Reference Wilson and Hunt 126 , Reference Channon, Qian and George 127 ).
In addition to regulating vascular tone, NO can facilitate many other important functions preventing the development of atherosclerosis, which include antiplatelet effects, anti-proliferative effects, anti-inflammatory, and antioxidant effects( Reference Channon, Qian and George 127 , Reference Simon, Stamler and Jaraki 129 , Reference Clapp, Hingorani and Kharbanda 130 ). Although the cellular pathways for these actions are yet to be clearly defined, it is clear that NO is capable of binding to or reacting with a variety of chemical modalities within the cellular environment, including metal-containing proteins, membrane receptors, ion channels, enzymes, transcription factors and oxygen species( Reference Channon, Qian and George 127 , Reference Vallance and Webb 131 ).
Other nitric oxides and possible mechanisms in the cardiovascular system
While NO is the most widely cited bioactive metabolite underpinning the cardiovascular therapeutic benefits of dietary inorganic nitrates and nitrites, it has been suggested that other nitric oxides also play a role( Reference Lundberg and Weitzberg 25 , Reference Weitzberg and Lundberg 93 ). This may be expected, given that dietary constituents in the stomach may react with each other in order to form a variety of bioactive compounds( Reference Lundberg and Weitzberg 25 ). Examples of such compounds include nitrated fatty acids, nitrosothiols and ethyl nitrite( Reference Lundberg and Weitzberg 25 ).
While the biological significance of these compounds is yet to be made clear, the following actions have been suggested:
-
(1) Ethyl nitrite. Rat models have shown that ethanol from alcoholic drinks can interact with salivary-derived nitrite in the acidic stomach, leading to the production of ethyl nitrite( Reference Lundberg and Weitzberg 25 , Reference Gago, Nyström and Cavaleiro 132 ). Ethyl nitrate is a potent smooth muscle relaxant and may have a vasodilatory role in the cardiovascular system( Reference Gago, Nyström and Cavaleiro 132 ).
-
(2) Nitrosothiols. In the stomach, nitrite has been shown to induce S-nitrosation within the gastric compartment. S-nitrosothiols are thought to represent a circulating endogenous reservoir of NO acting as an NO donor( Reference Lundberg and Weitzberg 25 ).
-
(3) Nitrated fatty acids (nitroalkenes). Nitric oxides can react with unsaturated fatty acids to produce nitroalkenes. Analysis of synthetic nitroalkenes derivatives of oleic, linoleic and arachidonic acids reveals that these species possess unique chemical reactions which may support multiple cell signalling events such as vasodilation and reduced inflammation( Reference Lundberg and Weitzberg 25 ). Such events may be mediated through their NO donor capabilities.
Currently the systemic capabilities of these bioactive N compounds remain uncertain; however, it highlights a possible whole-diet effect for exerting a beneficial effect on NO and other relevant cardiovascular signalling molecules. This notion is highlighted by Lundberg and Weitzberg( Reference Lundberg and Weitzberg 25 , Reference Weitzberg and Lundberg 93 ), indicating that various dietary constituents of the Mediterranean diet may interact in the stomach to produce these potentially therapeutic compounds, and may provide an additional explanation for the cardiovascular health benefits/protection seen with this dietary pattern.
Inorganic v. organic nitrate and nitrite
Organic nitrates such as glyceryl trinitrate and isosorbide mononitrate represent the first class of NO donors to reach the clinical setting and have been used extensively in the treatment of various cardiovascular conditions including angina, CAD and heart failure( Reference Omar, Artime and Webb 83 ).
Unlike inorganic nitrates which are relatively simple molecules and naturally occurring in fruits and vegetables, organic nitrates are synthetic compounds produced by a reaction between nitric acid and an alcohol group( Reference Omar, Artime and Webb 83 ). Organic nitrates are complex, non-polar hydrocarbon chains attached to a nitro-oxy-radical (–ONO2), which is responsible for its biological effects (Fig. 2)( Reference Omar, Artime and Webb 83 ).
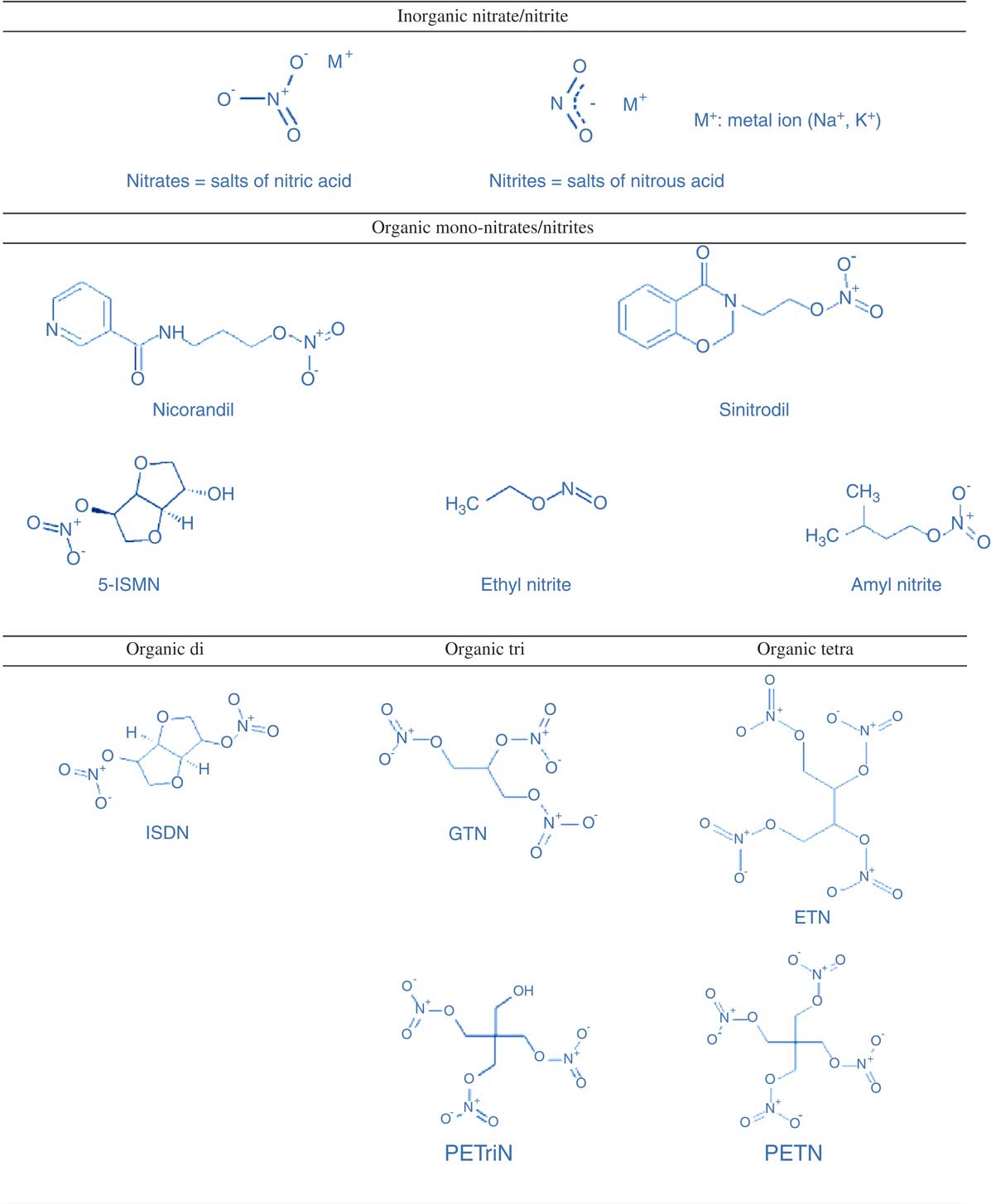
Fig. 2 Chemical structure of inorganic nitrate/nitrite compared with organic mono-, di-, tri- and tetra-nitrates/nitrites. 5-ISMN, isosorbide-5-mononitrate; ISDN, isosorbide dinitrate; GTN, glyceryl trinitrate; ETN, erythritol tetranitrate; PETriN, pentaerythrityl trinitrate; PETN, pentaerythritol tetranitrate. Reprinted from Omar et al. ( Reference Omar, Artime and Webb 83 ), with permission from Elsevier.
Once organic nitrates are introduced to the blood system, levels rise quickly leading to the rapid onset of their action( Reference Omar, Artime and Webb 83 ). At low doses (≤1·25 mg/kg body weight) organic nitrate has been demonstrated to dilate large conductance veins and large arteries, while at high doses (2·5–5 mg/kg body weight) organic nitrates can also induce dilation of the arterioles of the microcirculation( Reference Omar, Artime and Webb 83 ). These vasodilatory effects of organic nitrates have been shown to reduce cardiac work and lower myocardial oxygen requirements, which may alleviate or even prevent cases of MI( Reference Klemenska and Beresewicz 133 ). In addition, it has been suggested that organic nitrates have anti-aggregatory properties in patients with stable and unstable angina( Reference Klemenska and Beresewicz 133 ).
Today in clinical practice short-acting organic nitrates, most notably in the form of glyceryl trinitrate, are administered during the symptomatic treatment of MI and angina( Reference Omar, Artime and Webb 83 , Reference Klemenska and Beresewicz 133 ). Glyceryl tri-nitrates are generally administered in the form of either a mouth spray or intravenous infusion, to which onset of action is rapid (2–3 min)( Reference Klemenska and Beresewicz 133 ). Although short-term treatment with organic nitrates has some positive impact on endothelial function, acute side effects of their use include hypotension, dizziness, nausea and headache( Reference Omar, Artime and Webb 83 ). Also, despite the high potency of organic nitrates and their long history as being used to treat various CVD, nitrate tolerance is a huge limitation and an undesirable side effect of their use( Reference Omar, Artime and Webb 83 , Reference Klemenska and Beresewicz 133 ).
Nitrate tolerance is a complex phenomenon and is poorly understood; however, it is clearly a result of chronic organic nitrate use to which nitrovasodilator responsiveness is lost( Reference Omar, Artime and Webb 83 ). Nitrate tolerance has been reported to occur within 1–3 d of continuous glyceryl trinitrate treatment in patients with MI, stable angina and chronic congestive heart failure( Reference Klemenska and Beresewicz 133 ). Further, chronic organic nitrate use has also been linked to endothelial dysfunction, increased production of free radicals and the development of vascular tolerance to other endothelium-dependent vasodilators( Reference Omar, Artime and Webb 83 ). Although this phenomenon is poorly understood, recent animal and human studies indicate that increased vascular production of the superoxide anion (O2 –) underlies the mechanism for tolerance( Reference Klemenska and Beresewicz 133 ). This oxidative stress hypothesis of nitrate tolerance is supported by numerous reports demonstrating that the tolerance is prevented by co-administration of antioxidants (for example, vitamin C, vitamin E and folic acid) and interventions which inhibit reactive oxygen species formation (lipid- and blood pressure-lowering medications)( Reference Klemenska and Beresewicz 133 – Reference Fontaine, Otto and Fontaine 136 ).
It is interesting to note that the phenomenon of tolerance is not exhibited with the consumption of inorganic nitrates/nitrites; however, despite showing promise in preventing or treating certain cardiovascular conditions, such as hypertension, they have received little attention by the medical community( Reference Kapil, Weitzberg and Lundberg 27 ).
Inorganic nitrate and nitrite: from dietary contaminant to potential therapeutic nutrient
Throughout history, cases of accidental toxic exposure to nitrate and nitrite have been documented; however, the health risk of excessive inorganic nitrate and nitrite consumption appears specific to population subgroups( Reference Hord, Tang and Bryan 22 ). One of these subgroups includes infants aged less than 6 months, to which excessive nitrite exposure has been linked to cases of methaemoglobinaemia (blue baby syndrome)( Reference Greer and Shannon 137 ). As a result, strict regulatory limits have been established to govern the NOx content of the drinking water supply and their use as an additive to processed and cured meats in order to limit exposure to the population( 85 , 86 ).
Methaemoglobinaemia can occur when nitrite oxidises ferrous Fe (Fe2+) in Hb to the ferric state (Fe3+), resulting in methaemoglobin. Methaemoglobin is incapable of binding molecular oxygen, and impairs oxygen delivery to the tissues, causing hypoxia and cyanosis( Reference Greer and Shannon 137 ). While most cases of methomeoglobinaemia have been attributed to the consumption of well water (prone to high nitrate accumulation) used for the preparation of infant formula, there have been reported cases of nitrate poisoning in infants from the ingestion of plant nitrates( 86 , Reference Greer and Shannon 137 ). While Martinez et al. ( Reference Martinez, Sanchez-Valverde and Gil 138 ) found that the use of certain high-nitrate vegetables (herbs and green leafy vegetables) in infant homemade vegetable purée increased methaemoglobinaemia in infants (herbs: OR 5·2, 95% CI 1·1, 24·6; and green leafy vegetables: OR 2·0, 95% CI 0·4, 8·7), the most important factor increasing methaemoglobinaemia was the time lapse between vegetable purée preparation and consumption (OR 17·4, 95% CI 3·5, 86·3 if purée was prepared 24–48 h before; and OR 24·9, 95% CI 3·3, 187·6 if prepared >48 h before)( Reference Martinez, Sanchez-Valverde and Gil 138 ).
To date, human nitrate and nitrite exposure studies have failed to prove a direct link with methaemoglobinaemia, suggesting that NOx exposure alone may not be responsible for methaemoglobinaemia development( Reference Milkowski, Garg and Coughlin 139 , Reference Avery 140 ).
Another population subgroup that is thought to be at health risk due to excessive NOx exposure is high consumers of cured and processed meats( Reference Hord, Tang and Bryan 22 , Reference Bouvard, Loomis and Guyton 141 ). It has been theorised that nitrates and nitrites from processed meats generate N-nitroso compounds which can be carcinogenic( Reference Bingham 142 ).
In October 2015 the International Agency for Research on Cancer (IARC) summarised more than 800 studies conducted globally, and determined that 50 g of processed meat per d increased the risk of colorectal cancer by 18%, and therefore concluded that processed meats are carcinogenic( Reference Bouvard, Loomis and Guyton 141 ). In animal studies N-nitrosamines and related N-nitrosamides have been shown to be carcinogenic in a variety of molecular structures( Reference Gilchrist, Winyard and Benjamin 143 , Reference Magee and Barnes 144 ). However, such direct evidence demonstrating nitrate and nitrite as human carcinogens is severely lacking. This has been reflected in the conclusions of the FAO expert committee who found no consistent increased risk of cancer with increasing consumption of nitrate, as available epidemiological studies did not provide evidence that nitrate is carcinogenic to humans( 145 ).
Currently, researchers are interested in understanding whether the health risks associated with inorganic nitrates/nitrites outweigh the recently discovered health benefits; however, there is a growing consensus that any weak and inconclusive data on inorganic NOx and cancer associations are far outweighed by the potential health benefits of restoring NO homeostasis( Reference Hord, Tang and Bryan 22 , Reference Du, Zhang and Lin 84 , Reference Milkowski, Garg and Coughlin 139 , Reference Gilchrist, Winyard and Benjamin 143 ). In particular this has been demonstrated in various animal and human experimental studies, in which inorganic NOx has been shown to improve outcomes such as blood pressure, endothelial function, platelet function, ischaemia–reperfusion injury, exercise performance and host defence( Reference Gilchrist, Winyard and Benjamin 143 , Reference Kapil, Milsom and Okorie 146 – Reference Dykhuizen, Frazer and Duncan 151 ).
Evidence of cardiovascular benefit from animal studies
Intakes of dietary inorganic nitrate have been shown to be strongly cardioprotective in animal studies. Carlström et al. ( Reference Carlström, Persson and Larsson 152 ) indicated this in a four-arm dietary intervention trial in rats. The rats were placed on either a normal-salt diet (control), a high-salt diet, a high-salt diet supplemented with a nutritional (low) dose of nitrate, and a high-salt diet supplemented with a pharmacological (high) dose of nitrate for 8–11 weeks( Reference Carlström, Persson and Larsson 152 ). As expected, results demonstrated that chronic consumption of a high-salt diet develops hypertension; however, when combined with a low nitrate dose, blood pressure was non-statistically significantly lower( Reference Carlström, Persson and Larsson 152 ). On the other hand, the higher nitrate dose lowered blood pressure by a significant 24 mmHg compared with the plain high-salt diet, a magnitude of blood pressure reduction considerably magnified compared with blood pressure reductions observed in another study of healthy normotensive rats using the same nitrate dose( Reference Carlström, Persson and Larsson 152 , Reference Petersson, Carlström and Schreiber 153 ). Similar results were reported by Kanematsu et al. ( Reference Kanematsu, Yamaguchi and Ohnishi 154 ), finding that in hypertensive rats, antihypertensive effects were only apparent with the highest dose of nitrate, yet there was a strong tissue-protective effect seen with lower doses equivalent to modest dietary intakes. Ferguson et al. ( Reference Ferguson, Hirai and Copp 155 ) demonstrated clinically significant reductions in mean arterial pressure with beetroot juice supplementation in exercising rats (control: 137 (sem 3); beetroot juice: 127 (sem 4) mmHg; P<0·05), indicating that clinically significant blood pressure reductions may be achievable in doses attained from dietary sources( Reference Ferguson, Hirai and Copp 155 ).
In addition to significant blood pressure control, Carlström et al. ( Reference Carlström, Persson and Larsson 152 ) found that dietary nitrate supplementation can partly prevent the development of cardiac hypertrophy and high nitrate doses significantly reduced the fibrotic changes which were observed in the high-salt group, two factors which are major predictors of heart failure( Reference Carlström, Persson and Larsson 152 ). Two other studies found that mice ingesting inorganic nitrate led to a significantly reduced infarct size during myocardial ischaemia, an important finding given that reduced infarct size is associated with lower heart failure risk post-MI and mortality( Reference Bryan, Calvert and Elrod 156 – Reference Minicucci, Azevedo and Polegato 158 ).
When Baker et al. ( Reference Baker, Su and Fu 149 ) treated rats with an intravenous bolus of sodium nitrite across various doses (0·04, 0·4, 1·0, 4·0, 7·0 and 10·0 mg/kg), before initialising a blockage of the coronary artery, there was a clear dose-dependent effect of nitrite on infarct size. However, it was intriguing to note that protection was only found in doses up to 4·0 mg/kg, an effect which was absent at higher doses( Reference Baker, Su and Fu 149 ). Rats administered with 4·0 mg/kg nitrite exhibited a significant 32% reduction in infarct size compared with controls( Reference Baker, Su and Fu 149 ). Nitrite was also found most effective when administered before and/or during the ischaemic event, but not at the onset of reperfusion( Reference Baker, Su and Fu 149 ). Further, equivalent doses of sodium nitrate had no effect on infarct size, indicating that administration timing and doses are key considerations for nitrite protection from MI( Reference Baker, Su and Fu 149 ).
Thrombosis is largely a result of platelet adhesion, activation and aggregation, and is a common pathology underlying IHD and ischaemic stroke( Reference Nieswandt, Pleines and Bender 159 , Reference Raskob, Angchaisuksiri and Blanco 160 ). NO plays a key role in preventing thrombosis development( Reference Park, Piknova and Huang 161 ). Park et al. ( Reference Park, Piknova and Huang 161 ) demonstrates this notion upon discovering an inverse correlation between NOx levels and platelet activity/aggregation in mice. In addition, Apostoli et al. ( Reference Apostoli, Solomon and Smallwood 162 ) examined the effect of inorganic nitrite on platelet aggregation in endothelial NOS-deficient mice. This study found that inorganic nitrite exerts an antiplatelet effect during endothelial NOS deficiency and suggested that dietary nitrate may reduce platelet hyperactivity during endothelial dysfunction( Reference Apostoli, Solomon and Smallwood 162 ).
Pulmonary hypertension can lead to the remodelling of the artery wall, causing abnormalities of elastic fibres, intimal fibrosis and medial hypertrophy( Reference Moraes, Colucci and Givertz 163 ). This can result in vascular stiffness and is a condition linked to the development of chronic heart failure( Reference Moraes, Colucci and Givertz 163 ). Sodium nitrite interventions in lamb and mouse models have shown reductions in pulmonary hypertension specifically during hypoxic conditions( Reference Hunter, Dejam and Blood 164 , Reference Zuckerbraun, Shiva and Ifedigbo 165 ). However, Casey et al. ( Reference Casey, Badejo and Dhaliwal 166 ) found that intravenous injections of sodium nitrite during normoxic conditions could lead to reductions in pulmonary and systemic arterial pressure and increased cardiac outputs in adult male rats. This suggests that sodium nitrite may have a role in reducing the workload of the heart during pulmonary hypertension, thus protecting the heart and vascular system from associated damage and dysfunction( Reference Casey, Badejo and Dhaliwal 166 ).
Hendgen-Cotta et al. ( Reference Hendgen-Cotta, Luedike and Totzeck 167 ) pre-treated mice with nitrate before inducing chronic limb ischaemia, and nitrate supplementation was found to enhance revascularisation and increased mobilisation of circulating angiogenic cells (CAC), which are important for the recovery and maintenance of healthy endothelial function( Reference Hendgen-Cotta, Luedike and Totzeck 167 ). Heiss et al. ( Reference Heiss, Meyer and Totzeck 168 ), on the other hand, injected inorganic nitrite into healthy mice, and found that nitrite significantly increased CAC at 1 h compared with controls. It is interesting to note, however, that when this test was repeated in endothelial NOS-deficient mice, no CAC mobilisation was observed, indicating that NOS may be required to take part in nitrate-mediated CAC mobilisation( Reference Heiss, Meyer and Totzeck 168 ).
In a study conducted by Sindler et al. ( Reference Sindler, Fleenor and Calvert 169 ) the effect of nitrite in aged, but healthy, mice was investigated and high dietary nitrite doses were found to reverse age-related vascular dysfunction, arterial stiffness and reduce levels of oxidative stress. This is in line with Carlström et al. ( Reference Carlström, Persson and Larsson 152 ) who found that key plasma and urinary oxidative stress markers (malondialdehyde, type VI isoprostane (iPF2α-VI) and 8-oxo-2’-deoxyguanosine (8-OHdG)) were significantly reduced (despite co-consumption of a high-salt diet) with both low- (0·1 mmol nitrate/d) and high- (1·0 mmol nitrate/d) dose dietary nitrate supplementation, which may be useful in preventing NO degradation and endothelial dysfunction( Reference Carlström, Persson and Larsson 152 , Reference Cai and Harrison 170 ). This is an interesting finding, given that oxidative stress is directly linked with an inflammatory response which is thought to have a central role in the development of atherosclerosis( Reference Weitzberg and Lundberg 93 ).
Stokes et al. ( Reference Stokes, Dugas and Tang 171 ) found that mice fed cholesterol-enriched diets for 3 weeks tend to develop clear signs of vascular disease pathology, including elevated leucocyte adhesion and endothelial dysfunction, an effect which was prevented with nitrite supplementation in the drinking water. In another study by Carlström et al. ( Reference Carlström, Larsen and Nyström 172 ) it was demonstrated that several features of the metabolic syndrome (including visceral fat and circulating TAG, which are strong risk factors for CVD) can be reversed by dietary nitrate supplementation, in amounts which correspond to those derived from endothelial NOS under normal healthy conditions or a vegetable-rich diet( Reference Carlström, Larsen and Nyström 172 ).
Evidence of cardiovascular benefit from human studies
In 2003, Cosby et al. ( Reference Cosby, Partovi and Crawford 71 ) conducted one of the first studies demonstrating a relationship between inorganic nitrite supplementation and blood pressure reductions in healthy human subjects. This study chose to use sodium nitrite (NaNO2 –) infusions providing approximately 75 mg NaNO2 – over two 15-min periods, a dose which was found to significantly reduce mean blood pressure by 7 mmHg (P<0·01)( Reference Cosby, Partovi and Crawford 71 ). Similar findings were later established using sodium nitrate (NaNO3 –) in a study conducted by Larsen et al. ( Reference Larsen, Ekblom and Sahlin 173 ). In this study healthy subjects consumed NaNO3 – (8·5 mg/kg per d for 3 d) as a dietary supplement, and although systolic blood pressure was not changed during this time compared with placebo (sodium chloride), diastolic blood pressure was significantly reduced on average by 3·7 mmHg (P<0·02) and mean arterial pressure was lowered by 3·2 mmHg (P<0·03)( Reference Larsen, Ekblom and Sahlin 173 ). Soon after, Webb et al. ( Reference Webb, Patel and Loukogeorgakis 33 ) investigated this topic further using beetroot juice (containing approximately 1400 mg inorganic nitrate). Results from Webb et al. ( Reference Webb, Patel and Loukogeorgakis 33 ) showed a peak reduction in systolic blood pressure of 10·4 (sem 3) mmHg (P<0·01), a reduction in diastolic blood pressure of 8·1 (sem 2·1) mmHg (P<0·01) and mean arterial pressure reduction of 8·0 (sem 2·1) mmHg (P<0·01), thus indicating that significant blood pressure reductions are possible with the acute consumption of dietary inorganic nitrate in healthy subjects. This is a notion which has been further supported by a recently conducted systematic review and meta-analysis which found that inorganic nitrate and beetroot juice consumption was associated with greater changes in systolic blood pressure (–4·4 (95% CI –5·9, –2·8) mmHg; P<0·001) than diastolic blood pressure (–1·1 (95% CI –2·2, 0·1) mmHg; P=0·06)( Reference Siervo, Lara and Ogbonmwan 174 ). However, it is important to note that these findings have not been consistent across the literature, as a few recently conducted randomised controlled trials have found that inorganic nitrate consumption from either beetroot juice or from a high-nitrate diet (rich in green leafy vegetables) for 1–2 weeks had little/no effect on the blood pressure of study subjects( Reference Bondonno, Liu and Croft 57 , Reference Bondonno, Liu and Croft 175 , Reference Gilchrist, Winyard and Aizawa 176 ). The exact cause of this variation across studies remains unclear, yet could be due to methodological differences including the study population (for example, healthy subjects v. hypertensive subjects) or the conditions in which NOx was consumed (for example, food v. supplement, dosing or altered environmental conditions such as exercise stress). Nevertheless, this question remains unclear and will require further investigation, in order to better understand the usefulness of dietary/inorganic NOx within the general population.
While the acute effects of dietary inorganic nitrate on blood pressure have been extensively investigated, very few studies have investigated long-term effects. Sobko et al. ( Reference Sobko, Marcus and Govoni 23 ) investigated the effects of a traditional Japanese diet on blood pressure which provided approximately 1140 mg of nitrate per d for a 10 d period. The traditional Japanese diet led to a lower diastolic blood pressure than seen in the non-Japanese diet group (71·3 (sd 7·9) v. 75·8 (sd 7·8) mmHg; P=0·0066), indicating that dietary inorganic nitrate consumption for longer-periods of time may have some blood pressure-lowering effects in healthy individuals; however, a 10 d intervention can hardly be classified as a long-term intervention( Reference Sobko, Marcus and Govoni 23 ). In another 4-week intervention, Kapil et al. ( Reference Kapil, Khambata and Robertson 29 ) assigned hypertensive patients to receive a daily dose of either 250 ml of beetroot juice or placebo (nitrate-depleted beetroot juice). Notably, Kapil et al. ( Reference Kapil, Khambata and Robertson 29 ) found that daily dietary nitrate supplementation significantly reduced mean clinic blood pressure (7·7/2·4 mmHg (range 3·6–11·8/0·0–4·9 mmHg); P<0·001, P=0·05), mean 24 h ambulatory blood pressure (7·7/5·2 mmHg (range 4·1–11·2/2·7–7·7 mmHg); P<0·001 for both) and mean home blood pressure (8·1/3·8 mmHg (range 3·8–12·4/0·7–6·9 mmHg); P<0·001, P<0·01)( Reference Kapil, Khambata and Robertson 29 ).
Currently, the longest intervention study conducted in this area is a 10-week intervention trial from DeVan et al. ( Reference DeVan, Brooks and Evans 125 ). In this study, healthy 50- to 79-year-old subjects were recruited to consume either 0, 80 or 160 mg of sodium nitrite per d for a 10-week period( Reference DeVan, Brooks and Evans 125 ). Results indicated no significant changes in blood pressure at week 10 compared with baseline blood pressure values; however, a significant time × treatment effect for carotid diameter in the nitrite groups was detected, as well as improved endothelial function of the brachial artery, suggesting improved vascular function with chronic inorganic nitrite supplementation despite a lack of an effect seen with blood pressure( Reference DeVan, Brooks and Evans 125 ). However, it is worth noting that the only prospective cohort study on this topic conducted by Golzarand et al. ( Reference Golzarand, Bahadoran and Mirmiran 177 ) found that higher dietary intakes of nitrate-containing vegetables (about 427·6 g/d) in normotensive individuals may have a protective effect against the development of hypertension (highest tertile of nitrate-containing vegetables, OR 0·63 (95% CI 0·41–0·98); P=0·05).
Endothelial dysfunction is one of the key early events involved in the development of atherosclerosis( Reference Raitakari and Celermajer 178 ). Flow-mediated dilatation is commonly used as a measure of endothelial function as reduced flow-mediated dilatation is an indicator of endothelial dysfunction (caused by reduced NO bioavailability) and has been associated with increased severity and duration of blood pressure elevations( Reference Hadi, Carr and Suwaidi 179 ). More recently, dietary inorganic nitrate interventions have been shown to significantly improve flow-mediated dilatation in healthy and hypertensive human subjects consuming spinach, beetroot juice or sodium nitrate capsules( Reference Kapil, Khambata and Robertson 29 , Reference Heiss, Meyer and Totzeck 168 , Reference Bondonno, Yang and Croft 180 , Reference Rodriguez-Mateos, Hezel and Aydin 181 ). Joris & Mensink( Reference Joris and Mensink 182 ) tested the effects of beetroot juice (containing approximately 500 mg nitrate) with a dietary load of fat (56·6 g fat) in overweight and obese subjects (BMI 30·1 (sd 1·9) kg/m2). While the control drink group saw impaired flow-mediated dilatation with dietary fat intake, the consumption of beetroot juice appeared to attenuate this impairment (beetroot juice: –0·37 (sd 2·92) % v. control: –1·56 (sd 2·9) %; P=0·03)( Reference Joris and Mensink 182 ). Additionally, flow-mediated dilatation has been shown to be reduced by approximately 40% after vascular ischaemia; however, Ingram et al. ( Reference Ingram, Fraser and Bleasdale 183 ) demonstrated that sodium nitrite pre-conditioning (providing a nitrite dose before ischaemic event) will prevent ischaemic reperfusion injury by preventing reductions in flow-mediated dilatation and endothelial dysfunction. Similar findings have been reported by Kapil et al. ( Reference Kapil, Khambata and Robertson 29 ) and Webb et al. ( Reference Webb, Patel and Loukogeorgakis 33 ) with beetroot juice pre-conditioning, indicating that higher plasma NOx concentrations achieved by inorganic NOx consumption may have a role for improving cardiovascular outcomes after vascular ischaemic events( Reference Kapil, Khambata and Robertson 29 , Reference Webb, Patel and Loukogeorgakis 33 ).
In addition to flow-mediated dilatation, CAC have been identified as an important indicator of vascular endothelial function, as they have a critical role in vascular repair( Reference Heiss, Jahn and Taylor 184 ). The number of CAC have also been shown to predict the occurrence of CVD and death( Reference Heiss, Meyer and Totzeck 168 ). Therefore it is of interest to note that Heiss et al. ( Reference Heiss, Meyer and Totzeck 168 ) have indicated an important role for dietary nitrate for increasing CAC, showing that a single dose of sodium nitrate (12·7 mg/kg body weight) can double the number of CAC 1–2 h post-nitrate ingestion.
Pulse wave velocity and augmentation index are accepted measurements of arterial stiffness and atherosclerosis, to which higher readings are associated with increased CVD risk( 185 , Reference Chirinos, Zambrano and Chakko 186 ). The role of dietary inorganic nitrate in preventing arterial stiffness has been established, as Kapil et al. ( Reference Kapil, Khambata and Robertson 29 ) found that a 4-week beetroot juice intervention reduced pulse wave velocity and augmentation index in hypertensive subjects. Zamani et al. ( Reference Zamani, Rawat and Shiva-Kumar 187 ) also saw a significantly reduced augmentation index with beetroot juice consumption in patients with symptomatic heart failure (beetroot juice: 132·2 (sd 16·7) %; placebo: 141·2 (sd 21·9) %; mean change –9·1 (sd 15·4) %; P=0·03). Rammos et al. ( Reference Rammos, Hendgen-Cotta and Sobierajski 188 ) investigated the effect of a 4-week sodium nitrate supplementation trial in elderly volunteers with mild hypertension, and found that vascular stiffness was significantly improved in the nitrate-supplemented volunteers. This is a very significant finding given that vascular stiffness tends to naturally increase with age( Reference Liu, Bondonno and Croft 189 ).
In an randomized controlled trial conducted by Jones et al. ( Reference Jones, Pellaton and Velmurugan 190 ), participants prone to MI and undergoing primary percutaneous coronary intervention (non-surgical intervention to treat stenosis) were administered with either a high-dose bolus injection of NaNO2 – (1·8 µmol) or NaCl placebo. The nitrite group experienced a significantly (P=0·05) improved myocardial salvage index (established indicator of cardioprotective benefit) relative to placebo( Reference Jones, Pellaton and Velmurugan 190 ). In addition, a subset of participants who exhibited a blocked blood vessel experienced a 19% reduction in infarct size with nitrite treatment compared with placebo( Reference Jones, Pellaton and Velmurugan 190 ). A 1-year follow-up of study participants also found that the nitrite group experienced a significant reduction in major adverse cardiac events (NaNO2 –: 2·6% v. NaCl: 15·8%; P=0·04)( Reference Jones, Pellaton and Velmurugan 190 ).
Conclusion
CVD remains the major killer from any disease across the developed world. Currently the available evidence indicates a role for dietary nitrate for improving CVD risk factors, a highly valuable finding given that dietary nitrate from beetroot and green leafy vegetables could represent a relatively simple and cost-effective treatment/preventative strategy for reducing CVD and its sequelae. However, at present it remains unclear whether incidence of CVD morbidity or mortality can be reduced with long-term dietary intakes of inorganic nitrate, as such evidence investigating this question directly has not yet been published. At present, there is an overwhelming need for epidemiological research to be conducted to identify the potential long-term effects of sustained inorganic nitrate and nitrite consumption on the development of CVD and its consequences.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
We acknowledge the contribution of all authors to the writing of the present review and J. J. for conceiving the article. All authors approved the final manuscript.
There are no conflicts of interest.










